Abstract
Purpose
223Ra-Chloride (also called Alpharadin®) targets bone metastases with short range alpha particles. In recent years several clinical trials have been carried out showing, in particular, the safety and efficacy of palliation of painful bone metastases in patients with castration-resistant prostate cancer using 223Ra-chloride. The purpose of this work was to provide a comprehensive dosimetric calculation of organ doses after intravenous administration of 223Ra-chloride according to the present International Commission on Radiological Protection (ICRP) model for radium.
Methods
Absorbed doses were calculated for 25 organs or tissues.
Results
Bone endosteum and red bone marrow show the highest dose coefficients followed by liver, colon and intestines. After a treatment schedule of six intravenous injections with 0.05 MBq/kg of 223Ra-chloride each, corresponding to 21 MBq for a 70 kg patient, the absorbed alpha dose to the bone endosteal cells is about 16 Gy and the corresponding absorbed dose to the red bone marrow is approximately 1.5 Gy.
Conclusion
The comprehensive list of dose coefficients presented in this work will assist in comparing and evaluating organ doses from various therapy modalities used in nuclear medicine and will provide a base for further development of patient-specific dosimetry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
223Ra-Chloride targets bone metastases with high linear energy transfer (LET), short range (<100 μm) alpha particles. In recent years several clinical trials have been carried out showing, in particular, the safety and efficacy of palliation of painful bone metastases in patients with castration-resistant prostate cancer (CRPC) using 223Ra-chloride (also called Alpharadin®) [1–4]. Recently the results of the phase III, double-blinde, randomized, international ALSYMPCA study, which compared 223Ra plus best standard of care (BSC) vs placebo plus BSC in CRPC patients with bone metastases, were presented at the 2012 Congress of the American Society of Nuclear Medicine [5]. The authors stated that “223Ra is safe and straightforward to administer using conventional nuclear medicine equipment… ALSYMPCA demonstrated significantly improved overall survival and very low toxicity, suggesting that 223Ra may provide a new standard of care for patients with CRPC and bone metastases.”
Although data have been presented for several clinical trials [1–4], published data on dosimetry of 223Ra-chloride are sparse. There is at present only one publication providing a combined alpha-beta-gamma estimate of the equivalent dose for 223Ra-chloride [1] using a radiation weighting factor for alpha particles of five.
The aim of the present paper is, therefore, to provide detailed data on absorbed alpha and beta/gamma organ doses and on dose coefficients according to the latest model of the International Commission on Radiological Protection (ICRP) [6] for normal organs and tissues. These data could be a base for future risk estimates after treatment with 223Ra similar to what has been published before for 224Ra-chloride [7]. In addition, the data of this work will allow a comparison of patient organ doses of this treatment modality with a variety of other therapeutic nuclear medicine procedures such as radioimmunotherapies or other bone pain palliation therapies.
Materials and methods
Biokinetic behaviour of 223Ra-chloride
After intravenous injection, 223Ra-chloride as a calcium analogue is deposited mainly in the bone. According to ICRP Publication 67 [6], in an adult 25 % of radium in blood is transferred to skeleton. The regional uptake correlates with the intensity of bone metabolism. It has been shown that due to its affinity to osteoblasts the substance is concentrated in parts of the skeleton with increased bone formation [8]. It is, therefore, assumed to slow down synostotic formations and to have analgesic and antiphlogistic effects.
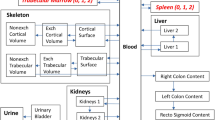
ICRP has developed an age-dependent biokinetic model for alkaline earth elements [6]; Fig. 1 shows the radium-specific model which is based on a model for adults proposed by Leggett [9]. According to this model, radium is transferred from blood to bone surfaces (25 %) and to soft tissues including liver (almost 45 %). Radium is excreted mainly via the gastrointestinal (GI) tract. While radium in the liver is retained with a biological half-time of 50 d before it is re-transferred to the blood, most of the radium in other tissues is quickly re-transferred to the blood with biological half-times of 0.1 d and 1 d, respectively. Radium on bone surfaces is quickly transferred to bone volume (17 %) or re-transferred to blood (83 %) with a biological half-time of 1 d. The activity in (exchangeable) bone volume is retained there with a biological half-time of 30 days and then re-transferred to the bone surface (80 %) or transferred to non-exchangeable bone volume (20 %) where it is retained for many years. However, due to the short half-life of 223Ra (11.4 days), these transfer routes are not considered as essential for the resulting dose contribution.
The biokinetic model of ICRP Publication 67 [6] for radium. The dashed arrows indicate pathways of less importance for the short-lived isotope 223Ra
In the ICRP model [6], the biokinetics of the radium daughter products are taken into consideration as being independent from those of the parent nuclide. For radon isotopes, the activity in soft tissues and on bone surfaces is assumed to be transferred to the blood with a biological half-time of 10 min, and then quickly exhaled. For the activity in bone volume, this process is slower (half-times of 0.46 and 1.9 days). The daughter product of 223Ra is 219Rn with a very short half-life of 4 s; nearly all 219Rn activity produced in bone volume stays with the parent 223Ra.
The next decay product, 215Po, with a very short half-life of <0.01 s, decays at the site of origin. The decay product following 215Po is 211Pb with a half-life of 36.1 min, showing biokinetics similar to that of radium, but with an uptake primarily in the liver and in the kidneys. The final radionuclides of the decay chain, 211Bi, 211Po and 207Tl, have half-lives of less than 5 min. Therefore, these isotopes will only marginally affect the biodistribution of radionuclides within the body.
Treatment schedule
In the recent ALSYMPCA trial, the recommended treatment schedule was six intravenous injections per week with 0.05 MBq/kg of 223Ra-chloride each [5].
Dosimetry
The calculations were performed using the program DOSAGEFootnote 1 which is one of the codes that have been used in the ICRP Task Group on Dose Coefficients to calculate doses for ICRP publications. In addition, the code is used for quality control of dose calculations by other contributors to ICRP. Therefore, its accuracy is constantly checked against other codes used for the same purpose.
The code takes into account the independent biokinetics of daughter nuclides by application of the second method described in ICRP Publication 71, Annex C.3 [10] and uses the dosimetric parameter values proposed by ICRP Publication 67 [6]. The calculations include the recycling of 223Ra and its decay products into the corresponding compartments. The current dose calculations were carried out for the case of intravenous injection for 25 organs and tissues, whereas the data published by ICRP [6, 10] contain results for inhalation and ingestion only.
As ICRP has not yet published specific absorbed fraction (SAF) values based on the new ICRP adult reference voxel phantoms [11], DOSAGE still uses the dosimetric parameters described in ICRP Publication 30 [12]. This implies that the target tissue bone endosteum is calculated as an average over tissue up to a distance of 10 μm from the bone surfaces instead of an assumed thickness of 50 μm for the target endosteal tissues considered in ICRP Publication 110 [11]. As the SAF values according to ICRP Publication 30 only include values for the upper and lower large intestine instead of right, left and rectosigmoid colon, the GI tract model of ICRP Publication 30 has been used instead of the new ICRP Human Alimentary Tract Model published in ICRP Publication 100 [13].
For the calculation of the dose coefficients Howell et al. suggest, based on their experimental data, a radiation weighting factor of 5.4 for 223Ra [14]. Based on a review of experimental literature Sgouros et al. report on a recommended relative biological effectiveness (RBE) value for alpha particles between 3 and 5 for cell killing [15]. Therefore, and for the sake of comparison of the combined alpha-beta-gamma values given by Bruland et al. [1], a radiation weighting factor of 5 has been used in this work for quantifying deterministic radiation effects.
Additionally a radiation weighting factor of 20 was applied as this is the official ICRP radiation weighting factor given in ICRP Publication 103 [16]. This value is strictly based on RBE values for stochastic (cancer) risks, and not for deterministic effects or tissue reactions.
Results
The results of the organ-specific dosimetric calculations for 25 organs or tissues are summarized in Table 1. Given are the absorbed doses (in Gy/Bq) for high LET alpha radiation as well as low LET beta/gamma radiation, and the dose coefficients using the radiation weighting factors of 5 (given in Gy/BqFootnote 2) and 20 (given in Sv/Bq) for alpha radiation and 1 for beta/gamma rays, respectively, as well as the relative contributions of beta/gamma doses to the total absorbed organ doses.
Table 1 reveals that bone endosteum and red bone marrow have the highest dose coefficients (equivalent doses) followed by liver, colon and lower large intestine (LLI) and upper large intestine (ULI). Alpha radiation plays a predominant role for the dose contribution to most of the organs, as indicated by the data of the relative contribution of the absorbed beta/gamma fraction to the total dose (Table 1). For the large intestine and colon compartments, however, due to self-absorption of alpha radiation within the contents, beta radiation originating from daughter nuclides, such as 212Pb, 212Bi and 208Tl, dominates the absorbed dose to the walls. Therefore, the radiation weighting factor for alpha radiation is of minor influence for the calculation of equivalent doses compared to other organs which have a higher dose contribution by alpha radiation.
A series of six treatments for a 70 kg person with an administered activity of 0.05 MBq/kg 223Ra-chloride each (overall: 21 MBq 223Ra-chloride) results in an absorbed alpha dose of approximately 16 Gy to the bone endosteum. The corresponding absorbed dose to the red bone marrow is approximately 1.5 Gy.
Discussion
Based on standardized biokinetics as described in ICRP Publication 67 [3], the results summarized in Table 1 provide a comprehensive set of data for dosimetry in adult patients. Our current absorbed dose calculations were done for a number of organs (see Table 1). The independent contributions of daughter nuclides were taken into account; calculation algorithms were used which determine the individual contributions of each daughter nuclide. In addition, the present work gives the contribution of doses to all organs caused by low LET as well as by high LET radiation. Dose coefficients were calculated applying for alpha particles, for a conservative risk assessment, a radiation weighting factor of 20 [16]. This value is most appropriate for the protection of radiation workers and might be too conservative for use in radionuclide therapy.
The use of SAF values based on the methodology of ICRP Publication 30 [12] instead of the new ICRP reference voxel models [11] has mainly an impact on doses to the bone endosteum, the red bone marrow and the large intestine (colon) compartments. While the impact on the skeletal doses can hardly be assessed at the moment, the colon doses might be lowered to the level of non-source tissue doses because no information on uptake of radium in the colon walls seems to be available and the alpha emissions in the colon contents do not reach the target cells in the colon walls defined in a depth of 280–300 μm [13].
For the sake of comparability to the data published by Bruland et al. [1], additional dose coefficients were calculated applying a radiation weighting factor of 5. As has been discussed recently in Medical Internal Radiation Dose (MIRD) pamphlet 22 [15] human studies using alpha emitters still have to be analysed for deterministic effects. Until these data become available the MIRD Committee recommends a radiation weighting factor of 5 for projecting the possible deterministic biological effects associated with an estimated absorbed dose by alpha particles [15]. Should a modification of the risk factors be available the absorbed doses listed in Table 1 will provide an easy way of recalculating the dose coefficients for 223Ra-chloride.
In Table 2, the dose estimates developed in this work (with the use of a radiation weighting factor of 5 for alpha particles) for selected organs are presented in comparison with those by Bruland et al. calculated for a 70 kg patient [1]. The resulting dose coefficients are quite similar. Data for the red bone marrow as one important potential organ at risk are not provided by Bruland et al. [1]. The organ with the highest dose after therapeutic application of 223Ra-chloride are the bone endosteal cells (16 Gy).
For comparison, the dose to the bone endosteal cells after e.g. application of 2.6 GBq 153Sm-ethylenediamine tetramethylene phosphonate (EDTMP) used in palliative therapy for osseous metastases, the absorbed dose to the bone endosteum is 17.6 Gy [17].
The red bone marrow dose calculated here is a dose averaged over the total red (active) marrow even if a heterogeneous dose distribution with higher doses near to endosteal tissues is expected according to the biokinetic model. In order to better understand potential bone marrow toxicity Hobbs et al. [18] developed a Monte Carlo-based model for cell-level dosimetry. The authors show (1) a heterogeneous distribution of cellular absorbed dose, strongly dependent on the position of the cell within the marrow cavity and (2) that increasing the average marrow cavity absorbed dose, or equivalently, increasing the administered activity resulted in only a small increase in potential marrow toxicity (i.e. the number of cells receiving more than 4 or 2 Gy), for a range of average marrow cavity absorbed doses from 1 to 20 Gy [18]. The consequences of the results of this work for the clinical application of 223Ra-chloride therapy are not clear yet, however.
Although the biokinetic data may have to be modified in order to give an improved representation of the individual patient dose, they provide a means for risk analysis in a broad patient population and in an individual patient of average weight and height. There are, however, additional sources of uncertainty related to the calculation of doses from intake of 223Ra-chloride. These are, among others, the uncertainty of the radiation weighting factor, the problem of localizing the sensitive cells in the skeleton, age- and sex-dependent differences and individual variation in the uptake in tissue. A potential solution to that problem could be the use of quantitative imaging for patient-specific dosimetry as suggested by Hindorf et al. [19]. However, dosimetry data based on these quantitative imaging procedures have not been published yet.
Tumour/lesion absorbed doses have not been calculated as there are no data available on uptake and biokinetics. However, it can be stated that the dose assessment of the absorbed doses to organs/tissues provided in this work represents an upper limit of the absorbed doses as substantial tumour/lesion uptake reduces the availability of 223Ra-chloride to normal organs and tissues.
If a curative therapy is intended, the dose coefficients provided in Table 1 could also be used for performing a long-term risk analysis of intravenous 223Ra-chloride application in patients with osseous metastases.
Conclusion
The comprehensive list of dose coefficients presented in this work, based on the biokinetic data of the ICRP, will assist in comparing and evaluating organ doses from various therapy modalities used in nuclear medicine and will provide a base for further development of patient-specific dosimetry for individual patients.
Notes
Developed at the Bundesamt für Strahlenschutz (BfS), Abteilung Strahlenschutz und Gesundheit, 85764 Oberschleißheim, Germany
As proposed by the ICRP in ICRP Publication 103 [16] as the unit for an RBE-weighted absorbed dose for deterministic biological effects
References
Bruland ØS, Nilsson S, Fisher DR, Larsen RH. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res 2006;12:6250s–7s.
Nilsson S, Franzén L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol 2007;8:587–94.
Nilsson S, Larsen RH, Fosså SD, Balteskard L, Borch KW, Westlin JE, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res 2005;11:4451–9.
Nilsson S, Strang P, Aksnes AK, Franzèn L, Olivier P, Pecking A, et al. A randomized, dose–response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer 2012;48:678–86.
Lewington V, Lamey R, Staudacher K, Vogelzang N. Radium-223 chloride: radiation safety, tolerability, and survival gain in patients with castration-resistant prostate cancer (CRPC) and bone metastases. J Nucl Med 2012;53(Suppl 1):222.
ICRP. Publication 67: Age-dependent doses to members of the public from intake of radionuclides: part 2 ingestion dose coefficients. Ann ICRP 1992;22.
Lassmann M, Nosske D, Reiners C. Therapy of ankylosing spondylitis with 224Ra-radium chloride: dosimetry and risk considerations. Radiat Environ Biophys 2002;41:173–8.
Salmon PL, Onischuk YN, Bondarenko OA, Lanyon LE. Alpha-particle doses to cells of the bone remodeling cycle from alpha-particle-emitting bone-seekers: indications of an antiresorptive effect of actinides. Radiat Res 1999;152:S43–7.
Leggett RW. A generic age-specific biokinetic model for calcium-like elements. Radiat Prot Dosimetry 1992;41:183–98.
ICRP. Publication 71: Age-dependent doses to members of the public from intake of radionuclides: part 4 inhalation dose coefficients. Ann ICRP 1995;25.
ICRP. Publication 110: Adult reference computational phantoms. Ann ICRP 2009;2.
ICRP. Publication 30 (part 1): Limits for intakes of radionuclides by workers. Ann ICRP 1979;2.
ICRP. Publication 100. Human alimentary tract model for radiological protection. Ann ICRP 2006;36:1–2.
Howell RW, Goddu SM, Narra VR, Fisher DR, Schenter RE, Rao DV. Radiotoxicity of gadolinium-148 and radium-223 in mouse testes: relative biological effectiveness of alpha-particle emitters in vivo. Radiat Res 1997;147:342–8.
Sgouros G, Roeske JC, McDevitt MR, Palm S, Allen BJ, Fisher DR, et al. MIRD Pamphlet No. 22 (abridged): radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J Nucl Med 2010;51:311–28.
ICRP. Publication 103: The 2007 recommendations of the International Commission on Radiological Protection. Ann ICRP 2007;2007:37.
Bodei L, Lam M, Chiesa C, Flux G, Brans B, Chiti A, et al. EANM procedure guideline for treatment of refractory metastatic bone pain. Eur J Nucl Med Mol Imaging 2008;35:1934–40.
Hobbs RF, Song H, Watchman CJ, Bolch WE, Aksnes AK, Ramdahl T, et al. A bone marrow toxicity model for 223Ra alpha-emitter radiopharmaceutical therapy. Phys Med Biol 2012;57:3207–22.
Hindorf C, Chittenden S, Aksnes AK, Parker C, Flux GD. Quantitative imaging of 223Ra-chloride (Alpharadin) for targeted alpha-emitting radionuclide therapy of bone metastases. Nucl Med Commun 2012;33:726–32.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lassmann, M., Nosske, D. Dosimetry of 223Ra-chloride: dose to normal organs and tissues. Eur J Nucl Med Mol Imaging 40, 207–212 (2013). https://doi.org/10.1007/s00259-012-2265-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2265-y