Abstract
Purpose
The study evaluated the role of preoperative 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT in the prediction of recurrent gastric cancer after curative surgical resection.
Methods
A total of 271 patients with gastric cancer who underwent 18F-FDG PET/CT and subsequent curative surgical resection were enrolled. All patients underwent follow-up for cancer recurrence with a mean duration of 24 ± 12 months. 18F-FDG PET/CT images were visually assessed and, in patients with positive 18F-FDG cancer uptake, the maximum standardized uptake value (SUVmax) of cancer lesions was measured. 18F-FDG PET/CT findings were tested as prognostic factors for cancer recurrence and compared with conventional prognostic factors. Furthermore, 18F-FDG PET/CT findings were assessed as prognostic factors according to histopathological subtypes.
Results
Of 271 patients, 47 (17 %) had a recurrent event. Positive 18F-FDG cancer uptake was shown in 149 patients (55 %). Tumour size, depth of invasion, presence of lymph node metastasis, positive 18F-FDG uptake and SUVmax were significantly associated with tumour recurrence in univariate analysis, while only depth of invasion, positive 18F-FDG uptake and SUVmax had significance in multivariate analysis. The 24-month recurrence-free survival rate was significantly higher in patients with negative 18F-FDG uptake (95 %) than in those with positive 18F-FDG uptake (74 %; p < 0.0001). In subgroup analysis, 18F-FDG uptake was a significant prognostic factor in patients with tubular adenocarcinoma (p = 0.003) or poorly differentiated adenocarcinoma (p = 0.0001). However, only marginal significance was shown in patients with signet-ring cell carcinoma and mucinous carcinoma (p = 0.05).
Conclusion
18F-FDG uptake of gastric cancer is an independent and significant prognostic factor for tumour recurrence. 18F-FDG PET/CT could provide effective information on the prognosis after surgical resection of gastric cancer, especially in tubular adenocarcinoma and poorly differentiated adenocarcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the most common cancer in Korea [1]. Although 5-year survival rates for gastric cancer have markedly increased recently, possibly due to an early diagnosis, advanced gastric cancer still carries a poor prognosis with high mortality rate [1–3]. The only available curative therapy for gastric cancer is surgical resection involving gastrectomy with radical lymph node (LN) dissection [4]. However, cancer recurrence can occur after surgical resection, with rates ranging from 12 to 49 %, and the often dismal results of treatment yield a poor prognosis [5–7]. The stage of gastric cancer, depth of tumour invasion and extent of LN metastasis are the most significant factors for predicting recurrence [6, 8–10].
18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET) has been widely used to evaluate various types of malignant tumours [11]. However, the role of 18F-FDG PET in gastric cancer is debatable. Although 18F-FDG PET is clinically useful in detecting recurrent gastric cancer after surgical resection [12, 13], the role of 18F-FDG PET in preoperative workup is limited due to its low sensitivity for primary tumour and LN metastasis [14, 15]. Furthermore, because only a few studies with a small number of patients have been performed, the role of 18F-FDG PET in predicting prognosis of patients with gastric cancer is still contentious [13, 16–18].
This study aimed to investigate the role of 18F-FDG PET/computed tomography (CT) as a prognostic factor for gastric cancer recurrence and to compare the predictive values with conventional prognostic factors. Furthermore, we also evaluated the predictive value of 18F-FDG PET/CT findings according to histopathological subtypes of gastric cancer.
Materials and methods
Patients
This study was approved by the Institutional Review Board in our medical centre. Between June 2006 and December 2010, the records of 299 patients with gastric cancer who underwent preoperative 18F-FDG PET/CT scan and subsequent curative surgical resection were retrospectively reviewed. Patients who had a previous history of another malignancy or received any neoadjuvant therapy prior to surgical resection of gastric cancer were excluded. Of the 299 patients, 14 patients were excluded from this study due to loss to follow-up, and 10 patients were excluded due to death from cancer-unrelated causes. Furthermore, four patients had rare pathological types of gastric cancer (adenosquamous carcinoma, squamous cell carcinoma and leiomyosarcoma) and were excluded from statistical analysis. The remaining 271 patients with gastric cancer were enrolled in this study.
18F-FDG PET/CT scan and image analysis
All 18F-FDG PET/CT scans were performed with a dedicated PET/CT scanner (Gemini, Philips, Milpitas, CA, USA) within 1 month before surgical resection of gastric cancer. All patients were instructed to fast at least 6 h before the 18F-FDG PET/CT scans. Furthermore, they were also requested to drink at least 500 ml of water just prior to scanning to distend the stomach. Patients were intravenously injected with 5.18 MBq/kg of 18F-FDG 1 h prior to imaging. At first, a CT scan was performed at 80 mA and 140 kVp for attenuation correction without contrast enhancement. Afterwards, an emission scan was performed from the skull base to the proximal thigh in one bed position for 2.5 min. Emission scan images were reconstructed into a matrix of 128 × 128 using an iterative algorithm (ordered subset expectation maximization), and attenuation as well as scatter correction was performed.
All of the 18F-FDG PET/CT images were evaluated by two nuclear medicine physicians. The evaluation of 18F-FDG PET/CT images was performed in two steps. First, 18F-FDG PET/CT images of all patients were visually assessed and the patients were classified as positive or negative with respect to 18F-FDG cancer uptake. Lesions showing focally increased 18F-FDG uptake exceeding the uptake of the surrounding normal stomach wall and corresponding with cancer lesions on contrast-enhanced CT images and gastroduodenoscopies were read as positive 18F-FDG uptake. No visible focally increased 18F-FDG uptake or diffusely increased 18F-FDG uptake that was unable to differentiate cancer uptake from physiological gastric wall uptake was judged to be negative 18F-FDG uptake. Furthermore, focally increased 18F-FDG uptake that did not correspond with cancer lesions on contrast-enhanced CT images, gastroduodenoscopies and histopathological findings were also read as negative 18F-FDG uptake. Afterwards, for quantitative analysis, the maximum standardized uptake value (SUVmax) was measured only in patients with positive 18F-FDG cancer uptake. The SUV was calculated as decay corrected activity (kBq) per tissue volume (ml)/injected 18F-fluoride activity (kBq) per body mass (g). The SUVmax was measured by drawing a circular region of interest (ROI) at the site of the maximum 18F-FDG uptake on the transaxial 18F-FDG PET images.
Surgery and follow-up
All patients underwent subtotal or total gastrectomy with regional LN dissection (at least D1+ dissection) according to the treatment guidelines of the Japanese Gastric Cancer Association (JGCA) [19]. In histopathological evaluation of surgical specimens, the JGCA system and the Lauren classification were applied [20, 21]. The histopathological subtypes of gastric cancer were categorized into papillary adenocarcinoma, tubular adenocarcinoma (TAC, well-differentiated and moderately differentiated types), poorly differentiated adenocarcinoma (PAC), signet-ring cell carcinoma (SRC) and mucinous adenocarcinoma (MAC) according to the JGCA system [20]. Furthermore, the Lauren classification was used to differentiate intestinal and non-intestinal tumours [21]. The categories “diffuse type”, “mixed type” and “non-classifiable” in the Lauren classification were included within the non-intestinal type [16, 21].
All 271 enrolled patients underwent clinical follow-up that included blood tests and diagnostic imaging studies after surgical resection of gastric cancer. The mean duration of follow-up was 24 ± 12 months (range 7–61 months). In the first 3 years after operation, all patients were clinically assessed every 3–4 months and blood tests, contrast-enhanced CT scan and gastroduodenoscopy were performed every 6–8 months. Afterwards, the patients were clinically assessed every 4–6 months and diagnostic studies were performed every 10–12 months. If the clinical assessment or diagnostic studies showed an abnormal finding, additional diagnostic studies and pathological confirmation were performed to assess cancer recurrence.
Statistical analyses
All enrolled patients were classified as patients with cancer recurrence and with no evidence of cancer recurrence. Tumour factors and the results of 18F-FDG PET/CT scans were compared between patients with recurrence and no recurrence using Student’s t test, chi-square test and Fisher’s exact test. Kaplan-Meier survival analysis was performed to calculate cumulative recurrence-free survival rates according to the tumour factors and 18F-FDG PET/CT findings. For tumour size and SUVmax, the optimal cutoff values for the Kaplan-Meier method were determined by receiver-operating characteristic (ROC) curve analysis. Survival time was defined as the time from the surgical resection to the day of detection of cancer recurrence or to the day of last clinical follow-up. The significance of the predictive value of the tumour factors and 18F-FDG PET/CT findings was analysed by log-rank test in univariate analysis and by Cox proportional hazards regression test in multivariate analysis.
Afterwards, all enrolled patients were categorized into three subgroups: patients with TAC, patients with PAC and patients with SRC or MAC (SRC/MAC). The values of SUVmax between these three subgroups were compared using the Kruskal-Wallis test. Further, Fisher’s exact test and Mann-Whitney test were used to compare 18F-FDG PET/CT findings between patients with recurrence and no recurrence for each subgroup. For each subgroup, the Kaplan-Meier method with log-rank test was used to calculate the cumulative recurrence-free survival rate according to 18F-FDG PET/CT findings. SPSS software for Windows (SPSS, Chicago, IL, USA) was used for all statistical tests and p values < 0.05 were considered statistically significant.
Results
Characteristics of the patients and 18F-FDG PET/CT findings
Of the 271 patients enrolled, 99 patients (37 %) were diagnosed with TAC, 141 patients (52 %) with PAC, 25 patients (9 %) with SRC and the remaining 6 patients (2 %) with MAC. During follow-up, cancer recurrence was found in 47 patients (17 %). Of these 47 patients, distant organ metastases and/or peritoneal carcinomatosis were observed in 35 patients, abdominal LN metastases in 5 patients, local recurrence with distant organ metastases or peritoneal carcinomatosis in 5 patients and only local recurrence in 2 patients. The characteristics of the enrolled patients are shown in Table 1. Of the 271 patients, 128 patients (47 %) has early gastric cancer (T1 tumours irrespective of LN metastasis). Overall, positive 18F-FDG uptake of primary tumours was shown in 149 patients (55 %), and the values of SUVmax were measured only in these 149 patients. Positive 18F-FDG uptake was observed in 44 patients (34 %) of 128 patients with early gastric cancer; meanwhile, positive 18F-FDG uptake was shown in 105 patients (73 %) of the remaining 143 patients with advanced gastric cancer. Furthermore, the ratio of patients with positive 18F-FDG uptake was 59 % in patients with intestinal type (55 of 93 patients) and 53 % in patients with non-intestinal type (94 of 178 patients).
In the comparison between the recurrence group and non-recurrence group, operation type, adjuvant chemotherapy, depth of tumour invasion, presence of regional LN metastases, TNM stage, tumour size, ratio of patients with positive 18F-FDG uptake and SUVmax showed significant differences (p < 0.05; Table 1). The distributions of SUVmax in the recurrence and non-recurrence group among 149 patients with positive 18F-FDG uptake are shown in Fig. 1. Of the 149 patients with positive 18F-FDG uptake, recurrence was found in 43 patients (29 %; Fig. 2). In contrast, only 4 patients (3 %) among 122 patients with negative 18F-FDG uptake had recurrence (p < 0.0001; Fig. 3).
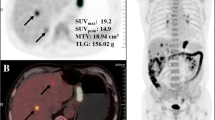
18F-FDG (a), CT (b) and fused 18F-FDG PET/CT (c) images of a 68-year-old male patient with early gastric cancer. Focal 18F-FDG uptake of gastric cancer lesion is shown in the antrum with an SUVmax of 3.6 (arrow). The patient was diagnosed as having poorly differentiated adenocarcinoma of T1 stage without regional LN metastasis. The cancer recurred 9 months after curative surgery
18F-FDG (a), fused 18F-FDG PET/CT (b) and contrast-enhanced CT (c) images of a 66-year-old female patient with advanced gastric cancer. Contrast-enhanced CT image shows well-enhanced gastric cancer lesion in the gastric body (arrow); however, no abnormal focal 18F-FDG uptake is seen in PET image. The patient was diagnosed as having moderately differentiated TAC of T3 stage with regional LN metastases. There was no recurrence during follow-up of 31 months after curative surgery
Prognostic factors in prediction of recurrence
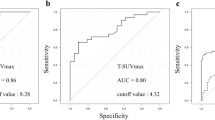
The significance of prognostic factors in univariate and multivariate analyses is shown in Table 2. Although operation type and adjuvant chemotherapy showed significant differences between patients with recurrence and non-recurrence, these factors were excluded from survival analysis. Because operation type and adjuvant chemotherapy are determined by other tumour factors such as tumour location, depth of tumour and TNM stage, they were not considered as independent factors. The optimal cutoff values of SUVmax and tumour size for the Kaplan-Meier method determined by ROC curve analysis were 8.2 and 2.7 cm, respectively. The depth of tumour invasion, presence of regional LN metastases, tumour size, positive 18F-FDG uptake and SUVmax were significant prognostic factors for tumour recurrence in univariate analysis (Table 2). In the multivariate analysis, only depth of tumour invasion and positive 18F-FDG uptake were determined to be significant in all patients. Furthermore, in 149 patients with positive 18F-FDG uptake, only depth of tumour invasion and SUVmax were significant prognostic factors (Table 2). The cumulative recurrence-free survival curve according to the 18F-FDG uptake, SUVmax and depth of tumour invasion by the Kaplan-Meier method is shown in Fig. 4a–c, respectively. Patients with negative 18F-FDG uptake showed better survival and higher 24-month recurrence-free survival rate (95 vs 74 %) than those with positive 18F-FDG uptake (p < 0.0001). Patients with T1 stage also showed higher 24-month recurrence-free survival rate (92 vs 78 %) than those with T2–T4 stage (p < 0.0001). Moreover, in 149 patients with positive 18F-FDG uptake, patients with SUVmax below the cutoff value had better survival and higher 24-month recurrence-free survival rate (79 vs 58 %) than those with SUVmax above the cutoff value (p = 0.001).
The cumulative recurrence-free survival curves according to 18F-FDG uptake (a), SUVmax (b) and depth of tumour invasion (T stage) (c). Patients with negative 18F-FDG uptake or T1 stage showed significantly better survival than those with positive 18F-FDG uptake or T2–T4 stage, respectively (p < 0.0001 for all). Furthermore, of 149 patients with positive 18F-FDG uptake, patients with SUVmax < 8.2 showed better survival than those with SUVmax ≥ 8.2 (p = 0.001)
Combined depth of tumour invasion with 18F-FDG uptake could enhance the predictive value of the patients (Table 3). In patients who showed T1 stage cancer and negative 18F-FDG uptake, there was no recurrence. However, even though the gastric cancer lesion was T1 stage, the recurrence rate was 16 % in patients with positive 18F-FDG uptake. Furthermore, in patients who showed T2–T4 stage cancer and positive 18F-FDG uptake, the recurrence rate was 34 %. The patients with negative 18F-FDG uptake in both T1 stage and T2–T4 stage showed significantly higher recurrence-free survival rate than those with positive 18F-FDG uptake (Fig. 5a, b; p = 0.001 for T1 stage and p = 0.01 for T2–T4 stage).
The cumulative recurrence-free survival curves according to 18F-FDG uptake in patients with early gastric cancer (T1 stage) (a) and in patients with advanced gastric cancer (T2–T4 stage) (b). In both patient groups, patients with negative 18F-FDG uptake showed significantly better survival than those with positive 18F-FDG uptake (p = 0.001 for early gastric cancer and p = 0.01 for advanced gastric cancer)
Subgroup analysis according to the histopathology
The 18F-FDG PET/CT findings according to histopathological subtypes are shown in Table 4. Although the ratios of patients with positive 18F-FDG uptake were higher in TAC (61 %) and PAC (55 %) groups than the SRC/MAC group (39 %), the values of SUVmax in patients with positive 18F-FDG uptake between the three groups showed no significant differences (p = 0.5). In the TAC and PAC groups, the ratio of patients with positive 18F-FDG uptake was significantly different between patients with recurrence and non-recurrence (p < 0.05); meanwhile, there was a marginal significant difference in the SRC/MAC group (p = 0.06).
The cumulative recurrence-free survival curve according to the 18F-FDG uptake in the subgroup patients is shown in Fig. 6a–c. In the TAC and PAC groups, patients with negative 18F-FDG uptake had a significantly higher recurrence-free survival rate than patients with positive 18F-FDG uptake (p = 0.003 for the TAC group and p = 0.0001 for the PAC group). In contrast, only marginal significance was shown in a comparison of the recurrence-free survival rate for the SRC/MAC group (p = 0.05).
The cumulative recurrence-free survival curves according to 18F-FDG uptake in subgroup patients with TAC (a), PAC (b) and SRC/MAC (c). In patients with TAC and PAC, those with negative 18F-FDG uptake showed significantly better survival than those with positive 18F-FDG uptake (p = 0.003 for TAC and p = 0.0001 for PAC). In contrast, marginal significance was shown in patients with SRC/MAC (p = 0.05)
Discussion
To the best of our knowledge, this is the largest clinical study to evaluate the role of 18F-FDG PET/CT for predicting prognosis in patients with gastric cancer after curative surgery. This study demonstrated that positive 18F-FDG uptake of a primary gastric cancer lesion is an independent and significant prognostic factor for cancer recurrence after curative surgical resection. Although the detection rate of 18F-FDG PET/CT for gastric cancer was only 55 %, in a comparison with various prognostic factors by multivariate analysis, positive 18F-FDG uptake and SUVmax showed significance in addition to the depth of tumour invasion. Furthermore, in a subgroup of patients with TAC or PAC, positive 18F-FDG uptake was a significant prognostic factor. The results of our study suggest that preoperative 18F-FDG PET/CT can play a significant role in predicting prognosis in patients with gastric cancer, especially in patients with TAC or PAC, although the diagnostic ability of 18F-FDG PET/CT is limited.
Presently, 18F-FDG PET/CT displays a low detection rate for primary gastric cancer (55 %), especially for early gastric cancer (34 %) and SRC/MAC (39 %). Sensitivity for detecting the primary tumour varies between 47 and 96 % due to the different characteristics of enrolled patients [15, 16, 18, 22–26]. Similar to the results of our study, previous studies have already documented very low sensitivity of 26–47 % for detecting early gastric cancer [18, 22, 26] and 25 % for SRC [16]. Furthermore, another study reported that 49 % (20 of 41 patients) of patients with SRC had SUVmax < 3.8 [24]. The variable and sometimes intense physiological 18F-FDG uptake in the normal gastric wall and differences of 18F-FDG uptake in cancer lesions according to histopathological subtypes of gastric cancer are the most significant contributing factors for the low detection rate of primary tumours. Normal gastric wall devoid of malignant lesions can display an SUV exceeding 2.5 and benign gastric mucosal inflammation can show focal intense 18F-FDG accumulation, which restricts detection of gastric cancer lesions [16, 27, 28]. 18F-FDG uptake in mucinous carcinoma can be positively correlated with tumour cellularity, but negatively correlated with the amount of mucin within the tumour mass, which accounts for low detectability of 18F-FDG PET for SRC and MAC [29]. Furthermore, an infiltrative growth pattern, high content of mucus and low concentration of cancer cells lead to low 18F-FDG uptake in poorly differentiated cancer and signet-ring cell cancer, in spite of their aggressiveness [14].
Previous studies also showed a lower detection rate for non-intestinal tumours (41–52 %) than that for intestinal tumours (66–83 %) [16, 26]. However, similar detection rates in both types of tumours have been noted previously (78 % for non-intestinal type and 72 % for intestinal type) [18] and presently (53 % for non-intestinal type and 59 % for intestinal type). The detection rate for the non-intestinal type can be influenced by the proportion of PAC, SRC and MAC because the detection rates of 18F-FDG PET for TAC and PAC were similar and higher than that for SRC/MAC in our study. Most of the non-intestinal tumours in our study were PAC and only 16 % of non-intestinal tumours were SRC/MAC. Hence, 18F-FDG PET findings according to the histopathological classification can reveal the characteristics of gastric cancer better than those according to the Lauren classification.
18F-FDG PET has a significant role in predicting prognosis for diverse malignancies [30–32]. In the present study, in addition to tumour size, depth of tumour invasion and presence of LN metastasis, 18F-FDG uptake in gastric cancer lesions was a significant prognostic factor in univariate analysis. The tumour size, stage and the status of LN metastasis are regarded as representative of the progression and aggressiveness of gastric cancer and have already been reported as significant prognostic factors [6, 8–10]. However, these prognostic factors have limitations because they cannot be exactly evaluated preoperatively. In contrast, 18F-FDG PET is noninvasive and feasible to use and can provide effective information on the prognosis before surgical resection. Furthermore, combined depth of tumour invasion with 18F-FDG PET findings could more appropriately predict prognosis of the patients. The association between 18F-FDG uptake of gastric cancer and prognosis can be explained by glucose transporter 1 (GLUT1) expression on gastric cancer cells. Previous studies have shown that the degree of 18F-FDG uptake in gastric carcinoma is related to GLUT1 expression, and GLUT1 expression in gastric carcinoma is associated with tumour aggressiveness and patient survival [25, 33]. Because 18F-FDG uptake differs between different histopathological subtypes [14, 34], we also investigated the role of 18F-FDG PET as a prognostic factor according to histopathological subtypes. In the subgroup of patients with TAC or PAC, patients with negative 18F-FDG tumour uptake showed better recurrence-free survival than those with positive 18F-FDG tumour uptake. Subgroup patients with SRC/MAC also showed a tendency toward better recurrence-free survival curve in patients with negative 18F-FDG tumour uptake, but failed to be significant in statistical analysis, which might be due to the small number of the subgroup. Because the proportion of patients with SRC is between 12 and 17 % in Korea [35, 36], 18F-FDG PET/CT can be effectively used in most of the patients with gastric cancer for predicting prognosis.
There have been a limited number of studies examining the role of 18F-FDG PET as a prognostic factor in patients with gastric cancer, and these studies have shown conflicting results. Stahl et al. [16] reported that the survival rate was not significantly different in patients with detectable tumours on 18F-FDG PET and patients with non-detectable tumours. In contrast, other previous studies showed that patients with high 18F-FDG uptake had a worse prognosis than those with low 18F-FDG uptake, and 18F-FDG PET could provide important information concerning the prognosis of gastric cancer [13, 18, 37]. Furthermore, Pak et al. [17] investigated the role of 18F-FDG PET in 41 patients with SRC and showed that the high SUVmax group had more frequent recurrence and a shorter relapse-free survival than the low SUVmax group. Our study also demonstrated that 18F-FDG uptake of gastric cancer was an independent and significant prognostic factor for cancer recurrence in multivariate analysis, in addition to the depth of the cancer lesion. The differences between our study and the study by Stahl et al. [16] could be derived from the different patient populations. All of the patients in our study and previous studies by Mochiki et al. [18] and Pak et al. [17] underwent curative surgical resection after 18F-FDG PET without any neoadjuvant treatment. However, the patients in the study by Stahl et al. [16] underwent chemotherapy after 18F-FDG PET, suggesting that the patients in their study had a more advanced stage of gastric cancer than patients in our study.
There were several limitations in the present study. First, because we only enrolled patients who underwent curative surgical resection, the proportion of patients with early gastric cancer was high (47 %), which produced an overall good prognosis. Second, the recurrence rate of patients with early gastric cancer in our study was 5 %, which is slightly higher than the results of previous studies performed in Korea (2–3 %) [38–40]. This difference could be due to the selection bias and relatively small number of patients in our study. Third, the number of patients with SRC and MAC was small in this study, and further studies with more patients will be needed to elucidate the role of 18F-FDG PET as a prognostic factor in patients with SRC and MAC. Finally, our study was a retrospective single-centre study. Further prospective multi-centre studies will be needed.
In conclusion, the results of our study demonstrated that 18F-FDG uptake in gastric cancer is an independent and significant prognostic factor for predicting cancer recurrence after curative surgical resection. Patients with negative 18F-FDG uptake in gastric cancer had significantly better recurrence-free survival than patients with positive 18F-FDG uptake. Furthermore, in patients with TAC and PAC, recurrence-free survival was significantly different between patients with positive and negative 18F-FDG uptake. Therefore, although the detectability of 18F-FDG PET/CT for gastric cancer is low, preoperative 18F-FDG PET/CT could provide effective information on the prognosis after curative surgical resection of gastric cancer.
References
Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer 2011;11:135–40.
Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 1999;83:18–29.
Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, et al. Recent patterns in gastric cancer: a global overview. Int J Cancer 2009;125:666–73.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113–23.
Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg 2000;87:236–42.
Wu B, Wu D, Wang M, Wang G. Recurrence in patients following curative resection of early gastric carcinoma. J Surg Oncol 2008;98:411–4.
Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439–49.
Shiraishi N, Inomata M, Osawa N, Yasuda K, Adachi Y, Kitano S. Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer 2000;89:255–61.
Liu Y, Chen XH, Meng XH, Liu CF, Zhao LL, Han JW, et al. Multivariate prognostic study on node-positive gastric cancer: is tumor size a prognostic indicator? Hepatogastroenterology 2012;59:623–6. doi:10.5754/hge11455.
Wang X, Wan F, Pan J, Yu GZ, Chen Y, Wang JJ. Tumor size: a non-neglectable independent prognostic factor for gastric cancer. J Surg Oncol 2008;97:236–40.
Kostakoglu L, Agress Jr H, Goldsmith SJ. Clinical role of FDG PET in evaluation of cancer patients. Radiographics 2003;23:315–40.
Bilici A, Ustaalioglu BB, Seker M, Kefeli U, Canpolat N, Tekinsoy B, et al. The role of (18)F-FDG PET/CT in the assessment of suspected recurrent gastric cancer after initial surgical resection: can the results of FDG PET/CT influence patients’ treatment decision making? Eur J Nucl Med Mol Imaging 2011;38:64–73.
De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging 2002;29:525–9.
Dassen AE, Lips DJ, Hoekstra CJ, Pruijt JF, Bosscha K. FDG-PET has no definite role in preoperative imaging in gastric cancer. Eur J Surg Oncol 2009;35:449–55.
Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging 2006;33:148–55.
Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging 2003;30:288–95.
Pak KH, Yun M, Cheong JH, Hyung WJ, Choi SH, Noh SH. Clinical implication of FDG-PET in advanced gastric cancer with signet ring cell histology. J Surg Oncol 2011;104:566–70.
Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg 2004;28:247–53.
Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer 2002;5:1–5.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma - 2nd English edition. Gastric Cancer 1998;1:10–24.
Hamilton SR, Aaltonen LA. Tumors of the stomach. In: WHO classification of tumors. Pathology and genetics. Tumors of the digestive system. Lyon: IARC Press; 2000. p. 38–52.
Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, et al. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med 2005;46:1582–8.
Oh HH, Lee SE, Choi IS, Choi WJ, Yoon DS, Min HS, et al. The peak-standardized uptake value (P-SUV) by preoperative positron emission tomography-computed tomography (PET-CT) is a useful indicator of lymph node metastasis in gastric cancer. J Surg Oncol 2011;104:530–3.
Hur H, Kim SH, Kim W, Song KY, Park CH, Jeon HM. The efficacy of preoperative PET/CT for prediction of curability in surgery for locally advanced gastric carcinoma. World J Surg Oncol 2010;8:86.
Yamada A, Oguchi K, Fukushima M, Imai Y, Kadoya M. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med 2006;20:597–604.
Mukai K, Ishida Y, Okajima K, Isozaki H, Morimoto T, Nishiyama S. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer 2006;9:192–6.
Koga H, Sasaki M, Kuwabara Y, Hiraka K, Nakagawa M, Abe K, et al. An analysis of the physiological FDG uptake pattern in the stomach. Ann Nucl Med 2003;17:733–8.
Takahashi H, Ukawa K, Ohkawa N, Kato K, Hayashi Y, Yoshimoto K, et al. Significance of (18)F-2-deoxy-2-fluoro-glucose accumulation in the stomach on positron emission tomography. Ann Nucl Med 2009;23:391–7.
Berger KL, Nicholson SA, Dehdashti F, Siegel BA. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol 2000;174:1005–8.
Sasaki R, Komaki R, Macapinlac H, Erasmus J, Allen P, Forster K, et al. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol 2005;23:1136–43.
Lee JW, Paeng JC, Kang KW, Kwon HW, Suh KS, Chung JK, et al. Prediction of tumor recurrence by 18F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med 2009;50:682–7.
Lee SM, Kim TS, Lee JW, Kim SK, Park SJ, Han SS. Improved prognostic value of standardized uptake value corrected for blood glucose level in pancreatic cancer using F-18 FDG PET. Clin Nucl Med 2011;36:331–6.
Kawamura T, Kusakabe T, Sugino T, Watanabe K, Fukuda T, Nashimoto A, et al. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer 2001;92:634–41.
Shimada H, Okazumi S, Koyama M, Murakami K. Japanese Gastric Cancer Association Task Force for Research Promotion: clinical utility of (18)F-fluoro-2-deoxyglucose positron emission tomography in gastric cancer. A systematic review of the literature. Gastric Cancer 2011;14:13–21.
Kim JP, Kim SC, Yang HK. Prognostic significance of signet ring cell carcinoma of the stomach. Surg Oncol 1994;3:221–7.
Park JC, Lee YC, Kim JH, Kim YJ, Lee SK, Shin SK, et al. Clinicopathological features and prognostic factors of proximal gastric carcinoma in a population with high Helicobacter pylori prevalence: a single-center, large-volume study in Korea. Ann Surg Oncol 2010;17:829–37.
Chung HW, Lee EJ, Cho YH, Yoon SY, So Y, Kim SY, et al. High FDG uptake in PET/CT predicts worse prognosis in patients with metastatic gastric adenocarcinoma. J Cancer Res Clin Oncol 2010;136:1929–35.
Lai JF, Kim S, Kim K, Li C, Oh SJ, Hyung WJ, et al. Prediction of recurrence of early gastric cancer after curative resection. Ann Surg Oncol 2009;16:1896–902.
Kim JW, Hwang I, Kim MJ, Jang SJ. Clinicopathological characteristics and predictive markers of early gastric cancer with recurrence. J Korean Med Sci 2009;24:1158–64.
Youn HG, An JY, Choi MG, Noh JH, Sohn TS, Kim S. Recurrence after curative resection of early gastric cancer. Ann Surg Oncol 2010;17:448–54.
Acknowledgment
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J.W., Lee, S.M., Lee, MS. et al. Role of 18F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Imaging 39, 1425–1434 (2012). https://doi.org/10.1007/s00259-012-2164-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2164-2