Abstract
Purpose
This study evaluated the usefulness of semiquantitative and volumetric PET parameters for predicting prognosis in patients with advanced gastric cancer (AGC).
Methods
We enrolled 213 patients who underwent 18F-fluoro-2-deoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) prior to curative surgery for AGC. Maximum standardized uptake value (SUVmax) and tumor-to-liver uptake ratio (TLR) were measured in all patients. Metabolic tumor volume (MTV) and total lesion glycolysis were measured in volume-measurable patients. For further quantification of FDG uptake, we developed PET prognostic scores by combining SUVmax and MTV (1: low SUVmax/low MTV; 2: high SUVmax/low MTV; 3: high SUVmax/high MTV). Comparison of PET parameters between recurrence and non-recurrence groups was performed. Univariate and multivariate analyses for recurrence-free survival (RFS) and overall survival (OS) were subsequently performed.
Results
The recurrence rate was 32.4% (69/213 patients). Mean SUVmax and mean MTV of the recurrence group were significantly higher than those of the non-recurrence group (p = 0.026 and p = 0.025). TLR showed marginal significance (p = 0.051). In multivariate analysis for RFS including all patients, SUVmax (p = 0.022), TLR (p = 0.010), and PET score (p = 0.003) were independent prognostic factors. In post hoc analysis of PET score, significant differences in RFS were observed between PET scores 2 and 3 as well as scores 1 and 3. No significant difference in RFS was observed between scores 1 and 2. Only PET score was statistically significant for OS in univariate analysis. None of the PET parameters were statistically significant for OS in multivariate analysis.
Conclusion
High SUVmax and high MTV of the primary tumor suggest a high risk of recurrence for AGC patients. Even if SUVmax is similar, the prognosis may vary depending on MTV. Combining PET parameters results in a better prediction for prognosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is one of the most common cancers in Korea, especially in men aged 35 to 64 years [1]. Despite the decreased incidence and mortality rates over the past decade, gastric cancer still ranks fifth in incidence rate and third in mortality rate globally [2,3,4]. Moreover, its incidence and mortality rates in both sexes are the highest in East Asia, and about half of the world’s patients are East Asians [3, 5]. The treatment for gastric cancer is a radical resection, but local recurrence or distant metastasis often occurs even after surgery. The prognosis is poor in case of recurrence.
18F fluoro-2-deoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is useful for initial staging and recurrence evaluation in most malignant tumors [6]. However, gastric cancer shows variable FDG uptake according to histopathologic type and tumor aggressiveness, with detection rates ranging from 40 to 90% [7,8,9,10]. Additionally, the gastric wall may show high physiologic uptake and may also show focally increased uptake under benign conditions such as visceral thickening or inflammation [9]. Despite its varying sensitivity, PET/CT shows high specificity and high positive predictive value for gastric cancer and it is excellent in evaluating distant metastasis [11, 12]. Therefore, PET/CT has diagnostic value in preoperative staging of gastric cancer.
The quantified values of FDG uptake are clinically important for malignancy assessment, treatment response evaluation, and prognostic prediction. In particular, prognostic prediction based on PET parameters helps cancer patients accurately understand their condition and also helps medical physicians determine the treatment direction [13]. There have been many studies on the prognostic role of 18F-FDG PET/CT in non-metastatic and metastatic gastric cancer [14,15,16,17,18,19,20]. These studies reported a significant correlation between metabolism and prognosis. However, most of these studies have investigated using FDG avidity or a semiquantitative parameter such as standardized uptake value (SUV). Few have dealt with volumetric PET parameters. This may be because FDG avidity and SUV have fewer inter-observer variations, and it is often difficult to measure metabolic volumes in case of FDG-negative tumors or infiltrative tumors. Despite these characteristics, volumetric parameters are useful in that they reflect the size and metabolic information of the entire tumor, not just the hottest point of the tumor. We hypothesized that the evaluation by combining FDG uptake intensity and metabolic volume enabled us to predict gastric cancer patients’ prognosis more precisely. Since semiquantitative and volumetric parameters have different advantages, we assumed that evaluating the two parameters together would complement each other.
This study aimed to investigate useful PET parameters for predicting recurrence and overall survival of advanced gastric cancer (AGC) and to verify the benefits of evaluating different types of PET parameters together.
Materials and Methods
Patients
Patients with gastric cancer who underwent 18F-FDG PET/CT within 1 month prior to curative resection were included. We excluded patients with early gastric cancer (EGC), gastrointestinal stromal tumor (GIST), recurred case, palliative surgery, neoadjuvant chemotherapy, and follow-up loss within 3 months after surgery (Fig. 1). EGC (T1 stage tumor) was inadequate to examine the relationship between metabolism and recurrence because of its low FDG uptake and low recurrence rate [7, 14, 21]. Finally, 213 patients between April 2008 and March 2016 were enrolled in this study. Ninety-six patients underwent total gastrectomy, 91 underwent subtotal gastrectomy, and 26 underwent distal gastrectomy. A total of 116 patients received postoperative adjuvant chemotherapy. This retrospective study was approved on August 24, 2017 by the Institutional Review Board of our institution (AN17196-001), and the requirement for informed consent was waived.
18F-FDG PET/CT Imaging
18F-FDG PET/CT images were acquired using a Gemini TF 16 PET scanner (Philips Medical Systems, Cleveland, OH, USA) with a 4-mm spatial resolution and 18-cm axial field of view. Patients fasted for at least 6 h and received 18F-FDG injections of approximately 5.18 MBq/kg when blood glucose levels were less than 200 mg/dL. PET/CT images were acquired 60 min after the injection. Low-dose CT scans (50 mA, 120 kVp, 512 × 512 matrix size) were acquired for attenuation correction. PET scans were subsequently acquired for 1 min per bed position. PET image reconstruction was performed using a three-dimensional iterative algorithm with TOF function.
Measurements of PET Parameters
It was considered a detectable case when the primary tumor uptake was clearly distinguished from the surrounding physiologic uptake, and its site matched the pathologically proven cancer location. It was considered an undetectable case when the primary tumor uptake was indistinct from the surrounding physiologic uptake. In detectable cases, the volume of interest (VOI) was manually adjusted to the tumor and maximum SUV (SUVmax) was obtained. The VOI was set slightly larger than the distinguishable tumor boundary and did not include adjacent organs or lymph nodes in the case of irregularly shaped or extensive tumor. Since even the low tumor uptake was visually slightly higher than the surrounding physiologic uptake in detectable cases, we carefully set the VOI along the uptake margin based on the tumor extent identified by abdominal CT. SUV is the accumulated radioactivity in the tissue of the VOI divided by the dose injected per body weight. SUVmax is the one-pixel value with the highest SUV. In undetectable cases, the tumor site was determined by enhanced abdominal CT finding and endoscopic finding. SUVmax was obtained by setting a 2-cm spherical VOI at the presumed location. We used this method because we considered that indistinguishable low uptake also reflected tumor aggressiveness [15]. We also measured tumor-to-liver uptake ratio (TLR) by dividing SUVmax of the tumor by SUVmean of the liver. SUVmean of the liver was obtained by setting a 3-cm VOI in the right hepatic lobe [22].
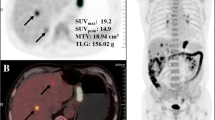
Additionally, in detectable cases, we measured metabolic tumor volume (MTV) which is the sum of the volume of voxels with SUVs exceeding 2.5 threshold [15]. The VOI was drawn to include the tumor, and the contour was set automatically along the voxel above SUV 2.5. Total lesion glycolysis (TLG) was calculated by multiplying MTV by SUVmean of the tumor. We also developed PET prognostic scores by combining SUVmax and MTV for further quantification of FDG uptake. The optimal cut-off values for SUVmax (3.49) and MTV (18.34) were obtained from receiver operating characteristic (ROC) curve analysis. We then classified all patients into three PET score groups (score 1: low SUVmax/low MTV; score 2: high SUVmax/low MTV; score 3: high SUVmax/high MTV) (Fig. 2). No patients were included in the low SUVmax/high MTV group. Patients with unmeasurable MTVs were included in the low MTV groups (68 patients in score 1 group and 3 patients in score 2 group). Image analysis was performed using a dedicated workstation (Extended Brilliance Workspace 4.0, Philips Healthcare).
Representative images according to PET scores. 18F-FDG PET images, PET/CT fusion images, and enhanced abdominal CT images are presented. The top row shows tumor uptake with a low SUVmax/low MTV (score 1) in an 82-year-old woman. The middle row shows tumor uptake with a high SUVmax/low MTV (score 2) in a 68-year-old man. The bottom row shows tumor uptake with a high SUVmax/high MTV (score 3) in a 71-year-old man. The patients with scores 1 and 2 had no recurrence but the patient with score 3 had a recurrence
Definition of the Recurrence Group and Clinical/Pathologic Factors
Patients were considered to have recurrence in the following cases: (1) Suspicious lesion was observed in follow-up images or endoscopy and the lesion was pathologically confirmed by biopsy or cytology. (2) Various imaging studies or serial imaging studies strongly suggested recurrence and the patient started chemotherapy under clinical decision. In the second case, there was an improvement or aggravation in follow-up images of the alleged lesion/serum tumor marker level during chemotherapy, which could confirm that the lesion was a recurrence without pathologic confirmation. Thirty-one patients were determined by pathology, and 38 patients were determined by non-pathologic proof. Recurrence-free survival (RFS) was defined as the period from the date of surgery to the date of first imaging or endoscopy of the recurrent lesion. Overall survival (OS) was defined as the period from the date of surgery to the date of death or to the date of last clinical visit without death. We reviewed electronic medical records for clinical information. Clinical factors included age, sex, type of gastric resection, and adjuvant chemotherapy. We also reviewed postoperative pathology reports to obtain histopathologic information of the primary tumor, pT stage, and pN stage. Pathologic factors included histologic type, Lauren classification, differentiation, Bormann type, lymphovascular invasion, tumor location, and tumor size. We used the seventh edition of American Joint Committee on Cancer (AJCC) staging manual to classify TNM stage [23].
Statistical Analysis
In the comparison between recurrence and non-recurrence groups, continuous variables were analyzed using Student’s t test and Fisher’s exact test. Categorical variables were analyzed using Pearson’s chi-square test and Kruskal-Wallis test. Univariate analysis for RFS and OS was performed using Cox proportional hazard test, including clinical/pathologic factors and PET parameters. Multivariate Cox regression models for each PET parameter (SUVmax, TLR, PET score) were subsequently performed to avoid multicollinearity between PET parameters. Cut-off values obtained from ROC curve analysis were used for dichotomization of continuous variables in univariate and multivariate analyses. Kaplan-Meier method and log-rank test were performed to calculate cumulative RFS in post hoc analysis of PET score. We used SPSS statistics software (version 19.0, SPSS Inc., Chicago, IL, USA) and considered p < 0.05 as statistically significant.
Results
Patient Characteristics
The average follow-up period was 36.3 ± 1.4 months (range, 4–96 months). The recurrence rate was 32.4% (69/213). Of them, 54 patients (78%, 54/69) had recurrence within 2 years. Mean RFS of the recurrence group was 15.2 ± 1.4 months (range, 2–58 months). The recurrence sites were as follows: anastomosis site/duodenal stump, 6 (9%); peritoneum/ascites, 29 (42%); LN, 10 (15%); liver, 8 (12%); lung, 2 (3%); bone, 3 (4%); leptomeninges, 1 (1%); liver and LN, 2 (3%); duodenal stump and liver, 1 (1%); anastomosis site and peritoneum, 1 (1%); peritoneum and pleura, 1 (1%); peritoneum, liver, adrenal gland, and LN, 1 (1%); anastomosis site, peritoneum, and liver, 1 (1%); anastomosis site, peritoneum, bone, and LN, 2 (3%); and anastomosis site, peritoneum, liver, and bone, 1 (1%). First, we compared the clinical and pathologic factors between groups with and without recurrence (Table 1). Mean age of the patients was 63.2 years. Mean age at diagnosis was significantly lower in the recurrence group than in the non-recurrence group (p = 0.023). We classified the histologic types into cohesive and non-cohesive types because the two types had different underlying pathophysiology [7, 24]. Cohesive types included 185 patients with tubular adenocarcinoma, one with papillary adenocarcinoma, one with papillotubular adenocarcinoma, and four with unclassified adenocarcinoma. Non-cohesive types included 12 patients with signet ring cell carcinoma and 10 with mucinous carcinoma. No difference was observed between these two groups (p = 0.367). For Lauren classification, non-intestinal type was significantly more frequent in the recurrence group than in the non-recurrence group (p = 0.005). The proportion of patients with lymphovascular invasion was significantly higher in the recurrence group (p < 0.001). Mean tumor size was also significantly larger in the recurrence group (p < 0.001). Differentiation was classified into three types: well, moderately, and poorly differentiated. Twenty-two patients with non-cohesive type, one with tubular adenocarcinoma, one with papillary adenocarcinoma, and two with unclassified adenocarcinoma were classified as non-classifiable according to pathology reports. There was no significant difference according to differentiation (p = 0.542). The proportions of patients with high pT, pN, and TNM stage were all significantly higher in the recurrence group (p < 0.001).
Comparison of PET Parameters Between Recurrence and Non-recurrence Groups
Intergroup comparisons of semiquantitative and volumetric PET parameters are presented in Table 2. Mean SUVmax of the recurrence group (6.2 ± 4.1) was significantly higher than that of the non-recurrence group (5.0 ± 3.4) (p = 0.026). TLR showed marginal significance (p = 0.051). Volumetric parameters were compared in 142 detectable cases (55 in the recurrence group and 87 in the non-recurrence group). Mean MTV of the recurrence group (43.2 ± 48.3) was significantly higher than that of the non-recurrence group (26.7 ± 30.0) (p = 0.025). TLG was higher in the recurrence group but showed no statistically significant difference (p = 0.071). Cut-off values of SUVmax, TLR, and MTV obtained from ROC curve analysis were 3.49 (sensitivity, 0.754; specificity, 0.431; area under the curve [AUC], 0.604), 1.86 (sensitivity, 0.754; specificity, 0.438; AUC, 0.602), and 18.34 (sensitivity, 0.673; specificity, 0.598; AUC, 0.615), respectively.
Prognostic Factors for RFS and OS
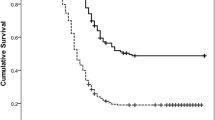
The recurrence rates of PET prognostic score groups were 21.5% (17/79) for score 1, 24.2% (15/62) for score 2, and 51.4% (37/72) for score 3. Univariate analysis for RFS including SUVmax, TLR, and PET score is presented in Table 3 and multivariate analysis for RFS in Table 4. Clinical and pathologic factors included age, Lauren classification, lymphovascular invasion, tumor size, T stage (depth of invasion), and regional lymph node (LN) metastasis. These factors were statistically significant in previous comparisons of recurrence and non-recurrence groups. In univariate analysis, six clinical/pathologic factors and three PET parameters were statistically significant (SUVmax, p = 0.004; TLR, p = 0.003; PET score, p < 0.001). In multivariate analysis, age, Lauren classification, and regional LN metastasis were independent prognostic factors. Tumor size was also statistically significant except for the PET score model. All three PET parameters were independent prognostic factors for RFS (SUVmax, p = 0.022; TLR, p = 0.010; PET score, p = 0.003). The risk of recurrence gradually increased by 1.737 times from PET score 1 to 3. In post hoc analysis using log-rank test, there were significant differences in RFS between PET scores 2 and 3 as well as scores 1 and 3 (Fig. 3). However, there was no significant difference in RFS between scores 1 and 2.
Univariate analysis for OS including SUVmax, TLR, and PET score is shown in Table 3 and multivariate analysis for OS in Table 5. In univariate analysis, age, lymphovascular invasion, tumor size, and regional LN metastasis were statistically significant. Among the three PET parameters, only PET score showed a significant difference in OS (p = 0.015). In multivariate analysis including four clinical/pathologic factors that showed significant differences in univariate analysis, age and regional LN metastasis were significant prognostic factors for OS. None of the PET parameters was statistically significant.
Discussion
This study suggested that AGC patients with high FDG uptake of the primary tumors had a higher risk of recurrence. SUVmax and TLR were significant prognostic factors in univariate and multivariate analyses for RFS. Several studies have shown that patients with high tumor uptake have a significantly higher recurrence rate and a lower survival rate compared with those with low tumor uptake, and our results are consistent with these studies [14,15,16,17]. It is generally known that FDG uptake of gastric cancer increases in tumors with large size, deep invasion, high cellularity, and low mucin content [14, 25]. Additionally, high FDG uptake is associated with elevated GLUT1 expression [26]. GLUT1 overexpression promotes glycolysis and increases tumor aggressiveness, which may result in lower patient survival [27]. In summary, high FDG uptake may be associated with higher tumor aggressiveness and worse prognosis.
Albeit only in volume-measurable cases, MTV was significantly higher in the recurrence group than in the non-recurrence group. We used SUV 2.5 as the cut-off value because this value was generally known as the reference value for discriminating malignancy, and this value was used in many previous studies [15]. We considered this method well reflected the amount of malignant parts of the tumor. Volume-based PET parameters are good indicators of prognosis by reflecting tumor burden. They are useful in many cancers including lung cancer, head and neck cancer, esophageal cancer, gallbladder cancer, ovarian cancer, and cervical cancer [13]. MTV was a significant prognostic factor also in AGC.
PET score was an independent prognostic factor for RFS in multivariate analysis. It implies that the risk of recurrence increases as the PET score increases. Score 3 showed a significantly poor prognosis in post hoc analysis as expected. This result strongly indicated that patients with high levels of both SUVmax and MTV have a high risk of recurrence. It was also noteworthy that there was a difference in prognosis between scores 2 and 3. Even if SUVmax is similarly high, the prognosis may vary depending on MTV. This result can explain when the prognosis is different despite the same SUV max. PET score suggested the possibility of a more accurate prognosis prediction when combining two parameters rather than using only one parameter. Additionally, PET score was the only significant prognostic factor among PET parameters in univariate analysis for OS. PET score demonstrated the superiority of combining various types of metabolic information.
In post hoc analysis of PET score, a significant difference in RFS was observed between PET scores 1 and 3. Score 2 was also expected to show a worse prognosis than score 1 because their SUVmax was higher than score 1. However, no significant difference in RFS was observed between scores 1 and 2. Because AGC generally has a poor prognosis [28, 29], patients without early recurrence eventually experienced recurrence over time, even with score 1. The recurrence rate of score 1 group was higher than we expected and the difference between scores 1 and 2 decreased especially after 2 years. We performed an additional 2-year RFS analysis (Fig. 4). Recurrence-free survival rate tended to decrease from score 1 to 3, but the difference between scores 1 and 2 was still not statistically significant (p = 0.571). Nevertheless, it is meaningful that the analysis showed a tendency for worse prognosis from score 1 to score 3, especially within 2 years.
In multivariate analysis for RFS, age, Lauren classification, tumor size, and regional LN metastasis were statistically significant. Lauren classification is a known prognostic factor and diffuse type generally shows a worse prognosis than intestinal type [30, 31]. TNM stage is a good prognostic factor for gastric cancer, and lymph node metastasis is one of the strong prognostic factors for long-term survival [10, 32]. These factors, however, have disadvantages in that their preoperative predictions are inaccurate. 18F-FDG PET/CT provides biological information before surgery unlike other factors such as pT stage, pN stage, exact tumor size, lymphovascular invasion, and Lauren classification [10, 14]. Tumor metabolism is a comprehensive indicator reflecting prognostic factors such as tumor size and T stage (invasive depth). Furthermore, 18F-FDG PET/CT can be performed easily and noninvasively. It can be a good imaging modality for preoperative prognostic prediction.
Unlike RFS, independent PET prognostic parameters were not identified in multivariate analysis for OS. In this study with a small-sized cohort, some patients survived for more than 20 months after recurrence and had a relatively long-term OS (10/69). This may have reduced the difference in OS between recurrence and non-recurrence groups. Additionally, we thought that OS could be affected by other factors not evaluated in this study (such as treatment after recurrence and patient's general condition), compared to RFS. This made it difficult for us to compare overall survival.
This study has several limitations. First, it was a retrospective small-sized study. Further studies including more patients with increased homogeneity are needed to derive advanced results for OS and PET score. Second, we did not use gastric distention method with water intake. FDG uptake can increase when the empty stomach shrinks or the gastric wall thickens. Gastric distention improves diagnostic accuracy by reducing such physiologic uptake and increasing tumor delineation [33, 34]. This method may be useful to improve the result of TLG by delineating tumor boundaries more accurately. Since PET score, including semiquantitative and volumetric information, was a significant prognostic factor, TLG of the same type is expected to be a significant factor in further studies with more volume-measurable patients.
Conclusion
High tumor metabolism implies a high risk of recurrence in patients with AGC. Combining SUVmax and MTV helps to make a better prediction for prognosis. Therefore, preoperative 18F-FDG PET/CT is useful for prognostic evaluation of patients with AGC.
References
Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018;50:303–16.
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Song M, Lee H-W, Kang D. Epidemiology and screening of gastric cancer in Korea. J Korean Med Assoc. 2015;58:183.
Eom BW, Jung KW, Won YJ, Yang H, Kim YW. Trends in gastric cancer incidence according to the clinicopathological characteristics in Korea, 1999-2014. Cancer Res Treat. 2018;50:1343–50.
Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singap Med J. 2014;55:621–8.
Kostakoglu L, Agress H Jr, Goldsmith SJ. Clinical role of FDG PET in evaluation of cancer patients. Radiographics. 2003;23:315–40 quiz 533.
Yamada A, Oguchi K, Fukushima M, Imai Y, Kadoya M. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med. 2006;20:597–604.
Stahl A, Ott K, Weber WA, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003;30:288–95.
Atay-Rosenthal S, Wahl RL, Fishman EK. PET/CT findings in gastric cancer: potential advantages and current limitations. Imaging Med. 2012;4:241–50.
Yun M. Imaging of gastric cancer metabolism using 18 F-FDG PET/CT. J Gastric Cancer. 2014;14:1–6.
Filik M, Kir KM, Aksel B, et al. The role of 18F-FDG PET/CT in the primary staging of gastric cancer. Mol Imaging Radionucl Ther. 2015;24:15–20.
Mukai K, Ishida Y, Okajima K, Isozaki H, Morimoto T, Nishiyama S. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer. 2006;9:192–6.
Moon SH, Hyun SH, Choi JY. Prognostic significance of volume-based PET parameters in cancer patients. Korean J Radiol. 2013;14:1–12.
Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004;28:247–53.
Kim J, Lim ST, Na CJ, et al. Pretreatment F-18 FDG PET/CT parameters to evaluate progression-free survival in gastric cancer. Nucl Med Mol Imaging. 2014;48:33–40.
Lee JW, Lee SM, Lee MS, Shin HC. Role of 18F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Imaging. 2012;39:1425–34.
De Potter T, Flamen P, Van Cutsem E, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging. 2002;29:525–9.
Coupe NA, Karikios D, Chong S, et al. Metabolic information on staging FDG-PET-CT as a prognostic tool in the evaluation of 97 patients with gastric cancer. Ann Nucl Med. 2014;28:128–35.
Chung HW, Lee EJ, Cho YH, et al. High FDG uptake in PET/CT predicts worse prognosis in patients with metastatic gastric adenocarcinoma. J Cancer Res Clin Oncol. 2010;136:1929–35.
Park JC, Lee JH, Cheoi K, et al. Predictive value of pretreatment metabolic activity measured by fluorodeoxyglucose positron emission tomography in patients with metastatic advanced gastric cancer: the maximal SUV of the stomach is a prognostic factor. Eur J Nucl Med Mol Imaging. 2012;39:1107–16.
Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer. Follow-up of 1475 patients and review of the Japanese literature. Cancer. 1993;72:3174–8.
Huang J, Huang L, Zhou J, et al. Elevated tumor-to-liver uptake ratio (TLR) from 18F-FDG-PET/CT predicts poor prognosis in stage IIA colorectal cancer following curative resection. Eur J Nucl Med Mol Imaging. 2017;44:1958–68.
Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–9.
Uchino S, Noguchi M, Ochiai A, Saito T, Kobayashi M, Hirohashi S. p53 Mutation in gastric cancer: a genetic model for carcinogenesis is common to gastric and colorectal cancer. Int J Cancer. 1993;54:759–64.
Berger KL, Nicholson SA, Dehdashti F, Siegel BA. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. Am J Roentgenol. 2000;174:1005–8.
Alakus H, Batur M, Schmidt M, et al. Variable 18F-fluorodeoxyglucose uptake in gastric cancer is associated with different levels of GLUT-1 expression. Nucl Med Commun. 2010;31:532–8.
Kawamura T, Kusakabe T, Sugino T, et al. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92:634–41.
Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomark Prev. 2014;23:700–13.
Cenitagoya GF, Bergh CK, Klinger-Roitman J. A prospective study of gastric cancer. ‘Real’ 5-year survival rates and mortality rates in a country with high incidence. Dig Surg. 1998;15:317–22.
Schauer M, Peiper M, Theisen J, Knoefel W. Prognostic factors in patients with diffuse type gastric cancer (linitis plastica) after operative treatment. Eur J Med Res. 2011;16:29–33.
Zheng H, Takahashi H, Murai Y, et al. Pathobiological characteristics of intestinal and diffuse-type gastric carcinoma in Japan: an immunostaining study on the tissue microarray. J Clin Pathol. 2007;60:273–7.
Deng J-Y, Liang H. Clinical significance of lymph node metastasis in gastric cancer. World J Gastroenterol. 2014;20:3967–75.
Kamimura K, Nagamachi S, Wakamatsu H, et al. Role of gastric distention with additional water in differentiating locally advanced gastric carcinomas from physiological uptake in the stomach on 18F-fluoro-2-deoxy-D-glucose PET. Nucl Med Commun. 2009;30:431–9.
Imperiale A, Cimarelli S, Sellem DB, Blondet C, Contantinesco A. Focal F-18 FDG uptake mimicking malignant gastric localizations disappearing after water ingestion on PET/CT images. Clin Nucl Med. 2006;31:835–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Hye Ryeong Kwon, Kisoo Pahk, Sungsoo Park, Hyun Woo Kwon, and Sungeun Kim declare that they have no conflict of interest.
Ethical Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
The institutional review board of our institute approved this retrospective study (AN17196-001) and the requirement to obtain informed consent was waived.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kwon, H.R., Pahk, K., Park, S. et al. Prognostic Value of Metabolic Information in Advanced Gastric Cancer Using Preoperative 18F-FDG PET/CT. Nucl Med Mol Imaging 53, 386–395 (2019). https://doi.org/10.1007/s13139-019-00622-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-019-00622-w