Abstract
Purpose
Patients with persistent elevated PSA and repeated negative prostate biopsy, that means having the prostate biopsied at multiple times, were investigated with 18F-choline PET/CT to delineate prostate cancer and guide renewed prostate biopsy.
Methods
Twenty patients with elevated PSA and negative prostate biopsies underwent 18F-choline PET/CT. We performed an early examination of the pelvic region 3-5 min after application. After 30 minutes a whole body PET/CT examination was performed. Image analysis was performed visually and by semi-quantitative analysis calculating the maximum standardised uptake value (SUVmax). 18F-choline uptake was defined as focal, multifocal or inhomogeneous. After the 18F-choline PET/CT, all patients underwent a repeated prostate biopsy, and in the cases where a focal or multifocal uptake was found, the biopsy was guided by the result of the examination.
Results
Qualitative image analysis revealed focal 18F-choline uptake in 13 out of 20 patients. In five patients, prostate cancer was revealed by repeated aspiration biopsy. None of the patients with a multifocal or inhomogeneous 18F-choline uptake had a malignant neoplasm in the prostate. Semiquantitative analysis performed with SUVmax was not helpful in the discrimination of malignancy but showed high values also in benign prostate diseases, as well as in normal prostate tissue. The dual-phase protocol delivered no clear benefit in discriminating malignancy from benign alterations.
Conclusion
The use of 18F-choline cannot be generally recommended for localising prostate cancer; however, in highly selected patients, we found useful additional information. In 25% of patients, 18F-choline PET/CT allowed the identification of neoplastic prostatic zones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Prostate cancer is the most common cancer in men in Europe, North America and some parts of Africa. Worldwide, this disease accounts for 9.7% of all cancers in men (15.3% in developed countries and 4.3% in developing countries) [1]. A curative treatment option is only possible for organ-confined prostate cancer and includes surgical radical prostatectomy and radiation therapy. Up to now, the only effective option for lowering mortality is to enhance early detection that means diagnosis at an organ-confined stage. The current work-up to diagnose prostate cancer includes the measurement of the serum prostate-specific antigen (PSA) level, digital rectal examination (DRE) and transrectal ultrasound (TRUS) guided biopsy.
By means of PSA, a screening for prostatic cancer is possible and was described already several years ago; however, using PSA alone, a 70-80% specificity and a 70% sensitivity is only mediocre [2, 3, 4]. In a recent study from Thompson et al., the prevalence of prostate cancer among men with a PSA level ≤ 4.0 ng/ml were investigated with the result that prostate cancer was diagnosed per biopsy in 15.2% [5]. That means that even at low levels of PSA, there is a risk for prostate cancer. Numerous studies have shown that a significant number of men with an initial negative prostate needle biopsy but persistently elevated serum PSA levels will have prostatic malignancy on subsequent biopsy [6, 7]. The false negative cancer detection rate is exceeding 20% for TRUS guided biopsy, which results in repeated biopsies in high risk patients [8].
To date, despite advances in the technology, it is not possible to sufficiently locate malignant sites within the prostate. Up to now, the morphological imaging methods as ultrasound (including transrectal, colour flow Doppler and power doppler) and magnetic resonance imaging (MRI) including magnetic resonance spectroscopic imaging (MRSI) are insufficiently accurate for localisation of prostate cancer, as these methods lack adequate specificity and sensitivity. Comparing greyscale ultrasound to colour Doppler Kravchick et al. reported a specificity of 97% and a very low sensitivity of 18% [9]. Regarding MRI a specificity of 46-61% and a sensitivity of 77-81% was found by Scheidler et al. in a study including 53 patients and two readers [10]. MRSI improved specificity to 70 to 80% but reduced sensitivity to 68 to 73%. As morphologic methods have their limitations in the ability to locate cancer in the prostate metabolic imaging methods are increasingly studied. PET is a uniquely suited diagnostic imaging modality to evaluate metabolic activity in neoplasms.
The most common tracer used in oncology is FDG, which has been found to have a low sensitivity and/or specificity for the assessment of prostate cancer [11, 12]. Hara et al. first described in 1998 the ability for imaging prostate cancer using carbon-11-choline [13]. Up to now, several studies have provided substantial evidence that the choline metabolic pathway is a promising imaging target for prostate cancer [14, 15]. In comparison to the commonly used oncologic tracer 18F-FDG, 18F-fluorocholine has demonstrated a higher avidity for both androgen-dependent and androgen-independent prostate cancer [16]. On the basis of these preliminary results, PET/CT with 18F-labeled choline analogues offers a clear potential for localising prostate cancer in high-risk patients. The aim of this study was to evaluate whether it was possible to increase the diagnostic accuracy of prostatic needle biopsy in high-risk patient by performing an 18F-fluorocholine PET/CT and limit the number of iterative biopsies.
Materials and methods
Patients
Twenty patients with persistently elevated PSA levels and repeated negative prostate needle biopsy were enrolled in this study between December 2005 and November 2006. The mean age was 63.5 years (range 47-78 years). The serum PSA was elevated in all patients (mean 14.06 ng/ml, range 5.75-70.8). All patients underwent repeated negative prostate biopsy, performed TRUS-guided on a sextant basis, between 2 and 5 times, mean 2.8. The characteristics of each patient are listed in Table 1.
18F-Choline PET/CT
PET/CT was performed using a combined PET/CT machine (Biograph®, Siemens), which includes a dual slice helical CT scanner (Somatom Emotion®; Siemens) and a dedicated full-ring lutetium oxyorthosilicate (LSO) PET scanner. One hour before the examination started, the patients were instructed to drink 11/2 l of water enriched with five packages (25g) of mucofalk® (psyllium husks, also known as Plantago ovata) as a negative oral contrast material for distending the stomach and the bowel loops at least in the upper and middle abdomen. After intravenous injection of 4 MBq 18F-choline per kilogram of body mass (Iasocholine®, Iason, Austria) a PET/CT examination of the pelvis with a low-dose CT was performed after 3-5 min to avoid interfering bladder activity. The low-dose CT protocol for the initial evaluation of the pelvis (one bed position) consisted of 130 kV, 35 mAs, pitch 1.5, and increment 3.3. Emission time was 2 min per field of view in the initial as well as in the delayed images. After 30 min whole body PET/CT was performed. Contrast-enhanced CT from the skull base to the upper thighs (whole body examination; 130 kV, 115 mAs, pitch 1.5) was acquired with 140 ml non-ionic contrast agent given intravenously (Visipaque 270®, Amersham). The contrast agent was administered intravenously at a flow rate of 3 ml/s for the first 80 ml and a flow rate of 2 ml/s for the remaining 60 ml (start delay 50 s) with an automated injector [XD 5500(Missouri); Ulrich Medical Systems]. Tracer uptake in the prostate on the initial and delayed images was measured. In the delayed images the whole body distribution of 18F-choline was also evaluated. The PET images were corrected for attenuation by the CT data, and iterative reconstruction algorithms with two iterations and eight subsets were performed (FORE 3 D rebinning method) using the software Somaris/5 VA40C. Data were also filtered and corrected for scatter.
Image analysis (data analysis)
The PET/CT scans were visually analysed by a nuclear physician for the PET part and a radiologist for the CT part of the examination. PET images were first assessed visually, using transaxial, sagittal, and coronal as well as 3D displays. 18F-choline uptake pattern in the prostate was defined as focal, multifocal or inhomogeneous on the attenuation-corrected fused PET/CT images. To describe the exact localisation of intraprostatic focal uptake, each lobe was trisected into an upper third, middle third and lower third. To allow an objective assessment of the amount of tracer uptake we performed a semiquantitative analysis using the maximum standardised uptake value (SUVmax) and the tumour to background ratio for each abnormal focus. SUVmax was calculated in the visually defined areas in the initial and delayed images. In the multifocal uptake pattern, the area with the highest SUVmax was used. In inhomogeneous uptake patterns, the region of interest covered the whole prostate. The background activity was considered as the uptake in the third with the lowest activity in each lobe. Prostate volume was assessed from the CT images determining the width, length and height and using the formula V = pi × abc/6.
Results
Fluorocholine PET/CT results are presented in Table 1. PET/CT visual analysis identified in the delayed images a focal 18F-choline uptake in 13 patients, a multifocal uptake in five patients and an inhomogeneous uptake pattern in two patients (Figs. 1 and 2 are examples for a focal uptake and Figs. 3 and 4 for a multifocal uptake). Of the 13 patients with a focal uptake pattern, repeated prostate biopsy revealed a prostate malignancy in five patients. No malignancy was depicted in the seven patients who had a multifocal or inhomogeneous 18F-choline uptake pattern, validated by repeated sextant biopsy. Regarding the PET images in none of the patients there was a focal pathologic 18F-choline uptake in the whole body with the exception of the prostate. Also on CT in none of the subjects a mentionable pathology was detected. The obtained histology of the prostate was as follows: prostate cancer in five patients, chronic prostatitis in eight patients, benign prostatic hyperplasia in four patients and no pathology indicating normal prostatic tissue in three patients. In five patients out of thirteen with a suspicious focal 18F-choline uptake prostate cancer was diagnosed in the area found in the fluorocholine PET/CT (25%). All these five patients demonstrated a focal uptake in the early as well in the delayed images. SUVmax was ranging between 1.68 and 6.22 (mean 3.39) on the initial images and 1.82 and 4.40 (mean 3.12) on the delayed images. The Gleason scores were 6 in three patients in each case 3 + 3, representing moderately differentiated tumours and 7 in the remaining two patients (3 + 4 in one case and 4 + 3 in one patient, respectively) indicating moderately to poorly differentiated tumours. The tumour to background ratio in these five patients ranged between 1.66 and 3.87 (mean 2.68) for the initial images and between 1.93 and 2.24 (mean 2.08) for the delayed images. The SUVmax increased in three out of the five patients between the initial and the delayed images and decreased in the remaining two. In one patient with a prostate cancer of Gleason Score 7, the SUVmax decreased from 2.19 to 1.82, and in the second patient, it decreased from 4.39 to 2.34. Of these five patients, one had undergone five biopsies at different time points previously, two had four biopsies, one of them had three biopsies, and one had two biopsies. In eight patients, the definitive result was chronic prostatitis. Four out of the eight subjects presented a focal uptake and three a multifocal uptake. The mean SUVmax of the early images was 3.25 (range 2.56-4.24) and 3.27 (range 2.47-4.20) for the delayed images. In the majority of patients, i.e. in five subjects, the SUVmax decreased, and in three patients, we observed an increase of the SUVmax. The third histological entity included four patients with benign prostate hyperplasia, presenting a focal and multifocal uptake pattern, in two and two cases, respectively. The mean SUVmax of the images was relatively stable and represented 2.95 for the initial images and 3.05 for the delayed images. Three of them presented a decrease of SUVmax comparing the initial and delayed images, only one patient showed an increase of the SUVmax from 2.59 to 3.59. In three patients, the repeated needle biopsy revealed normal prostate tissue with a relatively high mean SUVmax of 3.68 (range 3.45-4.13) for the initial images and mean SUVmax of 3.74 (range 3.37-3.94) in the delayed images. Regarding the uptake pattern, we observed a focal uptake in two patients and an inhomogeneous uptake pattern in one patient. In two patients, the SUVmax decreased between the two PET/CT acquisitions, and in one patient, it increased from 3.45 to 3.94. The detailed information regarding SUVmax and tumour to background ratio are listed in Table 2. In the group of patients who had a focal 18F-choline PET/CT finding, 18F-choline PET/CT had a sensitivity of 100%, a specificity of 46.7% and a positive predictive value of 38.5% for the detection of prostate cancer, however validated by biopsy and not histology.
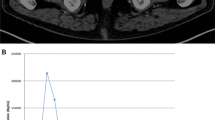
Initial PET images (maximum intensity projection, MIP) of a 59-year-old patient with histologically confirmed prostate cancer of the pelvis (one bed position) (a), corresponding axial slices (PET images and fused PET/CT images) (b) indicating a focal 18F-choline uptake in the left lobe of the prostate and no interfering bladder activity
Discussion
In high-risk patients with constantly elevated PSA, the combination of digital rectal examination, serum PSA determination and TRUS-guided biopsy are considered the standard methods for prostate cancer detection. PSA concentrations relate to age, prostate size, and the presence of prostate cancer, but can also be raised after ejaculation, prostate biopsy, surgery or prostatitis. Whatever the cut-point, up to 10% of men aged between 50 and 69 years will have a raised PSA level that will induce a biopsy. In the European Randomised Study of Prostate Cancer (ERSCP), 36.5% of detectable prostate cancers were identified in men with PSA concentrations lower than 4 ng/ml [17]. TRUS-guided biopsy often fails to locate prostate cancer; hence, repeated biopsies are the consequence. The incidence of a positive repeat biopsy in men with elevated serum PSA levels and in initial negative biopsy has been demonstrated to be 20%-40% [18]. Several studies suggest that the choline analogues are potentially useful as PET tracers for detection and localisation of prostate cancer [14, 15]. Two mechanisms have been suggested to explain the increased uptake in prostate cancer: increased cell proliferation and the up-regulation of choline kinase. Choline is a component of phosphatidycholine, an essential element of phospholipids in the cell membrane. Choline is transported into the cells and utilised for the synthesis of phospholipids and sphyngomyelin. Numerous studies assessed elevated levels of choline, as well as an up-regulated activity of CK in many types of cancer cells including also prostate cancer [19, 20]. Thus, the exact uptake mechanism is not precisely established up to now. 18F fluoromethyl-dimethyl-2-hydeoxyethyl-ammonium (18F-choline) closely mimics choline uptake by normal tissue and prostate cancer cells. 18F-choline offers a very rapid blood clearance resulting in an excellent tumour to background contrast by 3 min after injection, as described by DeGrado et al. PET imaging is allowed to be commenced as early as 4-5 min after injection with the advantage of absent 18F radioactivity in the urinary bladder [21]. A potential drawback comparing 18F-choline with 11C-choline may be the higher urinary excretion achieving 7.5% of injected 18F-choline [22]. Delayed imaging (30-60 min after injection) improved lesion contrast and the dual-phase protocol was described as being helpful in discriminating malignancy from benign tissue. Kwee et al. [23] demonstrated that malignancy demonstrated stable or increasing 18F-fluorocholine uptake, whereas most areas containing benign tissue demonstrated decreasing uptake. In this study, PET imaging was performed at two different time points: early images starting 7 min after administration and delayed images, acquired 1 h after application. A possible explanation of this observation may be that there exists a dephosphorylation of 18F-phosphorylfluorocholine by prostatic acid phosphatase, which is elevated in normal prostate tissue and in tissue affected by benign prostatic hyperplasia [24]. However, in our study, we did not observe these findings. We found an increasing uptake in eight patients (three patients with prostate cancer, one with normal prostate tissue, three with chronic prostatitis and one with benign prostate hyperplasia) and a decreasing activity in the remaining patients that means a decreasing uptake in two patients with prostate cancer and an increasing uptake in five patients with benign alterations. Our results are not exactly comparable with the results from this study as we performed early images starting 3 min after application and delayed scans after an uptake time of 30 min. DeGrado et al. described that there exists a rapid uptake of 18F-choline in prostate cancer beginning immediately after injection. After approximately 4 min, the uptake reaches a maximal value with stable intensity over a time span of 20 min [21]. However, the optimal timing for PET acquisition of 18F-choline has not yet been systematically evaluated, as well as the dual phase protocol is not generally recommended. There was no change between early and delayed images regarding the uptake pattern; therefore, we can conclude that a dual-phase protocol is not absolute necessary for the evaluation of the PET/CT images. The tumour background ratio decreased in the four different histological entities between the initial and delayed images. The only advantage we saw was the absent urinary bladder activity in the initial images, which degrades image quality and thus can confound the interpretation of findings in the prostate or in the pelvic region. Regarding the uptake pattern of 18F-choline a focal 18F-choline uptake was also found in normal prostate tissue, benign prostate hyperplasia, and prostatitis. Wyss et al. reported that 18F-choline accumulates in infectious tissue, in particular in granulocytes and macrophages with high SUV values between 3.0 and 4.0, however calculated in rats [25]. Schmid et al. already described in a study including 19 male patients with histologically confirmed adenocarcinoma that 18F-choline also accumulates in benign prostate hyperplasia and therefore is not well suited for T staging of prostate cancer [26]. In the majority of patients (n = 8), the result of the 18F-choline-supported biopsy was chronic prostatitis; five of them showed a focal uptake pattern. It is well known that in the diagnosis of chronic prostatitis, biopsy has limited sensitivity and specificity [27]. To draw a comparison to MRI and MRSI, Shukla-Dave et al. described an elevated choline peak in regions of chronic prostatitis, findings that mimic those of cancer [28]. In the literature, the results for visualisation of tumours in the prostate are controversial. Hara described that the SUVmax of 11C-choline is higher in prostate cancer than in normal prostate tissue or in benign prostate hyperplasia [29]. However Sutinen et al. concluded that the difference between the SUVs of the prostate neoplasm and benign disease such as prostatic hyperplasia was not statistically significant, although the highest SUVs were found in cancers [30]. As the behaviour of 18F-labelled analogues of choline is very similar to that of 11C-choline, it is possible to compare our results with these two studies. In addition, in a recent study performed by Scher et al. an increased uptake of the choline metabolite 11C-choline was observed in benign changes such as acute and chronic prostatitis, and benign prostate hyperplasia [31]. In our results, it was not possible to classify different histological entities regarding the SUVmax values. The lowest SUVmax was found in a prostate cancer at the early images (1.68) and relatively high SUVmax levels (4.13) was detected in normal prostate tissue. However in one patient with prostate cancer, we found a very high value of 6.22 at early images which decreased to 4.40 at the delayed images. Therefore, we can conclude that the determination of the SUVmax values is in most cases not helpful to distinguish between malignancy and benign prostate diseases. Only very high values, in our study greater than 4.2, may indicate a malignant neoplasm. In the study from Kwee et al., very high SUVmax values of 18F-fluorocholine in prostate cancer were identified [23]. The highest values were 19.66 after 7 min and 24.04 after 1 h, however in a patient with a poorly differentiated prostate cancer, i.e. Gleason score sum of 9 (5 + 4). It has to be considered that the reproducibility of the SUV measurement is limited because of different acquisition protocols; however, in this study, generally higher values were described in patients with prostate cancer comparable with our patients concerning cancer grading. Since we know that prostate cancer is often multifocal and the average patient has at least two distinct foci of cancer in the prostate, which can have different Gleason scores, it is not possible to exclude malignancy in a patient with an inhomogeneous or multifocal uptake pattern [32]. However, in our study, all five patients with prostate cancer presented a focal 18F-choline uptake and were identified visually. An important point should be considered when interpreting the study results. The reference standard used in our study was repeat prostate biopsy and not histology. In biopsy, only a small amount of tissue is available for histological examination and biopsies often identify only a few malignant glands among many benign. Although the biopsy was guided by the results of the 18F-choline PET/CT, meaning that supplementary biopsies were added to the routine sextant biopsy protocol on the 18F-choline PET/CT suspicious zones, we cannot generally rule out that there was a sampling error. In our study, in 14 patients out of 20, a single focal lesion in the prostate was identified, which indicates the most likely location of malignancy. Knowing this to be the most likely location, it is possible to direct biopsies toward these regions and therefore increasing the diagnostic accuracy of the biopsy. In our study, in 5 out of 20 patients, a new prostate biopsy performed after PET/CT confirmed prostate malignancy. As 18F-choline has been described to be of no indication for T-staging, there is evidence in our study that it may be an indication for high-risk patients with multiple negative prostatic biopsies for identification of the most likely location of malignancy. The drawbacks in our study were on the one hand the small sample size of 20 patients, and on the other hand, we do not know what a study with 18F-choline PET/CT looks like in men with normal PSA. But because of ethical reasons, it would be very difficult to perform 18F-choline PET/CT in volunteers. The question whether an inhomogeneous or multifocal 18F-choline uptake excludes malignancy or minimises the risk as indicated in our study has to be clarified in future studies.
Conclusion
Our study affirmed that 18F-fluorcholine is able to localise prostate cancer, but as the tracer is not specific, we also found an uptake in benign inflammatory changes like prostatitis, benign prostate hyperplasia as well as in normal tissue. Therefore, an 18F-choline PET/CT cannot be generally recommended for the localisation of prostate cancer. However, in highly selected patients who have undergone multiple TRUS-guided biopsies with negative findings it could be helpful and contribute valuable additional information regarding the detection of the primary tumour.
References
Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361(9360):859-64.
Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324(17):1156-61. Erratum in: N Engl J Med 1991;325(18):1324.
Brawer MK, Chetner MP, Beatie J, Buchner DM, Vessella RL, Lange PH. Screening for prostatic carcinoma with prostate specific antigen. J Urol. 1992;147(3 Pt 2):841-5.
Roscigno M, Scattoni V, Bertini R, Pasta A, Montorsi F, Rigatti P. Diagnosis of prostate cancer. State of the art. Minerva Urol Nefrol. 2004;56(2):123-45.
Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239-46.
Ellis WJ, Brawer MK. Repeat prostate needle biopsy: who needs it? J Urol. 1995;153(5):1496-8.
Fleshner NE, O'Sullivan M, Fair WR. Prevalence and predictors of a positive repeat transrectal ultrasound guided needle biopsy of the prostate. J Urol. 1997;158(2):505-8. discussion 508-9.
Rabbani F, Stroumbakis N, Kava BR, Cookson MS, Fair WR. Incidence and clinical significance of false-negative sextant prostate biopsies. J Urol. 1998 Apr;159(4):1247-50.
Kravchick S, Cytron S, Peled R, Altshuler A, Ben-Dor D. Using gray-scale and two different techniques of color Doppler sonography to detect prostate cancer. Urology. 2003;61(5):977-81.
Scheidler J, Hricak H, Vigneron DB, Yu KK, Sokolov DL, Huang LR, et al. Prostate cancer: localization with three-dimensional proton MR spectroscopic imaging-clinicopathologic study. Radiology. 1999;213(2):473-80.
Hoh CK, Seltzer MA, Franklin J, deKernion JB, Phelps ME, Belldegrun A. Positron emission tomography in urological oncology. J Urol. 1998;159(2):347-56.
Shreve PD, Grossman HB, Gross MD, Wahl RL. Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucose. Radiology. 1996;199(3):751-6. Jun.
Hara T, Kosaka N, Kishi H. PET imaging of prostate cancer using carbon-11-choline. J Nucl Med. 1998;39(6):990-5.
Ramirez de Molina A, Rodriguez-Gonzalez A, Gutierrez R, Martinez-Pineiro L, Sanchez J, Bonilla F, et al. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem Biophys Res Commun. 2002;296(3):580-3.
Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999 Jan 1;59(1):80-4.
Price DT, Coleman RE, Liao RP, Robertson CN, Polascik TJ, DeGrado TR. Comparison of [18 F]fluorocholine and [18 F]fluorodeoxyglucose for positron emission tomography of androgen dependent and androgen independent prostate cancer. J Urol. 2002;168(1):273-80.
Schroder FH, van der Cruijsen-Koeter I, de Koning HJ, Vis AN, Hoedemaeker RF, Kranse R. Prostate cancer detection at low prostate specific antigen. J Urol. 2000;163(3):806-12.
Keetch DW, Catalona WJ, Smith DS. Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values. J Urol. 1994;151(6):1571-4.
Negendank W. Studies of human tumors by MRS: a review. NMR Biomed. 1992;5(5):303-24.
Nakagami K, Uchida T, Ohwada S, Koibuchi Y, Suda Y, Sekine T, Morishita Y. Increased choline kinase activity and elevated phosphocholine levels in human colon cancer. Jpn J Cancer Res. 1999;90(4):419-24.
DeGrado TR, Coleman RE, Wang S, Baldwin SW, Orr MD, Robertson CN, et al. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: initial findings in prostate cancer. Cancer Res. 2001;61(1):110-7.
DeGrado TR, Reiman RE, Price DT, Wang S, Coleman RE. Pharmacokinetics and radiation dosimetry of 18F-fluorocholine. J Nucl Med. 2002;43(1):92-6.
Kwee SA, Wei H, Sesterhenn I, Yun D, Coel MN. Localization of primary prostate cancer with dual-phase 18F-fluorocholine PET. J Nucl Med. 2006;47(2):262-9.
Hakalahti L, Vihko P, Henttu P, Autio-Harmainen H, Soini Y, Vihko R. Evaluation of PAP and PSA gene expression in prostatic hyperplasia and prostatic carcinoma using northern-blot analyses, in situ hybridization and immunohistochemical stainings with monoclonal and bispecific antibodies. Int J Cancer. 1993;55(4):590-7.
Wyss MT, Weber B, Honer M, Spath N, Ametamey SM, Westera G, et al. 18F-choline in experimental soft tissue infection assessed with autoradiography and high-resolution PET. Eur J Nucl Med Mol Imaging 2004;43:187-99.
Schmid DT, John H, Zweifel R, Cservenyak T, Westera G, Goerres GW, et al. Fluorocholine PET/CT in patients with prostate cancer: initial experience. Radiology. 2005;235(2):623-8.
True LD, Berger RE, Rothman I, Ross SO, Krieger JN. Prostate histopathology and the chronic prostatitis/chronic pelvic pain syndrome: a prospective biopsy study. J Urol. 1999;162(6):2014-8.
Shukla-Dave A, Hricak H, Eberhardt SC, Olgac S, Muruganandham M, Scardino PT, et al. Chronic prostatitis: MR imaging and 1H MR spectroscopic imaging findings-initial observations. Radiology. 2004;231(3):717-24.
Hara T. 11C-choline and 2-deoxy-2-[18F]fluoro-D-glucose in tumor imaging with positron emission tomography. Mol Imaging Biol. 2002;4(4):267-73.
Sutinen E, Nurmi M, Roivainen A, Varpula M, Tolvanen T, Lehikoinen P, et al. Kinetics of [(11)C]choline uptake in prostate cancer: a PET study. Eur J Nucl Med Mol Imaging. 2004;31(3):317-24.
Scher B, Seitz M, Albinger W, Tiling R, Scherr M, Becker HC, et al. Value of (11)C-choline PET and PET/CT in patients with suspected prostate cancer. Eur J Nucl Med Mol Imaging. 2007;34(1):45-53.
Crawford ED, Miller GJ, Labrie F, Hirano D, Batuello J, Glode LM. Prostate cancer pathology, screening, and epidemiology. Rev Urol. 2001;3(Suppl2):S2-S10.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Igerc, I., Kohlfürst, S., Gallowitsch, H.J. et al. The value of 18F-Choline PET/CT in patients with elevated PSA-level and negative prostate needle biopsy for localisation of prostate cancer. Eur J Nucl Med Mol Imaging 35, 976–983 (2008). https://doi.org/10.1007/s00259-007-0686-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0686-9