Abstract
Currently, up to 50% of the operations in early-stage non-small cell lung cancer (NSCLC) are futile owing to the presence of locally advanced tumour or distant metastases. More accurate pre-operative staging is required in order to reduce the number of futile operations. The cost-effectiveness of fluorine-18 fluorodeoxyglucose positron emission tomography (18FDG-PET) added to the conventional diagnostic work-up was studied in the PLUS study. Prior to invasive staging and/or thoracotomy, 188 patients with (suspected) NSCLC were randomly assigned to conventional work-up (CWU) and whole-body PET or to CWU alone. CWU was based on prevailing guidelines. Pre-operative staging was followed by 1 year of follow-up. Outcomes are expressed in the percentage of correctly staged patients and the associated costs. The cost price of PET varied between €736 and €1,588 depending on the (hospital) setting and the procurement of 18FDG commercially or from on-site production. In the CWU group, 41% of the patients underwent a futile thoracotomy, whereas in the PET group 21% of the thoracotomies were considered futile (P=0.003). The average costs per patient in the CWU group were €9,573 and in the PET group, €8,284. The major cost driver was the number of hospital days related to recovery from surgery. Sensitivity analysis on the cost and accuracy of PET showed that the results were robust, i.e. in favour of the PET group. The addition of PET to CWU prevented futile surgery in one out of five patients with suspected NSCLC. Despite the additional PET costs, the total costs were lower in the PET group, mainly due to a reduction in the number of futile operations. The additional use of PET in the staging of patients with NSCLC is feasible, safe and cost saving from a clinical and from an economic perspective.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related death in Western society. In 1997 in the Netherlands, approximately 8,800 people were diagnosed with lung cancer and 8,650 patients died from the disease [1]. The 5-year survival rate was 13% [1]. The poor prognosis of lung cancer can be ascribed to the high rate of unresectable disease at diagnosis and the failure of other treatment modalities to cure metastatic disease. Non-small cell lung cancer (NSCLC), for which surgery is the only curative treatment option, represents approximately 85% of all primary lung tumours.
Diagnostic work-up is essential to confirm the diagnosis of NSCLC pre-operatively and to detect (mediastinal) lymph node involvement and distant metastasis. The aim is to restrict surgery to patients who will potentially benefit.
International guidelines have been formulated to optimise these efforts, but daily clinical practice remains variable [2]. The current diagnostic work-up cannot prevent futile surgery in up to 50% of patients with NSCLC [3]. Recently, the situation has improved significantly owing to the introduction of positron emission tomography with fluorine-18 fluorodeoxyglucose (18FDG-PET) [4]. Results of a randomised clinical trial showed that PET, employed in addition to conventional diagnostic work-up, could reduce futile operations by 50% [5].
The clinical relevance of PET in NSCLC is beyond doubt; however, health policy makers are concerned about the extra costs accruing from the introduction of this new technology. PET is a relatively expensive technique due to the high costs of acquisition, maintenance and the radioactive tracer [6, 7]. A number of economic evaluation studies have examined PET in various diagnostic pathways through modelling. Most of these variants showed that PET could be cost-effective in NSCLC when added to conventional work-up (CWU). However, all of these studies had to rely on many assumptions and were unable to consider how cumulative diagnostic patient information impacts on patient management and final outcomes.
In the PET in Lung Cancer Staging (PLUS) study, the effectiveness of PET added to the CWU compared with CWU alone was studied in a randomised fashion consistent with routine clinical practice [5]. A cost-effectiveness analysis was anticipated in this trial. The results of this analysis are presented here. The perspective of the study was the hospital's point of view, i.e. all hospital costs associated with PET and CWU were considered.
Materials and methods
Clinical study
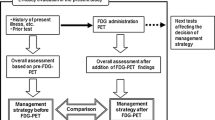
Between January 1998 and January 1999, patients with suspected or proven NSCLC, considered to be medically operable and to have potentially resectable disease by the referring physician on the basis of clinical staging, but not surgical staging, were invited to participate. Patients were randomly assigned either to PET followed by CWU (CWU+PET) or to CWU alone. CWU was based on existing guidelines and involved further invasive diagnostic and therapeutic procedures. Eight general hospitals and one university hospital recruited patients for the study.
PET scans were performed using a Siemens ECAT EXACT HR+ scanner (Siemens/CTI, Knoxville, Tenn., USA) at the Vrije Universiteit, Amsterdam. Referring clinicians were free to use the information and to act accordingly. However, potential distant or nodal metastases, which might have a major impact on patient management, had to be confirmed by other techniques. Unconfirmed PET findings were to be ignored. All procedures other than PET, including therapy and follow-up, were performed in the referring hospitals according to prevailing local standards. Follow-up consisted of regular visits (every 2–3 months) to the outpatient clinic for 12 months. The clinical paper contains a detailed description of the procedure [5].
Cost study
We focussed on the costs of diagnostic and therapeutic strategies. The cost items considered were hospital days, thoracotomies, and invasive and non-invasive diagnostic tests (including PET). Therapeutic interventions such as chemotherapy and radiotherapy were not taken into account.
The full costs of the various diagnostic procedures, hospital days and intensive care days and operation costs were calculated by using the bottom-up method [8, 9]. These costs included costs for personnel, materials, depreciation and overheads based on 1999 prices. The cost price study was performed in all hospitals. Where individual hospitals were not able to deliver details on specific procedures, average costs of similar procedures at the other hospitals were used. Table 1 contains the average cost prices for relevant procedures with ranges.
The costs of PET encompass the personnel costs, the depreciation and maintenance costs, the material (tracer) costs and overheads. A PET study requires a minimum level of radioactive activity, and because 18FDG has a half-life of 110 min, the quantity supplied depends on the period between production and usage in the patient. The PET centre was provided twice a day with enough 18FDG for the scheduled patients. The production and supply of 18FDG is therefore constrained by two factors, a maximum that can be injected in the patient and a minimum level of radioactivity necessary for PET to work.
The costs of PET also depend on the daily number of scans, the average time per scan, and the amount and expertise (e.g. salary) of the personnel involved.
Several scenarios were considered by varying the number of PET scans performed per day and varying the hospital setting. The "expensive" variant involved a PET study performed in a university hospital with on-site tracer production and with clinical (diagnostic) as well as research functions. In this calculation, it was assumed that eight PET scans were performed daily, requiring the presence of a nuclear medicine technologist and a nuclear medicine physician. This variant was based on real costs in one centre.
The next two variants were based on expert opinion, because in the Netherlands there were only two university hospitals with a PET centre at the time of our study. In the "cheap" variant, 12 PET scans per day were performed in a community hospital with a PET scanner which was incorporated in a nuclear medicine department, and merely used for clinical diagnostic work. Production and transport of 18FDG were done according to the satellite concept [7]. The "in-between" option considered a large community hospital in which eight PET scans were performed per day. In this setting, limited research was done and 18FDG was produced on site.
Table 2 shows a detailed specification of the cost of a PET scan. In this study we used the "in-between" variant. In this variant the costs of a whole-body FDG-PET scan amounted to €1,020. The upper and lower values were used in a sensitivity analysis.
Outcomes
Primary study outcome was the number of futile thoracotomies. A thoracotomy was classified as futile in cases of: (1) a benign lung lesion, (2) pathologically proven mediastinal lymph node (MLN) involvement (stage IIIA-N2) other than minimal N2 disease (i.e. intranodal involvement in a single lymph node established at mediastinal dissection), (3) stage IIIB disease, (4) explorative thoracotomy for any other reason, or (5) recurrent disease or death from any cause within 1 year after randomisation.
Patients were analysed according to the intention-to-treat principle. Secondary outcome was the cost associated with both strategies calculated as the average cost per (suspected) NSCLC patient.
Sensitivity analysis
Sensitivity analyses were performed on the efficacy of PET and the PET setting. For the efficacy the 95% confidence interval was used and for the setting the "expensive" variant and the "cheap" variant were taken.
Statistical analyses
The number of futile thoracotomies was tested by the chi-square test based on intention to treat. The costs of the various cost items were tested by means of the two-tailed Wilcoxon-Mann-Whitney test.
Results
Clinical results
Table 3 presents the baseline characteristics. Seventy-one percent of the CWU patients and 70% of the CWU+PET patients had clinical stage I or II. Pre-randomisation work-up was similar for both groups and included at least a chest computed tomography (CT) scan, which included the liver and adrenals (89%). In both groups, 58% of patients underwent at least one additional test to identify metastatic disease before randomisation.
The primary outcome is the proportion of patients who underwent futile thoracotomy: in the CWU arm, 41% (39/96) underwent futile surgery compared with only 21% (19/92) in the CWU+PET arm (P=0.003) (Table 4). This implies a relative reduction in the number of futile surgical procedures by 51%. The absolute difference of 20% corresponds to five patients (95% confidence interval 3–14) needing CWU+PET to avoid one futile thoracotomy. Eighteen patients in the CWU arm and 32 in the CWU+PET arm did not proceed to thoracotomy, mainly due to the presence of benign lesions, upstaging due to detection by PET or intercurrent morbidity and recurrence of metastases. Eighteen percent of the CWU patients and 32% of the CWU+PET patients were not operable owing to extensive co-morbidity or refusal of the patient. Unfortunately, in both arms several patients died due to surgery-related complications. Apparently justified surgery was done in a closely similar number of patients in each group, namely 39/96 in the CWU group and 41/92 in the CWU+PET group.
Cost analysis
Considering the groups of operative patients and all patients, the mean number of regular hospital days and number of days on the intensive care ward were in favour of the CWU+PET group. The number of imaging tests and (non-)invasive tests did not differ between the groups (except for the PET scan). Also the number of mediastinoscopies was similar in the two arms (63 patients in the CWU group and 67 patients in the CWU+PET group). The most important cost items are shown in Table 5.
The group of patients who were not operated on consisted of patients who refused an operation or patients who had severe co-morbidity (six patients), were down- or upstaged (41 patients) or were discovered to have a tumour type for which surgery was not the optimal treatment (three patients).
Overall, the average total costs were lower in the PET group owing to a reduction in (futile) operations and subsequent hospital days (Table 6). Patients who underwent surgery in the CWU arm cost €11,486 on average, versus €10,709 in the CWU+PET arm. Patients who underwent futile operations in the CWU group cost on average €12,473, compared with €13,689 in the CWU+PET group. This difference is mainly related to the cost of PET (€1,021) in the CWU+PET arm. Remarkably, non-futilely operated patients cost on average €10,489 in the CWU group and €9,487 in the CWU+PET group. This difference was mainly caused by a higher number of intensive care days due to one patient in the CWU group, who stayed 61 days on the intensive care ward.
The average costs of patients who were not operated on amounted to €1,287 in the CWU group and to €3,851 in the CWU+PET group. The costs in the CWU group were lower because the patients spent fewer days in the hospital and because of the cost of PET itself.
Sensitivity analysis
The results of the sensitivity analysis are presented in Table 7.
Varying the efficacy of PET
In this study adding PET to CWU resulted in a 20% absolute reduction (from 41% to 21%) in futile operations or, put another way, one prevented unnecessary surgical procedure for every five PET scans (95% CI: 3–14). The 95% confidence interval corresponded to a range of one prevented unnecessary surgical procedure for every three PET scans (9% futile operations remaining in the PET group) to one prevented procedure for every 14 PET scans (28% futile operations remaining). Taking into account the cost of €1,021 per PET scan, the more efficient PET result would result in higher savings compared with CWU. Preventing 28% of the futile operations would result in a difference of €2,123 in favour of CWU+PET. Even the upper boundary of the 95% CI would result in a difference of €759 in favour of CWU+PET.
Varying the setting of PET
The results of the cost analysis were rather sensitive to the price of PET. If the PET costs were lower than €1,021, the savings in favour of the PET+CWU arm would increase. Furthermore, the PET costs in the "expensive" setting were still in favour of the PET+CWU group. The break-even point, i.e. with no difference in costs between the groups, was at a PET cost of €2,350. If the costs of PET were higher than €2,350, then the CWU group would be cheaper.
Varying the setting and the efficacy of PET
Varying both the setting and the efficacy of PET, most results were robust and in favour of the PET+CWU group. When taking into account the highest price of PET and the worst efficacy outcome, the results were in favour of the CWU arm (namely €542).
Discussion
The introduction of PET as a new diagnostic technology for various indications is clinically an important step, but the costs may be relatively high. In this randomised controlled trial we directly measured the efficacy of PET as well as the associated costs. Our study shows that PET added to CWU in patients with suspected NSCLC is effective from either perspective. Since hospital days, the operation itself and postoperative intensive care were the major cost drivers, a decrease in futile operations would have a profound effect on the costs.
Until now, cost-effectiveness data on PET could only be derived from studies that applied modelling techniques to estimate the costs and benefits of PET in various diseases [10, 11, 12, 13, 14, 15, 16, 17], and mainly in (suspected) lung cancer. The results of these studies have been based on reviews of the sensitivity and specificity of CT and PET. Morbidity and mortality effects have scarcely been reported, and have been reliant upon additional assumptions made by the investigators. Diagnostic accuracy measures do not easily translate to the complete clinical decision-making and management of patients and subsequent clinical outcome. Further, one of the main deficiencies of these analyses is a failure to consider how cumulative diagnostic information impacts on patient management and final outcomes [15]. However, improved accuracy in pre-operative staging should provide an improvement in survival [18].
A limitation of our study is the neglect of pre-operative or neoadjuvant treatments for patients with locally advanced NSCLC or other treatment options for patients who were unsuitable for thoracotomy. However, it has been claimed that multimodality interventions are cost-effective [6].
The real costs of a PET scan are complicated to assess. In the Netherlands, the application is relatively new and no guidelines regarding indications for PET have been established at the national level. Furthermore, the acquisition cost of a PET scanner varies between €1,000,000 and €2,800,000, so that the depreciation per scan can vary considerably. The daily production of PET scans is an important determinant of the calculated price of a PET scan. Since clinical PET may comprise research as well as diagnostic activities, the daily production of diagnostic scans may range from 6 to 12. Therefore the cost of PET shows considerable variation within the hospital setting.
The possible configurations for PET and cyclotron have also been described elsewhere. In the United States, Keppler et al. found a range between $2,986 and $1,557 (i.e. between €2,706 and €1,411) (including radioactive FDG) for a whole-body PET scan [13, 14]. In our study, the break-even cost of PET (in which the average costs per NSCLC patient would have been equal in both arms) was €2,234, . This cost price of PET was much higher than the cost in the most expensive scenario (i.e. a university hospital with a research function). In this scenario the cost price amounted to €1,588.
In some countries, PET scans are reimbursed by insurance companies. In the USA, reimbursement amounts to approximately $2,000 (18FDG included) for PET staging in lung cancer. In Germany the reimbursement amounts to €1,227 [11]. In the Netherlands, the results of this study could be used for the reimbursement policy. Here, about 9,000 new lung cancers are detected annually. About 85% of these are NSCLC. After exclusion of those patients who prove to have irresectable disease after standard dissemination tests and those who are inoperable owing to co-morbidity precluding surgery, approximately 5,000 patients would be eligible for PET. Applying the results of this trial, the routine use of PET for NSCLC could save a great number of thoracotomies, resulting in a decrease in morbidity and resource use (i.e. intensive care), with a consequent saving of around 6.4 million euros (5,000 × €1,289).
Recently a second randomised controlled trial of PET in NSCLC was closed after including a total of 470 patients. The primary research question of this study is whether PET may substitute for other techniques in the conventional work-up. Again, data on costs will be collected in detail and analysed as a secondary endpoint.
On the basis of this study we conclude that the additional use of PET in the staging of patients with NSCLC is feasible and safe and saves costs from a clinical and an economic perspective.
References
Jansen-Heynen MLG, van Dijck JAAM, Schipper RM, Damhuis RAM. Longkanker in Nederland in de periode 1989–1997: de epidemie is nog niet voorbij. Ned Tijdschr Geneeskd 2001; 145:419–423.
Herder GJM, et al. Staging of non-small cell lung cancer in two large Dutch hospitals. Eur J Respir Dis 1999; 14:446.
van Tinteren H, Hoekstra OS, Smit EF, Verboom P, Boers M. Towards less futile surgery in non-small cell lung cancer? A randomised clinical trial to evaluate cost-effectiveness of positron emission tomography. Control Clin Trials 2001; 22:89–98.
Pieterman RM, van Putten JWG, Meuzelaar JJ, et al. Preoperative staging of non-small cell lung cancer with positron-emission tomography. N Engl J Med 2000; 343:254–261.
van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 2002; 359:1388–1393.
Evans WK, Will BP, Berthelot JM, Earle CC. Cost of combined modality interventions for stage III non-small cell lung cancer. J Clin Oncol 1997; 15:3038–3048).
Lottes G, Gorschlutter P, Kuwert T, Adam D, Schober O. Costs of F-18-FDG-PET. Experience with a satellite concept. Nuklearmedizin 1998; 37:159–164.
Verboom P, Herder GJM, Hoekstra OS, et al. Staging of non-small cell lung cancer and application of FDG-PET: a cost approach. Int J Technol Assess Health C 2002; 18:576–585.
Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996.
Gambhir SS, Hoh CK, Phelps ME, Madar I, Maddahi J. Decision tree sensitivity analysis for cost-effectiveness of FDG-PET in the staging and management of non-small cell lung carcinoma. J Nucl Med 1996; 37:1428–1436.
Dietlein M, Weber K, Gandjour A, et al. Cost-effectiveness of FDG-PET for the management of solitary pulmonary nodules: a decision analysis based on cost reimbursement in Germany. Eur J Nucl Med 2000; 27:1441–1456.
Keith CJ, Miles KA, Griffiths MR, et al. Solitary pulmonary nodules: accuracy and cost-effectiveness of sodium iodide FDG-PET using Australian data. Eur J Nucl Med Mol Imaging 2002; 29:1016–1023.
Keppler JS. Federal regulations and reimbursement for PET. J Nucl Med Technol 2001; 29:173–179; quiz 180–182.
Keppler JS, Conti PS. A cost analysis of positron emission tomography. Am J Roentgenol 2001; 177:31–40.
Miles KA. An approach to demonstrating cost-effectiveness of diagnostic imaging modalities in Australia illustrated by positron emission tomography. Australas Radiol 2001; 45:9–18.
Scott WJ, Shepherd J, Gambhir SS. Cost-effectiveness of FDG-PET for staging non-small cell lung cancer: a decision analysis. Ann Thorac Surg 1998; 66:1876–1885.
Dietlein M, Weber K, Gandjour A, et al. Cost-effectiveness of FDG-PET for the management of potentially operable non-small cell lung cancer: priority for a PET-based strategy after nodal-negative CT results. Eur J Nucl Med 2000; 27:1598–1609.
Vesselle H, Pugsley JM, Vallieres E, Wood DE. The impact of fluorodeoxyglucose F 18 positron-emission tomography on the surgical staging of non-small cell lung cancer. J Thorax Cardiovasc Surg 2002; 124: 511–519.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
A list of other participants from the PLUS Study group is shown in the Appendix.
Appendix
Appendix
In addition to the authors, the PLUS Study Group participants were as follows: A. Boonstra, B. Venmans, A.J.M. van Boxem, G. Sutedja, R.A. Manoliu and R. Golding at the Academic Hospital Vrije Universiteit, Amsterdam; W.F.M. Strankinga, P.M. Hooghiemstra, F.C. Crezée and P.L. Tolenaar at the BovenIJ Ziekenhuis, Amsterdam; H.B. Kwa, J.G. van Unnik, R. Hardjowijono and S.S. Wagenaar at the Onze Lieve Vrouwe Gasthuis, Amsterdam; C. Jie, M.C.T.B. Sie, R.M.J.M. Butzelaar and M.N. Weimann at the Lucas Ziekenhuis, Amsterdam; G. Visschers, P.I. van Spiegel, G.C. Collet and R.P. Rademakers at the Slotervaart Ziekenhuis, Amsterdam; W.G. Boersma, M. Deenstra, C.S. de Graaff, T. Haitjema, G.H. Ooms and J.H. Pot at the Medisch Centrum Alkmaar; J. Prins, P.M.J.M. de Vries, E. Scheijde and J. Dijkstra at the Westfries Gasthuis, Hoorn; J.P. Teengs and E. Boerma at the Kennemer Gasthuis, Haarlem; and J. Berkovits, R.P.A. Boom and J Krekt at the Ziekenhuis Amstelveen.
Rights and permissions
About this article
Cite this article
Verboom, P., van Tinteren, H., Hoekstra, O.S. et al. Cost-effectiveness of FDG-PET in staging non-small cell lung cancer: the PLUS study. Eur J Nucl Med Mol Imaging 30, 1444–1449 (2003). https://doi.org/10.1007/s00259-003-1199-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1199-9