Abstract
Objective
The aims of this study are to assess the diagnostic yield of image-guided biopsy for discitis-osteomyelitis (DO), identify factors associated with biopsy yield (laboratory, pre-defined MRI findings, and biopsy technique), and impact of biopsy on management of patients appropriately selected according to the Infectious Disease Society of America guidelines (IDSA).
Materials and methods
This is a retrospective review of patients who underwent biopsy for suspected DO from 2011 to 2019. Reference standards to establish diagnosis of DO in order were histopathology/microbiology from biopsy or subsequent surgical sampling, positive blood culture or serology, and imaging/clinical follow-up. Laboratory markers, pre-biopsy antibiotics and MRI features, procedural-related variables, and impact of biopsy on management were assessed. Multivariable logistic regression was also performed.
Results
Out of 97 included patients, 78 were diagnosed with DO. Overall sensitivity of biopsy for detecting DO was 41.0% (32/78), including 10 patients with positive histopathology only, 14 with positive biopsy culture only, and 8 with both. Elevated ESR (p < 0.001) and epidural collection on MRI (p = 0.008) were associated with higher biopsy yield (63.6% and 68.6%, respectively) in a multivariable model. Procedural variables were not associated with yield. Biopsy results impacted the management in 19/77 (24.7%) patients, of whom 15/19 (78.9%) had treatment de-escalation and 4/19 (21.0%) had treatment escalation including starting new anti-tuberculous and anti-fungal regimens.
Conclusion
Sensitivity of biopsy for detecting DO was 41.0%. When IDSA guidelines are followed, biopsy provided impactful information that changed the management in 24.7% of patients. Evaluation for elevated ESR and epidural collection can help improve yield and patient selection for biopsy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Discitis-osteomyelitis (DO) is a rare diagnosis, estimated to occur in only 4–24 patients per million per year [1]. The diagnosis of DO is difficult and often delayed due to nonspecific clinical presentation, such as insidious back pain; more specific symptoms like fever and neurologic deficits occur in a minority of cases. More common potential mimickers such as spine degenerative changes may also cloud the diagnosis [1,2,3,4,5]. Imaging, specifically MRI, is an essential part of the diagnostic workup, accurately characterizing the level and extent of disease [6]. The MRI features of DO have been well-described [7, 8]; however, literature is limited on specific imaging features predictive of diagnostic yield of image-guided biopsy, with one study finding association of paravertebral inflammation with biopsy yield [9].
Biopsy of the involved vertebral body, with variable yield of 31–91% reported in the literature for detecting an etiologic pathogen by culture, has been part of the diagnostic evaluation of DO, especially in a select group of patients as defined by the Infectious Disease Society of America (IDSA) in 2015. The IDSA strongly recommends percutaneous image-guided biopsy in all suspected cases of DO if a typical culprit pathogen (Staphylococcus aureus, Staphylococcus lugdenensis, or Brucella spp.) is not initially identified by serology or blood culture, which also has a widely reported yield of 40–89% [2, 10]. Yet, the clinical value and the impact of biopsy on treatment management has recently been questioned, with one study reporting that less than 10% of biopsies alter patient management [11, 12].
The goals of this study were to (1) assess overall diagnostic yield of image-guided biopsy for DO; (2) identify clinical factors, procedure-related variables, and MRI features that correlate with yield; and (3) evaluate the rate of clinically meaningful microbiologic data provided by biopsy results that impacts management by aiding tailored antibiotic therapy in candidates selected for biopsy as per the IDSA guidelines. We hypothesize that biopsy maintains considerable impact in current practice by providing microbiologic data undetected by noninvasive means.
Materials and methods
This investigation was approved by the institutional review board and was compliant with the guidelines of the Health Insurance Portability and Accountability Act (HIPAA). Informed consent was waived for the study due to its retrospective nature and minimal risk to participants.
Study participants
We performed a retrospective search of medical records from September 2011 to May 2019 in our institution using Primordial worklist manager advanced search (Primordial, San Mateo, CA). Consecutive patients who underwent percutaneous image-guided biopsy for suspected DO based on clinical and imaging findings, and also had microbiological and/or histopathological examination of the obtained tissue specimen, were included in the study (Table 1, Fig. 1). The reference standard for diagnosis of DO was histopathology at time of percutaneous biopsy, or subsequent surgical biopsy or debridement, matched with the clinical concern. If pathology was not conclusive or available, the reference standard was defined by positive blood culture with a typical pathogen (S. aureus, S. lugdenensis) or the same organism as biopsy culture, or positive serology with a typical pathogen (Brucella antibody assay) [10]. If the above criteria were not met, then clinical and imaging follow-up was used. For example, patients who were not treated for DO due to lower clinical suspicion, and showed stability or improvement in their follow-up clinical or imaging findings, were considered negative for DO. Those who had to receive antibiotic therapy due to high clinical suspicion for DO, and showed improvement in their clinical or imaging findings, were considered positive for DO.
Patients were excluded from the study if medical records did not include required laboratory and narrative notes, if final diagnosis of DO could not be ruled in or out based on the defined criteria, if they had facet joint septic arthritis without suspicion for vertebral body or intervertebral disc involvement, or if the procedure was aborted for any reason without sampling. If pre-biopsy MRI within 1 month was not available, the patient was excluded from the MRI feature analysis portion of the study.
MRI technique and evaluation
MRI studies were performed on either a 1.5 or 3.0 T MR scanner (Magnetom Aera or Skyra; Siemens Medical Solutions, Erlangen, Germany) using a spine array coil and standardized departmental protocol. The MRI sequences and parameters are summarized in Table 2.
Two musculoskeletal fellowship-trained radiologists (MS with 8 years and WW with 7 years of experience), blinded to the clinical history and biopsy results, independently reviewed anonymized MRI examinations of the cervical, thoracic, or lumbar spine. Consensus review was performed, where discrepancies were settled through discussion and review of the case. If needed, the opinion of a third musculoskeletal radiologist would be used to break a tie. Prior to imaging evaluation, a training session was conducted using a group of MRI studies not included in the cohort to standardize the imaging criteria, which included (1) disc edema defined as T2-hyperintense signal less than fluid and separate from normal nucleus pulposus evaluated on sagittal T2-weighted images, (2) disc fluid involving greater than 50% of the length of the disc on sagittal T2-weighted images, (3) epidural phlegmon as focal hyperintense signal on T2-weighted imaging, (4) epidural collection as loculated fluid signal, (5) paraspinal soft tissue edema, (6) paraspinal soft tissue fluid collection, (7) endplate erosion, and (8) disc or endplate enhancement if contrast media was administered.
Biopsy technique
Biopsies were performed by nine different musculoskeletal and neuroradiology physicians over the course of study with variable range of experience (3 to 18 years) in performing these procedures. Percutaneous image-guided biopsy was performed from posterolateral or transpedicular approaches under CT or fluoroscopic guidance, similarly to previously published techniques [13]. For CT-guided biopsies, localizer images at the level of suspected disease were obtained for entry site and trajectory planning purposes. Following aseptic preparation of the skin overlying the biopsy needle trajectory, local anesthesia was administered. A small skin incision was made, and the biopsy needle was subsequently introduced using bone biopsy kits ranging in size from 11 to 20 gauge (ten different biopsy kits were used in total), most commonly 11 gauge (54% of biopsies), then 13 gauge (10%). A transpedicular/transforaminal approach was chosen in 54/97 (56%) patients, and a posterolateral approach was chosen in 43/97 (44%) patients. CT or fluoroscopic confirmation of correct entry site and trajectory toward the target was obtained, and the biopsy device was advanced into the target. One or more core samples were then obtained via biopsy needle. Fine needle aspiration was performed from fluid collections found on pre-biopsy imaging (disk/bone or paraspinal regions) with needles ranging in size from 11 to 22 gauge and, in one case, a 5 French Yueh catheter (Fig. 5). If both core biopsy and fine needle aspiration were performed (n = 31), fluid aspiration was performed in 25/31 (80.6%) cases using the introducer sheath and, in 6/31 (19.4%), with an additional spinal needle under coaxial technique. Fluoroscopy-guided biopsies were performed in a similar technical fashion but using orthogonal images to guide placement of the biopsy needle into the target. A biopsy sample was sent for microbiological examination in all 97 patients, which included aerobic, anaerobic, mycobacterial, and fungal cultures. Additional formalin-fixed samples for histopathological analysis were sent in 64/97 (66.0%) patients.
Sampling sites were recorded, which included various combinations of the affected intervertebral disc, adjacent vertebral body endplates, paraspinal soft tissue, and fluid collections. If available in the procedure note, the following data were collected: core needle gauge, number of cores taken, and specific biopsy targets. If the biopsy sites were not described on the procedure note, images from the biopsy were used to identify the targets. When available on the histopathology report, biopsy core length (cm) was recorded.
Clinical and laboratory review
The data we recorded from medical record review included age, gender, history of present illness and relevant follow-up documentation, physical examination, presence of fever (maximum temperature > 100.3 °F within 2 weeks of biopsy), laboratory studies, presence of antibiotic therapy in the past 2 weeks, and relevant reports from radiology, pathology, and biopsy procedures. Recorded laboratory studies included presence of leukocytosis with white blood cell count > 10,800/μl, ESR (normal range 0–20 mm/h), and CRP (normal range 0–-9 mg/L) levels, and results of blood and urine cultures. The most recent results were considered and only if within 2 weeks prior to image-guided biopsy.
If clinical judgment favored non-septic etiologies but biopsy was positive with flora typical for contamination, patients would be assigned false-positive. Biopsy was considered true positive (TP) if a true organism was detected on biopsy culture or if histopathology demonstrated bacteria, acute inflammatory cellular infiltrate, or chronic inflammatory change (i.e., fibrosis, necrosis) deemed compatible with DO by the pathologist.
Determining clinical impact
Biopsy result provided meaningful information when changes in antimicrobial management were made directly attributed to the results of percutaneous image-guided biopsy in patients appropriately selected as per the IDSA criteria [10]. Unexpected organisms were defined as fungi or tuberculous pathogens uncovered by empiric antibiotics, or resistant organisms with suboptimal empiric coverage. Subsequent changes in management were defined as start or escalation of antimicrobial therapy for patients who were sub-optimally or inadequately covered based on susceptibility profiling from biopsy culture. De-escalation of empiric broad-spectrum antibiotics to a more narrow-spectrum antibiotic was also considered a change in management based on susceptibility profiling from biopsy culture. Furthermore, in three cases where histopathology showed DO and biopsy culture was negative, clinicians downgraded broad-spectrum coverage due to the absence of methicillin-resistant Staphylococcus aureus (MRSA) isolation—these cases were regarded as clinically meaningful.
Statistical analysis
Correlation of categorical variables to biopsy yield was determined using Fisher’s exact test, and correlation of continuous variables to yield was determined using Mann-Whitney’s test. Multivariable logistic regression was used to assess whether a set of independent factors can predict biopsy outcome. Since logistic regression cannot reliably estimate the independent effects of predictors in a multivariable model if the number of predictors is high relative to the number of positive outcomes, the multivariable analysis only examined models consisting of two predictors. For each outcome, only factors showing a significant association with the outcome in univariable analysis were considered for inclusion in a multivariable model to predict that outcome. All statistical tests were conducted with the two-sided 5% significance level using SAS software (SAS Institute, Cary, NC; version 9.4, 2013).
Results
Study participants
The retrospective search initially yielded a total of 183 patients who underwent spinal biopsy for suspected DO. On initial review of patients included in the search range, we excluded 81 patients with inadequate medical records required to determine the clinical outcome and the diagnosis of DO. Three patients were excluded due to facet joint septic arthritis without involvement of the vertebral body or intervertebral disc. Two patients were excluded due to aborted biopsies: one patient with intraprocedural hypertensive urgency and one patient by request. Therefore, 97 patients met inclusion criteria including 57 (58.8%) males and 40 (41.2%) females with an average age of 65.4 ± 14.9 years and range of 30–93 years (Table 1), and with clinical data available to establish the presence or absence of DO (Fig. 2). Of these, 15 patients were excluded from MRI analysis: 11 without pre-biopsy MRI available and 4 with MRI performed greater than 30 days prior to biopsy.
The diagnosis of DO according to our reference standard was based on clinical judgment in 50 patients, histopathology in 30 patients, tissue culture showing the same organism as blood culture in 6 patients, MRI improvement after antibiotic therapy in 6 patients, open surgical pathology in 4 patients, and brucellar serology in 1 patient. In total, 78/97 (80.4%) patients received the diagnosis of DO, and 19/97 (19.6%) patients were deemed negative for DO despite initial concern on imaging.
Biopsy review
Biopsy yield
There were 82/97 (84.5%) CT-guided and 15/97 (15.5%) fluoroscopic-guided biopsies. Biopsies included 57 (58%) with core sampling only, 31 (32.0%) with both core sampling and aspiration, and 9 (9.3%) with aspiration only. Out of 78 patients with DO, there were 32 patients with positive biopsy results, with overall sensitivity of 41.0% (Fig. 2). There were no false-positive results, resulting in a specificity of 100%. Among the 32 patients with positive biopsy, there were 10 patients positive for histopathology only, 14 patients positive for biopsy culture only, and 8 patients positive for both.
In total, fourteen different organisms were identified by biopsy culture (Table 3), most commonly Staphylococcus epidermidis and Escherichia coli (n = 3/20, 15.0%). The three patients with S. epidermidis growth were deemed to have significant infections by infectious disease specialist. Therefore, there were no false positive results based on the clinical scenario despite growth of typical skin flora. Eight of nine (88.9%) patients with a positive outcome on both biopsy and blood cultures demonstrated organism concordance (including the above patients with concordant S. epidermidis cultures). Four of six (66.7%) patients with a positive outcome on both biopsy and urine cultures demonstrated organism concordance. Other organisms identified on biopsy and blood cultures are summarized in Table 4. One patient was diagnosed with brucellar DO by serology subsequent to image-guided biopsy. Only one patient had methicillin-sensitive Staphylococcus aureus (MSSA) growth on pre-biopsy blood culture, which was replicated by biopsy culture.
Biopsy-related variables and biopsy yield
Biopsy yield did not correlate with modality of imaging guidance—CT (28/67 or 41.8%) or fluoroscopic (4/11 or 36.4%) guidance (p = 0.76). Choice of sampled tissue did not correlate with yield (p = 0.35 to 1.00), which included bone in 66 (68.0%) patients, disc in 65 (67.0%), paraspinal soft tissue in 10 (10.3%), and fluid aspirate in 39 (40.2%). Mean core sample length, needle size, and number of cores did not demonstrate significant association with biopsy yield (Tables 4 and 5).
MRI evaluation
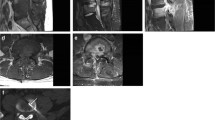
A total of 82 patients had MRI within 30 days of biopsy (67/78 MRI’s in the DO group, 53 with contrast; and 15/19 MRI’s in the DO-negative group, 9 with contrast). The sensitivities and specificities of MRI features for DO are summarized in Table 5. MRI features with highest sensitivity for diagnosing DO included disc edema (67/67, 100%), endplate enhancement (52/53, 98.1%), paraspinal phlegmon (64/67, 95.5%), and endplate erosion (65/67, 95.5%). Epidural collection offered the highest specificity of 14/15 (93.3%). There was a significant difference in presence of disc edema comparing patients with DO (67/67 or 100%) and those without DO (12/15 or 80.0%, p = 0.005). After discussion of studies needing consensus decisions, readers were able to resolve their initial records and disagreements; therefore, a third musculoskeletal radiologist opinion was not needed. Representative MRI images of each feature are illustrated in Figs. 3, 4, 5, and 6, with a representative true-negative case illustrated in Fig. 7.
Representative MRI of an 80-year-old female with discitis-osteomyelitis at T12-L1 characterized by epidural fluid collection and epidural phlegmon secondary to Mycobacterium tuberculosis on subsequent biopsy culture (true positive). a Sagittal contrast-enhanced T1-weighted image showing enhancing phlegmon in the epidural space (arrows). b Axial contrast-enhanced T1-weighted image showing enhancing epidural phlegmon with central, focal non-enhancing areas consistent with epidural collections (thin arrows)
Representative MRI of a 65-year-old male with discitis-osteomyelitis at L1-2 characterized by disc edema, disc fluid, endplate erosion, and endplate enhancement. Subsequent histopathology showed necrotic bone and rare gram-variable cocci without growth from biopsy culture (true positive). a Sagittal T1-weighted image showing hypointense signal involving the eroded L1-2 endplates. b Sagittal T2-weighted image showing fluid signal in intervertebral disc (arrow) and vertebral body edema. c Sagittal contrast-enhanced T1-weighted image showing endplate erosion, endplate enhancement (arrowheads), and vertebral body enhancement
Representative MRI of an 82-year-old female with discitis-osteomyelitis at T11-12 complicated by a large paraspinal collection. a Sagittal T2-weighted image showing a large fluid collection (asterisks) in the T11-12 disc space extending into anterior paraspinal space and near complete destruction of the T12 vertebral body. b Sagittal contrast-enhanced T1-weighted image showing rim enhancement of the fluid collection, extensive osseous destruction of the T11 and T12 vertebral bodies. c Axial T2-weighted image showing the extent of the paraspinal fluid collection. d Axial image from CT-guided biopsy showing a 5 French Yueh catheter targeting the left anterolateral aspect of the paraspinal collection from a posterolateral approach
Representative MRI of a 72-year-old male showing discitis-osteomyelitis characterized by disc edema, disc fluid, disc enhancement, and epidural phlegmon. Subsequent biopsy culture showed Staphylococcus epidermidis (true positive). a Sagittal T2-weighted image showing fluid signal in the T9-10 disc space (arrow). b Sagittal contrast-enhanced T1-weighted image showing enhancing epidural soft tissue (arrowhead) and enhancing soft tissue in the disc space (thin arrow). c Axial image from CT-guided biopsy of the bone and disc space using a transpedicular approach
Representative MRI of a true negative biopsy in a 79-year-old male with severe lower back pain, diagnosed through histopathology and clinical follow-up. a Sagittal STIR image showing fluid signal in disc (arrow) and vertebral bone marrow edema. b Sagittal T1-weighted image showing hypointense signal involving the endplates (arrows) with endplate irregularities. c Sagittal contrast-enhanced T1-weighted image showing endplate enhancement (arrowheads) and surrounding vertebral body enhancement (arrows). d CT-guided biopsy of intervertebral disc and endplates, which revealed sterile culture and lamellar bone debris on histopathology
MRI features and biopsy yield
When comparing TP and FN biopsies, epidural collection was a significant predictor of biopsy yield (p = 0.008), present in 15/29 (51.7%) TP and 7/38 (18.4%) FN biopsies (Table 5) for overall yield of 15/22 (68.1%). The other MRI features examined were not significantly different between patients with DO and without DO, and between TP and TN (Table 5).
Clinical and laboratory review
Among pre-biopsy clinical and laboratory variables, ESR was significantly elevated in patients with DO (73.6 ± 31.9 mm/h) versus those without (46.3 ± 37.3, p = 0.004). Mean CRP was also significantly elevated in those with DO (62.3 ± 38.5 mg/L) versus those without (44.3 ± 84.5, p = 0.005). Fever and leukocytosis were highly specific for DO (100% and 78.6%, respectively) but not sensitive (7.1% and 16.0%, respectively).
Clinical variables and biopsy yield
Elevated mean ESR was also associated with yield (p < 0.001—Table 4, Fig. 8). ESR greater than 75 mm/h was able to predict yield (p = 0.037), seen in 21/33 or 63.6%. Fever > 100.3 °F, and blood or urine culture positivity were seen at greater rates in TP versus FN biopsies, though not significantly (Table 4). Biopsy yield was not significantly different between patients who received (17/37 or 45.9%) and did not receive (13/31 or 41.0%) pre-biopsy antibiotics (p = 0.16).
The mean (bar) and standard deviation (error lines) of each numeric pre-clinical feature or biopsy technical factor among patients who were true positive (TP), false negative (FN), or true negative (TN) at percutaneous image-guided biopsy for suspected discitis-osteomyelitis. Blue bar = significantly different versus false negative by Mann-Whitney’s test with p < 0.05
Determining clinical impact
Image-guided biopsy provided clinically meaningful information that impacted clinical management in 19/77 (24.7%) patients meeting IDSA criteria for biopsy (Supplementary Table 1). All but one patient, who showed S. aureus on pre-biopsy blood culture, were appropriately selected according to the IDSA guidelines, and this patient was excluded from the clinical impact calculation. In 15 patients, broad-spectrum antibiotics were downgraded to narrower-spectrum antibiotics based on susceptibility testing of the isolated organism, which directly guided antibiotic therapy in 12/15 of these patients. In 4/19 patients, antimicrobial coverage was escalated due to suboptimal empiric coverage: one patient with methicillin-resistant S. epidermidis (added additional coverage with rifampin), two patients with tuberculous discitis-osteomyelitis (changed empiric antibiotics to anti-tuberculous regimen), and one patient with candida discitis-osteomyelitis (changed empiric antibiotics to anti-fungal regimen) identified on biopsy culture. In 3/15 patients with positive histopathology and negative biopsy culture, clinicians downgraded antibiotics based on negative MRSA isolation.
Of patients with TP biopsy, 9/32 (28.1%) underwent surgery subsequent to image-guided biopsy versus 4/46 (8.7%) of patients with FN biopsy. Of patients with TN biopsy, 3/19 (15.8%) underwent surgery, one paraplegic patient with Charcot arthropathy of the facet joint who underwent stabilization surgery, and two patients with discogenic radiculopathy.
Multivariable analysis
Multivariable analysis identified mean ESR (p = 0.003) and epidural collection (p = 0.020) as the only set of significant independent discriminators of TP from FN. The model with these factors predicted TP with an AUC of 0.800. When considered alone, ESR had an AUC of 0.757 and epidural collection had an AUC of 0.699.
Discussion
Our study showed overall sensitivity of image-guided biopsy of 41.0% and specificity of 100% for diagnosing discitis-osteomyelitis. The biopsy results changed clinical management in 19/77 (24.7%) of patients with DO after excluding one patient who did not meet the IDSA criteria for biopsy. This impact was critical in four patients whose biopsy culture and susceptibility testing revealed atypical organisms inadequately covered by empiric therapy, resulting in complete change in the therapy regimen for three patients with non-pyogenic DO—two Mycobacterium tuberculosis and one Candida albicans infections—and addition of rifampin to a patient with methicillin-sensitive S. epidermidis. Broad-spectrum antibiotics were safely narrowed based on unique microbiologic data from biopsy in 15 patients, consistent with the principles of responsible antibiotic stewardship in reducing adverse effects and the incidence of antimicrobial resistance [14]. Mean ESR and epidural collection on pre-biopsy MRI were independent predictors of biopsy yield and were included in multivariable analysis, which revealed increased accuracy when seen together. Mean ESR, CRP, and disc edema on MRI were significant differentiators of those with and without DO.
The overall yield of biopsy in our study is consistent with two previous meta-analyses reporting mean sensitivity of 48–49% and specificity of 99.9% [5, 15]. Our results show higher meaningful clinical impact compared to two prior studies that report that biopsy identified the culprit organism in 1/13 (7.7%) and 8/84 (9.5%) of total cases [11, 12]. There are several possible explanations for these differences. Tachibana et al. reported identification of a potentially causative organism in 7/13 of patients who underwent biopsy culture and in 10 patients from blood and other foci (non-specified), for a total of 15 identified organisms. In their cohort, the culture result revealed suboptimal treatment in 1/15 patients, and they concluded that identification of the organism could not provide a therapeutic benefit in a majority of the patients [11]. However, they did not consider the effect of downgrading antibiotics from broad-spectrum as clinically significant. The study also lacks specific information on the spectrum of antibiotic coverage, the percentage of patients who had positive blood culture before the biopsy, and the details of biopsy technique. Additionally, both these studies included patients with positive pre-biopsy blood culture in their analysis and considered concordant organism growth on blood culture as well as cultures from urine or other foci as reference standards when evaluating for the impact of biopsy culture. Our data showed that the rate of concordant organism growth on urine and biopsy cultures (4/6 or 67%) is lower than the rate of concordance on blood and biopsy cultures (8/9 or 89%). The IDSA guidelines also do not mention infectious foci other than peripheral blood as potential reference standards for a microbiological diagnosis. In contrast, two studies found higher rates of change in clinical management: 7/20 (35%) and 15/64 (23%) among all patients, and 15/20 (75%) among those with positive biopsy results [16, 17]. Kasalak et al. included patients who had pre-biopsy positive blood culture, which comprised 20% of their patients. Of these, almost half were also culture-positive on CT-guided biopsy and were included in their calculation of impact of biopsy on clinical management—including the calculation of 75% (15/20) impact for those with positive biopsy culture—which is not in accordance with the IDSA guidelines.
Our cohort demonstrated 89% rate of concordance when both blood and biopsy cultures are positive, consistent with a prior study reporting 80% concurrence rates [12]. Only 2/78 patients (2.6%) in our cohort showed MSSA as the etiology, an organism consistently reported in the literature as the most common causative organism (42–58% of DO cases) [2]. No cases of MRSA were found, which is reported to have an incidence of 24.9% [18]. The low number of S. aureus isolation in our cohort is likely, in part, related to the adherence of our clinicians to the IDSA guidelines in that they did not refer those patients who had blood culture positivity for typical pathogens, including S. aureus, for biopsy. As with most prior reports studying patients with DO, all of our positive biopsy cultures were considered truly positive, with only 2/33 studies in a large meta-analysis reporting false positive results [15]. The three patients with S. epidermidis identification in our cohort were clinically regarded as true infections.
We found that elevated ESR predicted yield similar to other prior studies [19, 20], providing quantitative stratification of patients more likely to yield positive results. The presence of epidural collection on pre-biopsy MRI also predicted biopsy yield (present in 15/29 or 51.7% in TP versus 7/38 or 18.4% in FN with p = 0.004). Our results were not able to replicate a prior study of 34 patients that found paraspinal infiltration on MRI as a predictor of successful pathogen detection (present in 29/29 or 100% of TP patients and 35/38 or 92% of FN patients) [9]. We did not find other clinical, biopsy-related, or MRI factors in our study significantly correlating with yield. Choice of tissue target did not predict a higher yield, consistent with a prior study reporting no statistical difference between the yields of bone-disc, disc-only, and paraspinal soft tissue biopsies [21].
Disc edema, paraspinal phlegmon, endplate enhancement, and endplate erosion all demonstrated high sensitivities for DO of greater than 95% but limited specificities of up to only 20%. Only disc edema was seen in significantly greater rates in patients with DO versus without. Our imaging data is consistent with a prior report of MRI feature analysis which showed 90–100% sensitivities for disc edema, paravertebral phlegmon, endplate erosion but additionally identifies endplate enhancement as a sensitive (98.1%) but nonspecific (6.7%) feature of DO [9].
Our study has several limitations. It was a retrospective study with inherent selection bias and a relatively small sample size, which may have been underpowered for the detection of differences for some of the included measures. We were also unable to control for factors such as MRI technique, biopsy technique variables including operator experience, and pre-sampling antibiotic administration due to the retrospective nature of the study. We had to use different criteria for the diagnosis of DO when anatomical pathology was not available; therefore, the reference standard was evaluated through a combination of endpoints. Finally, we did not specifically assess the value of biopsy in true negative subjects following ruling out DO. We acknowledge that there are varying degree of imaging and clinical overlap between DO and other spinal pathologies such as erosive osteochondritis and seronegative spondylodiscitis. We had several true-negative subjects in our study with final diagnoses of long-standing degenerative disc disease, erosive osteochondritis, and renal osteodystrophy. Given poor specificity of inflammatory marker elevation in some of these cases, biopsy retains meaningful impact as a tool in these ambiguous scenarios to exclude infection, which can further highlight the value of biopsy in these particular scenarios.
Overall, our study shows that image-guided biopsy provided clinically meaningful information independent of blood culture in 24.7%, allowing clinicians to provide tailored narrow-spectrum antibiotic therapy in 19.5% and identifying atypical organisms inadequately covered by empiric antibiotics in 5.2%. Elevated ESR and presence of epidural collection on MRI are significant independent predictors of biopsy yield in a multivariable model. Therefore, evaluation of serologic inflammatory markers and spine MRI can improve patient selection for biopsy referral.
Abbreviations
- CRP:
-
C-reactive protein
- DO:
-
Discitis-osteomyelitis
- ESR:
-
Erythrocyte sedimentation rate
- FN:
-
False negative
- IDSA:
-
Infectious Disease Society of America
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- MSSA:
-
Methicillin-sensitive Staphylococcus aureus
- TN:
-
True negative
- TP:
-
True positive
References
Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65(Suppl 3):iii11–24.
Nickerson EK, Sinha R. Vertebral osteomyelitis in adults: an update. Br Med Bull. 2016;117(1):121–38.
Dunbar JA, Sandoe JA, Rao AS, Crimmins DW, Baig W, Rankine JJ. The MRI appearances of early vertebral osteomyelitis and discitis. Clin Radiol. 2010;65(12):974–81.
Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39(1):10–7.
Pupaibool J, Vasoo S, Erwin PJ, Murad MH, Berbari EF. The utility of image-guided percutaneous needle aspiration biopsy for the diagnosis of spontaneous vertebral osteomyelitis: a systematic review and meta-analysis. Spine J. 2015;15(1):122–31.
Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157(1):157–66.
Ledermann HP, Schweitzer ME, Morrison WB, Carrino JA. MR imaging findings in spinal infections: rules or myths? Radiology. 2003;228(2):506–14.
Maiuri F, Iaconetta G, Gallicchio B, Manto A, Briganti F. Spondylodiscitis. Clinical and magnetic resonance diagnosis. Spine (Phila Pa 1976). 1997;22(15):1741–6.
Spira D, Germann T, Lehner B, et al. CT-guided biopsy in suspected spondylodiscitis—the association of paravertebral inflammation with microbial pathogen detection. PLoS One. 2016;11(1):e0146399.
Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26–46.
Tachibana T, Moriyama T, Maruo K, Inoue S, Yoshiya S. Therapeutic impact of organism isolation in management of patients with pyogenic vertebral osteomyelitis. Springerplus. 2014;3:62.
Garg V, Kosmas C, Young PC, Togaru UK, Robbin MR. Computed tomography-guided percutaneous biopsy for vertebral osteomyelitis: a department's experience. Neurosurg Focus. 2014;37(2):E10.
Peh W. CT-guided percutaneous biopsy of spinal lesions. Biomed Imaging Interv J. 2006;2(3):e25.
Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc. 2011;86(2):156–67.
McNamara AL, Dickerson EC, Gomez-Hassan DM, Cinti SK, Srinivasan A. yield of image-guided needle biopsy for infectious discitis: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017;38(10):2021–7.
Rankine JJ, Barron DA, Robinson P, Millner PA, Dickson RA. Therapeutic impact of percutaneous spinal biopsy in spinal infection. Postgrad Med J. 2004;80(948):607–9.
Kasalak O, Wouthuyzen-Bakker M, Adams HJA, et al. CT-guided biopsy in suspected spondylodiscitis: microbiological yield, impact on antimicrobial treatment, and relationship with outcome. Skeletal Radiol. 2018;47(10):1383–91.
Park KH, Kim DY, Lee YM, et al. Selection of an appropriate empiric antibiotic regimen in hematogenous vertebral osteomyelitis. PLoS One. 2019;14(2):e0211888.
Lee HT, Pukenas BA, Sebro R. Change in bone CT attenuation and C-reactive protein are predictors of bone biopsy culture positivity in patients with vertebral discitis/osteomyelitis. Spine (Phila Pa 1976). 2020.
Ang MT, Wong GR, Wong DR, Clements W, Joseph T. Diagnostic yield of computed tomography-guided biopsy and aspiration for vertebral osteomyelitis. J Med Imaging Radiat Oncol. 2019;63(5):589–95.
Chang CY, Simeone FJ, Nelson SB, Taneja AK, Huang AJ. Is biopsying the paravertebral soft tissue as effective as biopsying the disk or vertebral endplate? 10-year retrospective review of ct-guided biopsy of diskitis-osteomyelitis. AJR Am J Roentgenol. 2015;205(1):123–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Lim, E., Walter, W., Gyftopoulos, S. et al. Does image-guided biopsy of discitis-osteomyelitis provide meaningful information to impact clinical management?. Skeletal Radiol 50, 1325–1336 (2021). https://doi.org/10.1007/s00256-020-03675-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-020-03675-7