Abstract
A stress fracture is a focal failure of bone induced by the summation of repetitive forces, which overwhelms the normal bone remodeling cycle. This review, the first of two parts, discusses the general principles of stress fractures of the foot and ankle. This includes bone structure, biomechanics of stress applied to bone, bone remodeling, risk factors for stress fracture, and general principles of imaging and treatment of stress fractures. Cortical bone and trabecular bone have a contrasting macrostructure, which leads to differing resistances to externally applied forces. The variable and often confusing imaging appearance of stress fractures of the foot and ankle can largely be attributed to the different imaging appearance of bony remodeling of trabecular and cortical bone. Risk factors for stress fracture can be divided into intrinsic and extrinsic factors. Stress fractures subject to compressive forces are considered low-risk and are treated with activity modification and correction of any modifiable risk factors. Stress fractures subject to tensile forces and/or located in regions of decreased vascularity are considered high risk, with additional treatment options including restricted weight-bearing or surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A stress fracture is a focal failure of bone caused by the summation of repetitive forces, which are relatively strenuous or new in comparison with the individual’s baseline activity. Stress fractures can be divided into fatigue fractures and insufficiency fractures [1]. A fatigue fracture is due to repeated stress on a normal bone, and an insufficiency fracture represents fracture of a weakened bone in response to relatively normal activity. This distinction is somewhat arbitrary because it is not always clear where to draw the line between normal or abnormal bone and between strenuous or expected/normal activity. For consistency with the orthopedic literature, this review uses the term stress fracture synonymously with fatigue fracture. Insufficiency fractures are not discussed.
Structure of bone
Owing to the strength and permanence of the human skeletal system, an individual’s bones may remain intact for thousands of years. Because bones may be the only remnant of a deceased individual’s existence, it is not surprising that bones have been imprinted with the stigma of death since the earliest human civilizations; however, despite this ancient misconception, bone is in fact a remarkably dynamic, living organ.
The primary function of bones is to provide a means for locomotion and complex movements, by functioning as lightweight yet rigid levers for the muscles to pull against [2]. In the adult, most of the skeleton is composed of lamellar bone, which consists in large part of type I collagen stiffened by calcium hydroxyapatite crystals [3]. In contrast, woven bone is an immature form of bone that is predominant in fetal development [4] and its presence in adults is limited to regions of rapid bone turnover, such as healing fractures or metabolic bone disease such as Paget disease or hyperparathyroidism [5].
The two distinct macrostructures comprising human lamellar bone are cortical bone and trabecular (cancellous, medullary) bone (Fig. 1) [5, 6]. Cortical bone surrounds the periphery of the bone, is predominant in the diaphyses of the long bones, and is made of individual cylindrical-shaped structures called osteons, formed of concentric lamellae with a central Haversian canal [7]. Neurovascular elements are present within the central Haversian canal. Each osteon contains several osteocytes, each located in a lacuna, which are connected by thin canaliculi. A cement line marks the boundary of an osteon, and functions to prevent microcrack propagation [8]. Cortical bone is dense (with a porosity of less than 5%), and is less metabolically active than trabecular bone [7], with a turnover up to eight times slower [4]. The outer surface of cortical bone is lined by the dual-layered fibrocellular periosteum, with an inner layer populated with highly osteogenic cells and an outer fibrous layer [9]. The inner surface of cortical bone is the endosteal surface, a nonmembranous space comprised of a thin discontinuous layer of osteoprogenitor cells [9]. In physiological bone remodeling, new bone is predominantly formed on the periosteal surface, and bone is resorbed from the endosteal surface (Fig. 2) [10].
In contrast, trabecular bone is located in the central aspect of the bone and is formed by a network of bone struts and plates, which are oriented in lines optimized to withstand stress. These struts and plates are also composed of osteons, but unlike cortical bone, the central Haversian canal is not present. Similar to cortical osteonal units, there is a concentric lamellar organization, with osteocytes located within lacunae interconnected by canaliculi. Trabecular bone is much less dense than cortical bone, with a porosity of 50–90% [5]. Trabecular bone accounts for only 20% of the total bone mass [5], but has a much greater surface area than cortical bone, and therefore has a much higher rate of remodeling and bone turnover [3].
The bones in the foot and ankle are composed of varying fractions of cortical and trabecular bone, dependent on their complex shapes. Cortical bone provides most of the biomechanical support for long bones, such as the tibial and fibular diaphyses, whereas the metaphyseal ends of the long bones and the tarsal bones are composed predominantly of trabecular bone.
The three major types of bone cells are osteoblasts, osteocytes, and osteoclasts, all of which are present in both trabecular and cortical bone. Osteoblasts line the surface of bones and predominantly function to produce new bone matrix. Osteocytes are former osteoblasts that are located in lacunae and are surrounded by calcified bone mineral matrix. Osteoclasts function to resorb bone by dissolving the hydroxyapatite crystals and deconstructing the collagen. The noncellular component of bone is composed of a mixture of mineral, organic, and water components, comprising approximately 60, 35, and 5% respectively [4]. The mineral component of bone predominantly consists of calcium hydroxyapatite, and is responsible for the compressive strength of bone. The organic component largely consists of type I collagen, and contributes to the tensile strength of bone.
Bone remodeling
Wolff’s law states that a bone responds to stress by continually remodeling, resulting in an increase in strength over time (Fig. 3) [11]. In fact, remodeling is an essential part of skeletal development, initially occurring in utero with fetal movement directly affecting bone growth [10]. Stress fractures result when the dynamic response of skeletal remodeling in response to progressive overload is not able to keep up with the repetitive external force (Fig. 4). Most stress factors are associated with the triad of activity that is new or increased for the individual, relatively strenuous, and repeated [12].
Stress fractures result from a complex interplay of bending, compressive, tensile, shearing, and torsional forces (Fig. 5) applied to bone from direct muscle action [6, 12] and indirect transmission of ground-contact forces [13]. Cortical bone and trabecular (cancellous) bone react differently to the application of force. Cortical bone has a higher modulus of elasticity than trabecular bone, resulting in its ability to withstand compressive loads, but it is relatively intolerant of bending in comparison to trabecular bone [5]. In contrast, trabecular bone is relatively intolerant of compression. Of these forces, the bending force is the most significant biomechanical factor related to the development of a stress fracture in the tibia [13], which is one of the most frequently studied sites of stress fracture. A bending force applies tensile stress to the convex aspect of the bone and compressive force to the concave aspect. Static bending forces applied to long bones do not lead to bony remodeling, but intermittent bending does lead to new bone deposition in regions of high bending stress [14].
Illustration demonstrates the various types of forces that can be applied to bone in vivo. Of these forces, the bending force is thought to be the most important in the development of a stress fracture. A bending force leads to a compression force on the concave aspect of the bone and a tensile force on the convex aspect
Both cortical and trabecular bone respond to mechanical loading by remodeling. Bone remodeling can occur on any of the four discrete bone surfaces: the periosteal, intracortical, and endosteal surfaces of cortical bone, and the trabecular surface of trabecular bone. Bone remodeling can be classified as stochastic (random) or targeted [15]. Stochastic remodeling occurs when osteocytes randomly resorb bone without a local signaling event, and is usually driven by calcium homeostasis. In contrast, targeted remodeling, which is the form of remodeling incited by external stress, occurs in response to cellular signaling, local microdamage, and/or osteocyte apoptosis.
The remodeling pathway (Fig. 6) begins with microcrack (also called microfracture or microdamage) formation in response to applied stress. Microcrack creation is considered a normal physiological phenomenon to allow energy dissipation and plastic deformation of bone. In experimentally produced stress fractures in rabbit tibias, microscopic damage was evident as small cracks with a mean length of 346 μm [16]. The microcracks sever canaliculi connecting the osteocytes, and in combination with calcium channel signaling [17] result in osteocyte apoptosis and the release of local and hematological factors. This signaling environment stimulates osteoclasts to form resorption cavities [6], especially targeting the sites of microcracks. After osteoclast resorption, osteoblasts create new bone at the resorption cavities, completing the remodeling pathway. The complete remodeling pathway (Fig. 6) can take 2–8 months [18], and there is a period of relatively decreased strength of bone early in the remodeling cycle when resorption cavities predominate owing to the delay of 10–14 days before osteoblasts begin the process of new bone formation.
Although the basic remodeling pathway encompassing microcrack formation, resorption cavity creation, and deposition of new bone is similar in cortical and cancellous bone, there are a few key differences in the remodeling pathways of these two macrostructures. Specifically, the periosteum investing cortical bone may become inflamed [19], whereas there is no associated periosteal envelope to provide ancillary support to trabecular injury. A distinguishing feature of remodeling in trabecular bone is the ability of the supporting struts to change orientation to better withstand applied stresses (Fig. 7), which strengthens the bone along the axis of the applied stress. Microcallus formation [20] is an integral component of this trabecular remodeling owing to osteoblastic new bone deposition along the trabecular struts.
Risk factors for stress fracture
The risk factors for stress fracture can be divided into intrinsic and extrinsic factors [21]. Intrinsic factors are related directly to the individual’s metabolic and anatomical characteristics, including hormonal milieu, bone quality, cardiovascular fitness, muscular strength, and anatomical alignment. Extrinsic factors include the individual’s training regimen, dietary intake, and equipment such as footwear and training surfaces. Women with low bone mineral density due to exercise-induced menstrual abnormalities or premature menopause are at increased risk for stress fractures [22], in addition to women with a body mass index less than 21 kg/m2 [23]. The female athlete triad, a syndrome of deficient caloric intake, amenorrhea, and osteoporosis, can increase the risk for stress fracture by 15–50% [24]. Decreased serum vitamin D may also be a predisposing factor for stress fracture [25]. Female military recruits sustain stress fractures more frequently than men, although this sex-based difference in stress fracture rate in the civilian population has not been as strongly proven [26]. Amongst military recruits, individuals with the slowest running times at the initiation of basic training were up to 7.7 times more likely to experience lower extremity stress fracture in comparison with the fastest runners [27]. However, in the civilian population, long-distance endurance athletes such as marathon runners and cyclists may have relatively decreased bone density and increased risk for stress fracture [22].
The neuromuscular hypothesis states that proper neuromuscular function can dissipate the forces on bones and joints that occur with athletic activity, and that muscle fatigue, relative muscle imbalance, or decreased muscle bulk are associated with stress fractures [28]. Several specific factors related to an individual’s gait, training, and musculoskeletal morphology may predispose to stress fractures, including variants in posture, gait, and foot strike [29], such as pes planus [30]. A longer running stride length also slightly increases the risk for stress fracture by 3–6% [31]. A decreased tibial cortical thickness and smaller muscle cross-sectional area are also shown to be risk factors for tibial stress fracture in runners [32, 33].
General principles of treatment for stress fracture
The cornerstone of stress fracture treatment is an understanding and correction of the factors that led to the injury [34]. Specifically, the extrinsic factors (training regimen, potential dietary deficiency, and equipment insufficiencies) must be addressed in a manner that is acceptable to the individual. Intrinsic factors are much more difficult to modify. Stress fractures can be classified into low-risk and high-risk types [35–38] to help guide treatment. It is important to make the distinction between these types of stress fractures, as overtreatment of a low-risk stress fracture may result in deconditioning and unnecessary time away from activity or sport, and undertreatment of a high-risk fracture can lead to complete fracture or non-union [37].
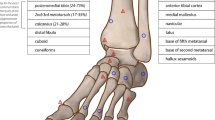
Mechanically, low-risk stress fractures are subject to compressive forces, and generally heal well with activity modification while maintaining normal weight-bearing. In contrast, high-risk stress fractures are often subject to tensile forces, may be located in regions of relatively decreased vascularity, or a combination of these two factors may be involved. The three most common stress fractures of the foot and ankle [39] are low-risk and include the posteromedial distal tibia, the calcaneus, and the metatarsals (excluding the fifth). Less common low-risk stress fractures include the distal fibula, and very rare cuboid and cuneiform stress fractures are also considered low risk. For low-risk stress fractures, an athlete may be allowed to continue the offending activity at a reduced level, with a gradual increase in intensity dependent on symptoms.
In comparison, a high-risk stress fracture is prone to delayed union or non-union, and more aggressive treatment is typically advised [37]. High-risk stress fractures include the anterior tibial cortex, the navicular, the base of the fifth metatarsal, the medial malleolus, the hallux sesamoids, and the talus. These fractures can be challenging to treat and may require restricted weight-bearing or possibly surgery [37]. High-risk stress fractures tend to be subject to tensile force, resulting in a lack of intrinsic stability and eventual formation of a fracture gap [40], owing to cortical bone’s low resistance to tensile loading [8]. In addition to activity modification, rest, and surgical intervention, extracorporeal shock wave therapy has also been reported to be an effective non-invasive technique for speeding up fracture healing, and to nonsurgically treat stress fracture non-union [41].
Imaging of suspected stress fracture
A typical imaging pathway in the clinical setting of suspected stress fracture (Fig. 8) is to perform radiographs initially. If radiographs are negative and there is persistent concern for a stress fracture, imaging options include MRI or repeat radiographs in 2–3 weeks after treating the injury as a stress fracture [42], although earlier MRI is preferred for high-level athletes or if a high-risk stress fracture is suspected. If the repeat 2- to 3-week follow-up radiographs do not show stress fracture then MRI can be performed at that time, to confirm the diagnosis of stress fracture or to offer an alternative etiology. CT can be helpful as a problem-solving tool in cases of an inconclusive MRI, but CT is not routinely used in the diagnostic evaluation of stress fracture if MR is available [42].
The clinical symptoms of stress fracture typically precede the radiographic findings by 2–3 weeks, and radiographs are insensitive for the detection of early stress injuries, with a sensitivity of 15% reported for the detection of tibial stress injury [43]. Radiographic findings are dependent on the chronicity, specific bone involved, and even the location within each bone [44]. If a stress fracture occurs in cancellous bone, such as the tarsal bones or metaphysis of the long bone, the initial radiographic finding is faint trabecular sclerosis due to microcallus formation (Fig. 9) [6].
Radiographic and MR images demonstrating the typical imaging appearance of stress fractures in three different patients. a Lateral radiograph of the heel demonstrates a stress fracture of the calcaneus, a trabecular-predominant bone, with sclerosis (arrows) oriented perpendicular to the calcaneal trabeculae. b Frontal radiograph of the forefoot (different patient from a) demonstrates faint periosteal reaction of the medial cortex of the third metatarsal (arrows), an early sign of stress fracture in a cortex-predominant region of bone. A metal marker has been placed at the site of pain. c Frontal radiograph of the ankle (different patient from a and b) demonstrates the lucency of the medial and lateral distal fibular diaphyseal cortices (arrows), with a frank cortical break laterally, a late finding of a stress fracture in a cortex-predominant region of bone. d Coronal T2-weighted MRI with fat suppression (same patient as c) shows periosteal edema, bone marrow edema, and cortical irregularity and signal change (arrows), consistent with a high-grade stress fracture
In contrast, if the cortex of a long bone is involved, the initial radiographic finding is subtle cortical lucency followed by periosteal reaction and endosteal callus formation [12, 43, 45], with a frank cortical break evident in high-grade injuries (Fig. 9). Many stress fractures of the foot and ankle can be notoriously difficult to detect on radiographs, especially stress fractures of the cuboid, cuneiforms, and navicular [46], which are often associated with a resultant delay in diagnosis.
Before the widespread use and availability of MRI, radionuclide bone scanning was considered the gold standard test for the detection of stress fracture, with a sensitivity close to 100% [47]. However, scintigraphy is nonspecific, imparts radiation exposure, and treatment decisions cannot generally be made solely on the scintigraphic findings. For these reasons, radionuclide bone scanning is currently seldom performed if MRI is not contraindicated.
Ultrasound is sensitive in detecting early stress fractures in superficial bones such as the metatarsals [21] or anterior tibia, but evaluation of the marrow space is not possible. Sonographic findings of stress fracture may include focal buckling of the cortex and surrounding hypoechoic callus [48]. The sensitivity of ultrasound in the detection of stress fractures ranges from 43 to 99%, with a specificity of 13–79% [42].
If a stress fracture is suspected clinically and initial radiographs are negative, MRI is the next single best technique [42, 49, 50]. MRI findings of stress fracture include periosteal and bone marrow edema, with intracortical signal changes or intramedullary low-signal intensity fracture line only able to be visualized relatively late in the pathogenesis of stress fracture (Fig. 9) [51]. The periosteal changes may be the only MRI manifestations of early cortical injury. It is important to note that the initial stages of cortical injury are generally only evident with specialized imaging techniques that are not in routine clinical use, such as high-resolution micro-CT [52], or possibly ultra-short TE MRI [53].
A stress reaction represents a clinical syndrome of exertional pain, thought to be due to early accumulation of microdamage [37] and likely representing an early stress injury. Distinct from a stress fracture, radiographs are normal in a stress reaction. Specifically, there is no cortical break, cortical thickening, or periosteal reaction. The MRI findings of a stress reaction include bone marrow edema-like signal, without a fracture line. A stress reaction becomes a stress fracture once a cortical break develops; however, this distinction is arbitrary and based largely on the inability of routine imaging to visualize the cortical microdamage that characterizes early stress fractures. If intramedullary bone marrow edema-like signal is seen on MRI, it is important to correlate it with the site of pain, as bone marrow edema changes may be seen in asymptomatic patients with altered weight-bearing [54] or those exposed to other osseous stresses [49] thought to represent early subclinical remodeling. This pattern can appear identical to a stress reaction. In one study, 43% of asymptomatic college distance runners were found to have bone marrow edema changes, and the bony marrow signal alterations were not predictive of the development of future tibial stress fractures [55].
Conclusion
A stress failure is a focal failure of bone in response to the summation of repetitive forces overwhelming the remodeling pathway. Lamellar bone is composed of osteons, which are tightly packed in cortical bone, and arranged in struts in trabecular bone. Both macrostructures of bone are constantly undergoing remodeling, which can be stochastic or targeted to a specific site of injury. Targeted remodeling is instigated by cortical or trabecular microdamage, cellular signaling, and osteocyte apoptosis, which attracts osteoclasts and leads to resorption cavities and subsequently new bone formation by osteoblasts. There is a temporary weakening of bone early in the remodeling cycle, when resorption cavities predominate. The contrasting macrostructures of cortical and trabecular bone contribute to the varying appearances of stress fractures of the foot and ankle. In diaphyseal locations, cortical bone predominates and the stress changes are initially centered on the cortex, although routine imaging is not well able to directly visualize these early cortical changes. In the metaphyseal region of long bones and tarsal bones, trabecular bone predominates and the stress changes are centered in the medullary space. Treatment depends on the specific location of the stress fracture, with low-risk types treated with activity modification, and additional treatment options for high-risk types including restricted weight-bearing or surgery. Radiographs are relatively insensitive in early injury, and MRI is the single best imaging modality for the assessment of stress fractures.
References
Stafford SA, Rosenthal DI, Gebhardt MC, Brady TJ, Scott JA. MRI in stress fracture. AJR Am J Roentgenol. 1986;147(3):553–6.
Turner CH. Three rules for bone adaptation to mechanical stimuli. Bone. 1998;23(5):399–407.
Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354(21):2250–61.
Pepper M, Akuthota V, McCarty EC. The pathophysiology of stress fractures. Clin Sports Med. 2006;25(1):1–16. vii.
Pathria MN, Chung CB, Resnick DL. Acute and stress-related injuries of bone and cartilage: pertinent anatomy, basic biomechanics, and imaging perspective. Radiology. 2016;280(1):21–38.
Anderson MW, Greenspan A. Stress fractures. Radiology. 1996;199(1):1–12.
Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3 Suppl 3:S131–9.
Ritchie RO, Kinney JH, Kruzic JJ, Nalla RK. A fracture mechanics and mechanistic approach to the failure of cortical bone. Fatigue Fract Eng Mater Struct. 2005;28(4):345–71.
Burr DB, Akkus O. Bone morphology and organization. In: Basic and applied bone biology. Amsterdam: Elsevier; 2013. p. 3–25.
Carter DR, Van Der Meulen MC, Beaupré GS. Mechanical factors in bone growth and development. Bone. 1996;18(1 Suppl):5S–10.
Robling AG, Fuchs RK, Burr DB. Mechanical adaptation. In: Basic and applied bone biology. 2014. p. 175–204.
Daffner RH, Pavlov H. Stress fractures: current concepts. AJR Am J Roentgenol. 1992;159(2):245–52.
Milgrom C, Giladi M, Simkin A, Rand N, Kedem R, Kashtan H, et al. An analysis of the biomechanical mechanism of tibial stress fractures among Israeli infantry recruits. A prospective study. Clin Orthop Relat Res. 1988;231:216–21.
Churches AE, Howlett CR, Waldron KJ, Ward GW. The response of living bone to controlled time-varying loading: method and preliminary results. J Biomech. 1979;12(1):35–45.
Allen MR, Burr DB. Bone modeling and remodeling. In: Basic and applied bone biology. Amsterdam: Elsevier; 2014. p. 75–90.
Burr DB, Milgrom C, Boyd RD, Higgins WL, Robin G, Radin EL. Experimental stress fractures of the tibia. Biological and mechanical aetiology in rabbits. J Bone Joint Surg (Br). 1990;72(3):370–5.
Batra NN, Li YJ, Yellowley CE, You L, Malone AM, Chi HK, et al. Effects of short-term recovery periods on fluid-induced signaling in osteoblastic cells. J Biomech. 2005;38(9):1909–17.
Kini U, Nandeesh BN. Physiology of bone formation, remodeling, and metabolism. In: Fogelman I, Gnanasegaran G, van der Wall H, editors. Radionuclide and hybrid bone imaging. Berlin: Springer; 2012. p. 29–57.
Berger FH, de Jonge MC, Maas M. Stress fractures in the lower extremity. The importance of increasing awareness amongst radiologists. Eur J Radiol. 2007;62(1):16–26.
Fazzalari NL. Trabecular microfracture. Calcif Tissue Int. 1993;53:S143–7.
Shindle MK, Endo Y, Warren RF, Lane JM, Helfet DL, Schwartz EN, et al. Stress fractures about the tibia, foot, and ankle. J Am Acad Orthop Surg. 2012;20(3):167–76.
Dugan S, Sosa SMA. Chapter 79: stress fractures of the lower limb. In: Essentials of physical medicine and rehabilitation. Amsterdam: Elsevier; 2015. p. 405–10.
Taunton JE, Ryan MB, Clement DB, McKenzie DC, Lloyd-Smith DR, Zumbo BD. A retrospective case–control analysis of 2002 running injuries. Br J Sports Med. 2002;36(2):95–101.
Barrack MT, Gibbs JC, De Souza MJ, Williams NI, Nichols JF, Rauh MJ, et al. Higher incidence of bone stress injuries with increasing female athlete triad-related risk factors: a prospective multisite study of exercising girls and women. Am J Sports Med. 2014;42(4):949–58.
Ruohola J-P, Laaksi I, Ylikomi T, Haataja R, Mattila VM, Sahi T, et al. Association between serum 25(OH)D concentrations and bone stress fractures in Finnish young men. J Bone Miner Res. 2006;21(9):1483–8.
Nelson BJ, Arciero RA, Maffulli N. Stress fractures in the female athlete. Sports Med Arthrosc Rev. 2002;10(1):83.
Jacobs JM, Cameron KL, Bojescul JA. Lower extremity stress fractures in the military. Clin Sports Med. 2014;33(4):591–613.
Miller TL, Best TM. Taking a holistic approach to managing difficult stress fractures. J Orthop Surg Res. 2016;11(1):98.
Daffner RH, Martinez S, Gehweiler JA, Harrelson JM. Stress fractures of the proximal tibia in runners. Radiology. 1982;142(1):63–5.
Sullivan D, Warren RF, Pavlov H, Kelman G. Stress fractures in 51 runners. Clin Orthop Relat Res. 1984;187:188–92.
Brent Edwards W, Taylor D, Rudolphi TJ, Gillette JC, Derrick TR. Effects of running speed on a probabilistic stress fracture model. Clin Biomech. 2010;25(4):372–7.
Popp KL, Hughes JM, Smock AJ, Novotny SA, Stovitz SD, Koehler SM, et al. Bone geometry, strength, and muscle size in runners with a history of stress fracture. Med Sci Sports Exerc. 2009;41(12):2145–50.
Popp KL, McDermott W, Hughes JM, Baxter SA, Stovitz SD, Petit MA. Bone strength estimates relative to vertical ground reaction force discriminates women runners with stress fracture history. Bone. 2017;94:22–8.
Raasch WG, Hergan DJ. Treatment of stress fractures: the fundamentals. Clin Sports Med. 2006;25(1):29–36.
Boden BP, Osbahr DC, Jimenez C. Low-risk stress fractures. Am J Sports Med. 2001;29(1):100–11.
McInnis KC, Ramey LN. High-risk stress fractures: diagnosis and management. PM R. 2016;8(3):S113–24.
Diehl JJ, Best TM, Kaeding CC. Classification and return-to-play considerations for stress fractures. Clin Sports Med. 2006;25(1):17–28.
Niva MH, Sormaala MJ, Kiuru MJ, Haataja R, Ahovuo JA, Pihlajamaki HK. Bone stress injuries of the ankle and foot: an 86-month magnetic resonance imaging-based study of physically active young adults. Am J Sports Med. 2007;35(4):643–9.
Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46–58.
McCormick F, Nwachukwu BU, Provencher MT. Stress fractures in runners. Clin Sports Med. 2010;29(3):399–416.
Taki M, Iwata O, Shiono M, Kimura M, Takagishi K. Extracorporeal shock wave therapy for resistant stress fracture in athletes: a report of 5 cases. Am J Sports Med. 2007;35(7):1188–92.
Wright AA, Hegedus EJ, Lenchik L, Kuhn KJ, Santiago L, Smoliga JM. Diagnostic accuracy of various imaging modalities for suspected lower extremity stress fractures: a systematic review with evidence-based recommendations for clinical practice. Am J Sports Med. 2016;2015:255–63.
Kijowski R, Choi J, Mukharjee R, de Smet A. Significance of radiographic abnormalities in patients with tibial stress injuries: correlation with magnetic resonance imaging. Skeletal Radiol. 2007;36(7):633–40.
Savoca CJ. Stress fractures. A classification of the earliest radiographic signs. Radiology. 1971;100(3):519–24.
Hopson CN, Perry DR. Stress fractures of the calcaneus in women marine recruits. Clin Orthop Relat Res. 1977;128:159–62.
Coris EE, Lombardo JA. Tarsal navicular stress fractures. Am Fam Physician. 2003;67(1):85–90.
Muthukumar T, Butt SH, Cassar-Pullicino VN. Stress fractures and related disorders in foot and ankle: plain films, scintigraphy, CT, and MR imaging. Semin Musculoskelet Radiol. 2005;9(3):210–26.
Wall J, Feller JF. Imaging of stress fractures in runners. Clin Sports Med. 2006;25(4):781–802.
Gaeta M, Minutoli F, Scribano E, Ascenti G, Vinci S, Bruschetta D, et al. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235(2):553–61.
Kiuru MJ, Pihlajamaki HK, Hietanen HJ, Ahovuo JA. MR imaging, bone scintigraphy, and radiography in bone stress injuries of the pelvis and the lower extremity. Acta Radiol. 2002;43(2):207–12.
Fredericson M, Bergman AG, Hoffman KL, Dillingham MS. Tibial stress reaction in runners. Correlation of clinical symptoms and scintigraphy with a new magnetic resonance imaging grading system. Am J Sports Med. 1995;23(4):472–81.
Burghardt AJ, Link TM, Majumdar S. High-resolution computed tomography for clinical imaging of bone microarchitecture. Clin Orthop Relat Res. 2011;469(8):2179–93.
Manhard MK, Horch RA, Harkins KD, Gochberg DF, Nyman JS, Does MD. Validation of quantitative bound- and pore-water imaging in cortical bone. Magn Reson Med. 2014;71(6):2166–71.
Schweitzer ME, White LM. Does altered biomechanics cause marrow edema? Radiology. 1996;198(3):851–3.
Bergman AG, Fredericson M, Ho C, Matheson GO. Asymptomatic tibial stress reactions: MRI detection and clinical follow-up in distance runners. AJR Am J Roentgenol. 2004;183(3):635–8.
Acknowledgements
This review is based on an educational exhibit presented at the Radiological Society of North America annual meeting in 2016.
The authors are grateful for the advice of Dr. Mini Pathria regarding a draft of this manuscript, and of Dr. Christopher Chiodo regarding his assistance with the educational exhibit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Mandell, J.C., Khurana, B. & Smith, S.E. Stress fractures of the foot and ankle, part 1: biomechanics of bone and principles of imaging and treatment. Skeletal Radiol 46, 1021–1029 (2017). https://doi.org/10.1007/s00256-017-2640-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-017-2640-7