Abstract
The aim of this study was to describe the histological features of erosive hand osteoarthritis (EHOA), which is considered an aggressive subset of hand osteoarthritis (OA) characterized by severe local inflammation and degeneration of the distal and proximal interphalangeal joints. Two patients with EHOA underwent replacement with a cement-free press fit ceramic prosthesis of a proximal interphalangeal joint (PIPJ). Clinical and radiological data were collected and histological examination was performed. Radiological examination with histological correlation showed complete erosion of the articular cartilage with focal presence of peripheral fibrocartilaginous resurfacing, sclerosis, and remodeling of the exposed bone, osteoclastic activity with resorptive lacunae in the subchondral bone and around degenerative fibromyxoid pseudocysts, coarse trabeculation of the cancellous bone, and marginal osteophytes. The synovial membrane showed non-specific mild hypertrophy and mildly cellular fibromyxoid stroma. The histological findings in patients with EHOA suggest a pathogenesis of cartilage resorption from the subchondral bone, via osteoclastic-mediated activity and formation of periarticular reactive fibrocartilaginous proliferation with partial resurfacing of the articular surface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Erosive hand osteoarthritis (EHOA) is considered an aggressive variant of hand osteoarthritis (OA) characterized by severe local inflammation and degeneration of the distal (D) and proximal (P) interphalangeal joints (IPJs) [1–3]. The common clinical features of EHOA include inflammatory flares with severe pain, joint swelling, and important functional impairment. EHOA differs from the classical form of hand OA because of clinical features characterized by more than one joint simultaneously affected, instead of a “monoarthritis multiplex” pattern [4], and radiographic parameters showing subchondral erosions with central collapse, cortical destruction, and the consequent reparative process leading to ankylosis [5]. Although clinical and radiological features of EHOA have been well described and summarized by our group in previous articles [4, 5], the mechanisms involved in the initiation and progression of joint degeneration have not been clearly identified. Although cartilage destruction is the hallmark of OA, it is now well established that OA is not only a disorder of cartilage homeostasis, but a whole-joint disease involving all the articular tissues, including the synovial membrane and subchondral bone [6–8]. EHOA is considered to have a greater inflammatory onset compared with non-erosive hand OA. As matter of fact, in EHOA, more inflammatory signs are found on the clinical, ultrasound, and magnetic resonance imaging (MRI) evaluations compared with non-erosive hand OA [9, 10] and an increase in a soluble interleukin-2 receptor has been described [11]. Moreover, increasing ultrasensitive CRP has been correlated with disease activity measured as the number of affected joints [12–14]. Although imaging and biochemical evidence supports a synovial-based inflammatory mechanism for the development of EHOA, these observations do not fully explain or address the subchondral bone findings (joint space narrowing, osteophytes, subchondral sclerosis, central erosion and pseudo-widening, pseudocyst, and mal alignment) [15]. No histological studies have been carried out to examine the whole joint (synovium, articular cartilage, and subchondral bone) in EHOA in the past three decades [16]. The aim of the present study was to describe the histological findings of the affected joints in two patients with the clinical and radiological appearance of EHOA who underwent replacement of a proximal interphalangeal joint (PIPJ) with a cement-free press fit ceramic prosthesis.
Materials and methods
Clinical aspects
A total of 156 outpatients attending the Hand OA Clinic, Rheumatology Unit, University of Padova, Italy, from 2010 to 2015, fulfilling the American College of Rheumatology criteria for hand OA [17], were studied. Criteria for inclusion in the EHOA group were at least two areas of erosion in any IPJ without the presence of erosions in the metacarpophalangeal joints, resulting in a total of 109 patients.
Two women aged 65 and 61 years respectively, underwent prosthetic surgery of PIPJ. The joint involved was the right index finger PIPJ in one and the right long finger PIPJ in the other. The following clinical data were collected: age, sex, BMI, comorbidities, familial predisposition for hand OA, disease duration, treatments, pre-operative VAS, pre-operative C reactive protein (PCR), erythrocyte sedimentation rate (ESR), and joint target of the surgery. The ethics committee of the Padova University Medical Centre approved the study. Informed consent was obtained from all individual participants included in the study. The main clinical characteristics of the two patients are reported in Table 1. Pre-operative CRP and ESR were within the normal range in both patients. VAS was 50 in the first patient and 70 in second. Both were treated with hydroxychloroquine, cartilage protectors, and non-steroidal anti-inflammatory agents.
Radiological aspects
Hand radiographs were performed in antero-posterior and coronal–oblique views and evaluated by an experienced musculoskeletal radiologist. The following radiological parameters were recorded, according to the combined Altman and Oslo radiological scoring systems: joint space narrowing (JSN), osteophytes, erosions, presence of central erosion and pseudo-widening, subchondral sclerosis, pseudocysts, and mal alignment [18, 19].
Surgical procedure and implant type
The surgical procedure was carried out under regional anesthesia. Access was obtained dorsally via a longitudinal incision in the PIPJ region. Through the longitudinal division of the extensor tendon the PIPJ was exposed and transversally resected proximally and distally by careful osteotomy preserving as many collateral ligaments as possible. The cement-free press fit Moje ceramic prosthesis was inserted using an impactor [20]. Radiograph fluoroscopy was carried out to check the exact seating of the implants. Longitudinal closure of the central slip of the extensor tendon was carried out with interrupted sutures.
Histological aspects
Joint articular and synovial tissues removed at surgery were submitted for histological examination. Joint samples were fixed in formalin, decalcified, processed through a standard cycle, embedded in paraffin, cut into 5-μm slices, and stained with hematoxylin and eosin. Picosirius red and trichrome stains for examination of bone and collagen structure were also performed. The histological examination was carried out by two experienced musculoskeletal pathologists (GP and MLV). The following histological parameters were examined: degree of articular cartilage erosion according to the OARSI scoring system [21], subchondral bone sclerosis defined as dense thickening of the cancellous bone trabeculae below the cartilage tide mark with loss of orientation, the presence of osteophytes defined as peripheral growth of reactive osteochondral tissue through endochondral ossification, and degenerative fibromyxoid pseudocysts defined as subchondral spaces without identifiable cell lining completely or partially filled with reactive fibroblasts embedded in a myxomatous matrix, osteoclast activation, vascular crossing of the tide mark, and synovial inflammation (superficial cell layer thickness with or without fibrinous exudate, stromal proliferation, the presence of perivascular and interstitial lymphoplasmacytic inflammatory infiltrate, and an eosinophilic/neutrophilic component). Histological findings correlated with the radiographic features of the EHOA patients.
Results
Radiological examination
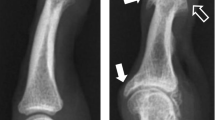
The radiographs of the first case showed large, central erosions in the proximal part of the joint and smaller ones in the distal part (Fig. 1a) and the implanted prosthesis (Fig. 1b). The radiographs of the second case showed moderate erosions in both proximal and distal parts with a pseudo-widening appearance (Fig. 1c) and the removal of the failed prosthesis 2 years after implantation (Fig. 1d). Both had large osteophytes in the proximal and distal parts of the joint and severe joint space narrowing with bone-to-bone contact, proximal and distal subchondral bone sclerosis, degenerative pseudocysts, and mal alignment (Fig. 1a, c, and e).
Radiographs of a finger with erosive hand osteoarthritis (EHOA) underwent prosthesis (antero-posterior [A-P] and coronal–oblique [C-O] views). a Index finger proximal interphalangeal joint (PIPJ; first patient) severe joint space narrowing and moderate osteophytes on both articular sides, several small/moderate erosions with a total size of large erosions on both sides, a small cyst proximally, and mal alignment on the frontal view. b Index finger PIPJ (first patient) with an implanted prosthesis. c Long finger PIPJ (second patient) severe joint space narrowing and beak osteophyte (long thin arrow), severe erosions on both articular sides, pseudowidening on the proximal and volar sides, subchondral sclerosis, degenerative pseudocysts proximally (short thick arrow) and mal alignment on the frontal view. d Prosthesis of the long finger PIPJ (second patient) failed and was removed after 2 years
Histological examination
The histological examination showed complete erosion of the articular cartilage with sclerosis and remodeling of the exposed bone and focal presence of fibrocartilaginous resurfacing (Fig. 2a, b). Osteoclastic activity with resorptive lacunae was evident in the subchondral bone (Fig. 2c) and around degenerative fibromyxoid pseudocysts. Vascular proliferation with crossing of the tide mark could not be assessed because of the complete loss of articular cartilage. Periarticular, moderate cellular proliferation of fibrocartilaginous tissue was also present (Fig. 2d). Large, marginal osteophytes were also identified in separate fragments (Fig. 2e). Synovial membrane, although with limited sampling, showed nonspecific mild hypertrophy and mildly cellular fibromyxoid stroma with an absence of fibrinous exudate, proliferation of the lining cell layer, interstitial mast cells, and perivascular or interstitial lymphoplasmacytic inflammation (Fig. 2f).
Histological characteristics of a–f EHOA and g, h juvenile idiopathic chondrolysis. a PIJP proximal articular bone of the second patient showing loss of cartilage, remodeling, and resorptive osteoclastic activity of the subchondral bone, formation of a large degenerative fibromyxoid pseudocyst, and focal fibrocartilaginous surface proliferation (picrosirius red stain, ×25). b Same section as in a showing osteoid formation (red), mineralized bone (blue), and surface fibrocartilaginous tissue (blue) (Mallory’s trichrome stain, ×25). c Subchondral osteoclastic erosive activity (detail in the insert at ×400) and fibrocartilaginous surface proliferation (Mallory’s trichrome stain ×40). d Marked periarticular fibrocartilaginous proliferation (Mallory’s trichrome stain, ×100). e Beak osteophyte (picrosirius red stain, ×40). f Synovial lining showing mild hypertrophy with slight myxomatous cell proliferation, abundant matrix, and slight reactive capillary proliferation with mild ectasia (H–E stain, ×200). g Juvenile idiopathic chondrolysis showing loss of cartilage with reparative surface fibrocartilaginous proliferation (Safranin-O stain, ×40). h Juvenile idiopathic chondrolysis showing subchondral resorptive osteoclastic activity (safranin-O stain, ×400)
Radiological–histological correlation
The histological features showed correlation with the radiological findings, although the descriptive terms used in radiology and pathology for the same lesion can be different, in addition to the resolution power of the image:
-
1.
Complete loss of cartilage with sclerosis of the subchondral bone is evident in Figs. 1a, c and 2a
-
2.
The degenerative pseudocysts observed in both cases radiologically (Fig. 1c, short thick arrow) correspond to the large pseudocyst evident in Fig. 2a
-
3.
The subchondral radiological erosions correspond histologically to the subchondral osteoclastic erosive activity shown in Fig. 2c and/or to the coarse trabeculation of the cancellous bone, with variation in size of the intervening marrow spaces and bone density more evident in Fig. 2e in the area of osteophyte formation, although in the quiescent phase (end-stage) there was no increase in osteoclastic/osteoblastic activity
-
4.
The pseudo-widening of the joint is secondary to bone remodeling and osteophytic formation and it is evident radiologically and not histologically because of the separation of the proximal and distal parts of the joint at surgery
- 5.
-
6.
Only indirect correlation can be provided for the examination of the formation of reactive fibrocartilage and synovial reaction because they are not visualized by radiographic examination and would require a CT scan or MRI studies for a meaningful comparison
Discussion
The pathogenesis of this aggressive form of OA is still largely unknown [1]. Any morphological and structural study of the changes that occur in EHOA should be interpreted with the purpose of better understanding the mechanisms involved in cartilage loss and bone erosion.
In our two cases, both patients had an erosive pattern on radiology, with some peculiar features of this disease such as central erosion and pseudo-widening. Large peripheral osteophytes, severe joint space narrowing, and mal alignment were also present. The reason why patients underwent joint replacement was intense pain not relieved by the pharmacological treatment and marked joint deformity.
The histological examination confirmed the radiological findings of a severe disruption of articular architecture with the complete loss of the cartilage and sclerosis of the exposed bone, erosions of the subchondral bone by osteoclastic resorption, formation of degenerative fibromyxoid pseudocysts, and formation of large marginal osteophytes. These findings reflect the end stage of a degenerative process common to all types of OA [21]. Histological features that can be related to the specific onset and progression of EHOA were the presence of erosive subchondral osteoclastic activity with formation of resorptive lacunae in the superficial layer of the exposed bone and coarse trabeculation of the cancellous bone indicative of extensive remodeling activity, which correlates with the central, subchondral bone erosions observed radiologically, and the formation of moderately cellular periarticular fibrocartilaginous proliferation with focal substitution of the articular surface. Of note was the absence of lining cell layer proliferation and perivascular and/or interstitial inflammatory infiltrate of the synovium in addition to the presence of a mildly proliferative fibromyxoid stroma, in stark contrast to the findings observed in inflammatory joint diseases of rheumatic disorders. These observations are to some extent in contrast with the previous histological evidence reported in two publications from the same research group [22, 23]. The two studies examined only the synovium at different stages of the disease and the findings of the synovial structure are not dissimilar to those we have observed in our cases, with the exception of an interstitial lymphocytic inflammatory infiltrate of variable intensity and an exudative component, which may be features of an earlier, acute stage of the disease, and subsided at the late stage of our observations. The subchondral bone was not analyzed and therefore no correlation is possible with our observations. In the second report [22], the inflammatory findings in the synovium of EHOA cases are reported to be quite similar to those of rheumatoid arthritis (RA) patients; our observations, albeit limited, do not support it, because villous hypertrophy and synovial sclerosis produce a bulky synovitis, which was absent in both of our cases. Observations corroborating our hypothesis of a primary subchondral erosion of the cartilage is the absence in the cases previously reported of either subchondral inflammation and/or marginal invasion of the subchondral bone by the inflamed synovium and the absence of an erosive synovial pannus.
The observed findings are suggestive of a contribution of cartilage resorption from the subchondral bone via osteoclastic mediated activity and formation of periarticular reactive fibrocartilaginous proliferation with partial resurfacing of the articular surface. Several studies report that there is a crosstalk between cartilage and subchondral bone [24]. Moreover, the potential role of the subchondral bone is also confirmed by the finding that the levels of C-telopeptide of type I collagen, a specific marker sensitive to bone resorption, is increased in patients with EHOA compared with non-EHOA [25]. We have observed a similar histological pattern in the hip joints of pre-adolescents affected by idiopathic chondrolysis, in which there is resorption of the cartilage with subchondral osteoclastic activity and formation of proliferative fibrocartilaginous tissue on the articular surface and synovium within normal limits (Fig. 2g, h), associated with the rapid onset of crippling pain, stiffness, and a marked decrease in the range of motion with no significant response to therapeutic agents (unpublished data).
In conclusion, more observations are needed, possibly with samples at various stages of development of the process, with studies of immunohistochemistry coupled with gene expression characterization to shed light on this clinically relevant subset of patients affected by EHOA.
References
Punzi L, Frigato M, Frallonardo P, Ramonda R. Inflammatory osteoarthritis of the hand. Best Pract Res Clin Rheumatol. 2010;24(3):301–12.
Ramonda R, Lorenzin M, Modesti V, Campana C, Ortolan A, Frallonardo P, et al. Serological markers of erosive hand osteoarthritis. Eur J Intern Med. 2013;24(1):11–5.
Ramonda R, Musacchio E, Campana C, Frigato M, Frallonardo P, Barbieri V, et al. Immunogenetic aspects of erosive osteoarthritis of the hand in patients from northern Italy. Scand J Rheumatol. 2011;40(2):139–44.
Punzi L, Ramonda R, Sfriso P. Erosive osteoarthritis. Best Pract Res Clin Rheumatol. 2004;18(5):739–58.
Ramonda R, Frallonardo P, Musacchio E, Vio S, Punzi L. Joint and bone assessment in hand osteoarthritis. Clin Rheumatol. 2014;33(1):11–9.
Goldring SR. The role of bone in osteoarthritis pathogenesis. Rheum Dis Clin N Am. 2008;34(3):561–71.
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–707.
Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–57.
Kortekaas MC, Kwok WY, Reijnierse M, Huizinga TW, Kloppenburg M. In erosive hand osteoarthritis more inflammatory signs on ultrasound are found than in the rest of hand osteoarthritis. Ann Rheum Dis. 2013;72(6):930–4.
Vlychou M, Koutroumpas A, Alexiou I, Fezoulidis I, Sakkas LI. High-resolution ultrasonography and 3.0 T magnetic resonance imaging in erosive and nodal hand osteoarthritis: high frequency of erosions in nodal osteoarthritis. Clin Rheumatol. 2013;32(6):755–62.
Punzi L, Bertazzolo N, Pianon M, Michelotto M, Todesco S. Soluble interleukin 2 receptors and treatment with hydroxychloroquine in erosive osteoarthritis. J Rheumatol. 1996;23(8):1477–8.
Oliviero F, Ramonda R, Punzi L. New horizons in osteoarthritis. Swiss Med Wkly. 2010;140:w13098.
Punzi L, Ramonda R, Deberg M, Frallonardo P, Campana C, Musacchio E, et al. Coll2-1, Coll2-1NO2 and myeloperoxidase serum levels in erosive and non-erosive osteoarthritis of the hands. Osteoarthritis Cartilage. 2012;20(6):557–61.
Punzi L, Ramonda R, Oliviero F, Sfriso P, Mussap M, Plebani M, et al. Value of C reactive protein in the assessment of erosive osteoarthritis of the hand. Ann Rheum Dis. 2005;64(6):955–7.
Ramonda R, Favero M, Vio S, Lacognata C, Frallonardo P, Belluzzi E, et al. A recently developed MRI scoring system for hand osteoarthritis: its application in a clinical setting. Clin Rheumatol. 2016;35(8):2079–86.
Anandarajah A. Erosive osteoarthritis. Discov Med. 2010;9(48):468–77.
Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990;33(11):1601–10.
Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):A1–56.
Haugen IK, Lillegraven S, Slatkowsky-Christensen B, Haavardsholm EA, Sesseng S, Kvien TK, et al. Hand osteoarthritis and MRI: development and first validation step of the proposed Oslo Hand Osteoarthritis MRI score. Ann Rheum Dis. 2011;70(6):1033–8.
Wesemann A, Flugel M, Mamarvar M. Moje prosthesis for the proximal interphalangeal joint. Handchir Mikrochir Plast Chir. 2008;40(3):189–96.
Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29.
Marmor L, Peter JB. Osteoarthritis of the hand. Clin Orthop Relat Res. 1969;64:164–74.
Peter JB, Pearson CM, Marmor L. Erosive osteoarthritis of the hands. Arthritis Rheum. 1966;9(3):365–88.
Findlay DM, Atkins GJ. Osteoblast-chondrocyte interactions in osteoarthritis. Curr Osteoporos Rep. 2014;12(1):127–34.
Rovetta G, Monteforte P, Grignolo MC, Brignone A, Buffrini L. Hematic levels of type I collagen C-telopeptide in erosive versus nonerosive osteoarthritis of the hands. Int J Tissue React. 2003;25(1):25–8.
Acknowledgements
We would like to acknowledge Irina Shuleshko for technical assistance in the preparation of the histological slides.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Favero, M., Perino, G., Valente, M.L. et al. Radiological and histological analysis of two replaced interphalangeal joints with active subchondral bone resorption in erosive hand osteoarthritis: a novel mechanism?. Skeletal Radiol 46, 385–391 (2017). https://doi.org/10.1007/s00256-016-2560-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-016-2560-y