Abstract
In end-stage osteoarthritis (OA) of the hip, the effect of bone metabolism with and without cartilage is unclear. In this study, we aimed to investigate histomorphology and microdamage in the subchondral bone of the femoral head in areas with and without articular cartilage in patients with end-stage OA. Nineteen femoral heads were evaluated in 10 women who underwent total hip arthroplasty for OA and in nine cadaveric controls (CNT). Chondral thickness and subchondral bone plate thickness (SBP.Th) were measured in 5-mm-wide areas where cartilage was lost (area A) or preserved (area B) in OA and in corresponding areas in the load-bearing portion of the femoral head in the CNT. Histomorphometry and microdamage in 5 × 5-mm areas of cancellous bone were assessed. SBP.Th and bone volume were significantly greater in area A than in area B or in the CNT. Osteoid volume was significantly greater in area A than in area B or in the CNT. There was no significant difference in eroded surface between area A and CNT. Microcrack density was significantly greater in area A than in area B or in the CNT. Although accumulation of microdamage was caused by concentration of stress on the subchondral bone in the cartilage loss area in end-stage OA, remodeling for microdamage repairing mechanism was not enhanced. It was considered that the subchondral cancellous bone volume was increased because of modeling, not remodeling, by stress concentration due to articular cartilage loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a serious disorder of the joints associated with aging [1] and has become a substantial medical and public health problem [2]. The morbidity of OA increases with age [3] and includes slowly progressive destruction and degeneration of cartilage, subchondral bone, and surrounding tissues [4].
There have been many reports on the changes that occur in the articular cartilage and subchondral bone in OA [5,6,7,8,9,10,11,12]. A relationship between OA and microdamage has also been described, but the details are unknown [13,14,15,16,17]. Reduction of subchondral bone mass in response to increased remodeling in early-stage OA has been reported [8], although microdamage has never been examined in early-stage OA. More microdamage of subchondral bone has been documented in osteoporotic patients with femoral neck fracture than in patients with end-stage OA [16]. Microdamage occurs in the subchondral bone in early- and progressive-stage OA and was reported to be an initiation of targeted remodeling [13]. OA is associated with degeneration and abrasion of cartilage, but its relevance to microdamage in the subchondral bone in end-stage OA is unclear.
The purpose of this study was to reveal the pathophysiology of end-stage OA by measuring histomorphometry and microdamage in the subchondral bone at the femoral head in areas with and without articular cartilage in patients with end-stage OA of the hip.
Materials and methods
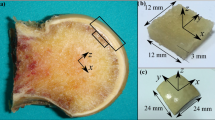
Nineteen femoral heads were collected from 10 women (mean age 73.7 years) who underwent total hip arthroplasty for end-stage OA of the hip (Kellgren–Lawrence classification [18] grade 4). Non-osteoarthrotic femoral heads were collected from nine cadaveric controls (CNT; 4 men, 5 women; mean age 83.1 years). There was no significant difference in age between OA patients and CNT (Fig. 1). The femoral heads were cut coronally 15 mm wide at the center (Fig. 2a). Using the method reported by Burr et al. [19], bone samples were stained en bloc with 1% basic fuchsin and embedded in methyl methacrylate. Ground sections of 100 µm thickness were obtained for histomorphometry and microdamage analysis. Chondral thickness and subchondral bone plate thickness were measured in areas where cartilage was lost (area A; Fig. 2b) or preserved (area B; Fig. 2b) in patients with OA and in corresponding areas in the load-bearing part of the femoral head in the CNT (Fig. 2c). Measurements were performed in the order of chondral thickness, subchondral bone plate thickness, and subchondral bone (Fig. 2d). Histomorphometry and microdamage were assessed in 5 × 5 mm areas of cancellous bone under the subchondral bone plate (Fig. 2d, square). Histomorphometric measurements were performed using a semi-automated digitizing image analyzer, consisting of a light or epifluorescent microscope and a digitizing pad connected to a computer with histomorphometric software (System Supply Co., Nagano, Japan). All measurements were performed in a blinded manner by one histomorphometrist. Microdamage was measured at 100× magnification. Microdamage in the bone was defined as a typical crack shape with a certain depth of field and a surrounding halo of increased basic fuchsin staining. Crack density, mean crack length, and crack surface density were measured.
Femoral heads were cut coronally 15 mm wide at the center. a Measurements of chondral thickness and subchondral bone plate thickness where cartilage is lost (area A) and preserved (area B) in patients with osteoarthritis b and corresponding areas in the load-bearing portion of the femoral head in cadaveric controls c. Histomorphometry and assessment of microdamage were performed in 5 × 5 mm areas of cancellous bone under the subchondral bone plate d. C.Th chondral thickness, SBP.Th subchondral bone plate thickness
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). Differences between the groups were tested for statistical significance by one-way analysis of variance. If a significant difference was found, the difference between the means of two groups was tested using Tukey’s multiple comparison test. A p value < 0.05 was considered statistically significant.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study design was approved by the Ethics Committee at Kagawa University. Informed consent was obtained from all individual participants included in the study.
Results
As shown in Table 1, Chondral thickness was significantly lower in area A than in area B or the CNT (p < 0.0001). Subchondral bone plate thickness was significantly greater in area A than in area B or in the CNT (p < 0.0001). Trabecular bone volume in the cancellous bone under the cartilage was significantly greater in area A than in area B or the CNT (p < 0.0001). Trabecular number was also significantly higher in area A than in area B (p = 0.033). Trabecular separation was significantly lower in area A than in area B (p = 0.014). Osteoid volume was significantly greater in area A than in area B or in the CNT (p = 0.0008). Eroded surface was significantly lower in area B than in the CNT (p = 0.0008). There was no significant difference in microcrack length between any of the areas sampled, although microcracks tended to be longer in area A. Microcrack density was significantly greater in area A than in area B or the CNT (p < 0.0001). This shows that microcracks accumulated more in area A than in area B (Fig. 3). Microcrack surface density was also significantly greater in area A than in area B or in the CNT (p < 0.0001).
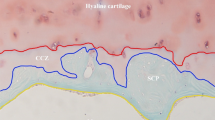
Microcracks in areas of the cancellous bone under the subchondral bone plate. a End-stage osteoarthritis of the hip (Kellgren–Lawrence classification grade 4). b A section of the surface of the femoral head showing a cartilage-lost area (area A) and cartilage-preserved area (area B). c In area A, the bone volume is increased and many microcracks (indicated by black arrows) can be observed. Scale bar 100 µm. d In area B, only a few microcracks are evident. Scale bar 100 µm
Discussion
The primary aim of this study was to examine bone metabolism in end-stage OA of the hip by measuring chondral thickness and subchondral bone plate thickness, as well as histomorphometry and microdamage at the femoral head in areas with and without articular cartilage and in corresponding areas in the load-bearing part of the femoral head in CNT. There were several reports on the loss of articular cartilage and subchondral bone in end-stage OA [2,3,4, 6, 7, 12, 13]. Although a relationship between OA and microdamage in the load-bearing part of the femoral head has been described [13,14,15,16], there have been no reports on microdamage in areas where cartilage is lost or preserved in patients with end-stage OA of the hip. To our knowledge, this is the first study to find an increase in the subchondral bone plate thickness and a decrease in chondral thickness in areas of lost cartilage compared with areas of preserved cartilage or the corresponding areas in the CNT, along with an increase in cancellous bone volume and significant accumulation of microdamage. In areas of lost cartilage, we found an increased osteoid volume but not an increase in the eroded surface, which is different from normal remodeling changes.
Burr et al. [13] reported that both subchondral bone plate thickness and cancellous bone volume decreased in early-stage OA because of increased remodeling and that subchondral bone plate thickness increased because of a decrease in the amount of cartilage and increased stress as the disease progresses. In the present study, we investigated patients with end-stage OA, whose disease was more advanced than in the patients described by Burr et al. We found an increase in subchondral bone plate thickness with decreasing chondral thickness, along with an increase in the cancellous bone volume under the subchondral bone plate. The relationship between cartilage and subchondral bone plate thickness was similar to that in the study of patients with progressive osteoarthritis by Burr et al. However, there was a marked difference in the subchondral cancellous bone volume between our patients with end-stage OA and those with progressive OA as reported by Burr et al.
Li et al. [11] reported that remodeling and bone volume increased more in the subchondral bone at the load-bearing portion of the femoral head than in the cancellous bone at the deeper trabecular bone in patients with OA of the hip. However, Kumarasinghe et al. [5] reported that bone erosion was decreased in the absence of reduced bone formation in end-stage OA of the hip. In our study, we observed an increased osteoid volume but not an increase in the eroded surface, which does not suggest involvement of the normal coupling between bone resorption and formation. Kuliwaba et al. [20] reported significantly elevated expression of mRNA for osteocalcin in the trabecular bone of the proximal femur in end-stage OA of the hip. Therefore, it is possible that the bone volume in the subchondral bone increases because of modeling in response to load bearing rather than normal remodeling of the bone. Excessive load bearing has been found to cause bone modeling and an imbalance between bone formation and bone resorption. Cox et al. [10] reported that bone volume was greater and that there was less bone matrix mineralization in the subchondral cancellous bone in areas of lost cartilage than in areas where cartilage remained in knees with OA. Moreover, whether this was in response to remodeling or modeling is unknown, but it seems possible that the amount of immature bone tissue was increased in response to excessive load bearing in areas without cartilage. Therefore, it is suggested that bone shape changes by modeling due to mechanical loading [21], and the same phenomenon is considered to occur in the subchondral bone of end-stage OA.
Microdamage in the form of microscopic cracks occurs in the bone because of physical repetitive loading in daily activities [22]. Targeted remodeling plays the role of repairing microdamage caused by load bearing, in vivo [23, 24]. Although the relationship between end-stage OA and microdamage in subchondral cancellous bone is not clear, Burr et al. [13] mentioned the possibility that repair of microdamage may cause remodeling in early OA. Coughlin et al. [14] reported that microdamage in post-traumatic OA causes resorption of subchondral bone and becomes key in progression of OA. Furthermore, Ramme et al. [17] found an accumulation of microdamage and increased remodeling followed by degeneration of articular cartilage in the subchondral bone in a rat model of OA created by rupture of a cruciate ligament. However, they did not investigate end-stage OA.
Frost et al. [25] reported that accumulation of microdamage occurs due to an increase in loading stress, due to suppression of damage repair, or both. Loss of cartilage damages the underlying bone [26]; thus, it is considered that the load-bearing stress of the subchondral bone increases due to cartilage loss. In this study, accumulation of microdamage was not observed in the cartilage-preserved area but in the cartilage-lost area. If accumulation of microdamage occurred, targeted remodeling should be enhanced. However, in this study, enhancement of remodeling was not observed even if microdamage increased. Based on these findings, accumulation of microdamage was suspected not as an initiation of remodeling but the result of increasing load bearing in end-stage OA. Increasing load bearing increases modeling, which is different from past reports [13, 17] that remodeling was enhanced in early-stage and progressive OA. In this study, increased bone volume was observed in end-stage OA of the hip.
Generalization of the study findings is limited by the small sample size. This study only assessed end-stage OA of the hip, and we did not investigate progression of OA.
In conclusion, we assessed histomorphologic features and bone microdamage in the subchondral bone in areas of the femoral head where articular cartilage area is lost or preserved in end-stage OA of the hip. Although accumulation of microdamage was caused by concentration of stress on the subchondral bone in the cartilage loss area in end-stage OA, remodeling for microdamage repairing mechanism was not enhanced. It was considered that the subchondral cancellous bone volume was increased because of modeling, not remodeling, by stress concentration due to articular cartilage loss.
References
Arden NK, Griffiths GO, Hart DJ, Doyle DV, Spector TD (1996) The association between osteoarthritis and osteoporotic fracture: the Chingford study. Br J Rheumatol 35:1299–1304
Bailey AJ, Mansell JP (1997) Do subchondral bone changes exacerbate or precede articular cartilage destruction in osteoarthritis of the elderly? Gerontology 43:296–304
Amir G, Pirie CJ, Rashad S, Revell PA (1992) Remodelling of subchondral bone in osteoarthritis: a histomorphometric study. J Clin Pathol 45:990–992
Grynpas MD, Alpert B, Katz I, Lieberman I, Pritzker KP (1991) Subchondral bone in osteoarthritis. Calcif Tissue Int 49:20–26
Kumarasinghe DD, Perilli E, Tsangari H, Truong L, Kuliwaba JS, Hopwood B, Atkins GJ, Fazzalari NL (2010) Critical molecular regulators, histomorphometric indices and their correlations in the trabecular bone in primary hip osteoarthritis. Osteoarthritis Cartilage 18:1337–1344
Bobinac D, Marinovic M, Bazdulj E, Cvijanovic O, Celic T, Maric I, Spanjol J, Cicvaric T (2013) Microstructural alterations of femoral head articular cartilage and subchondral bone in osteoarthritis and osteoporosis. Osteoarthr Cartil 21:1724–1730
Radin EL, Rose RM (1986) Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res 231:34–40
Radin EL, Paul IL, Tolkoff MJ (1970) Subchondral bone changes in patients with early degenerative joint disease. Arthritis Rheum 13:400–405
De Pedro JA, Martin AP, Blanco JF, Salvado M, Perez MA, Cardoso A, Collía F, Ellis SS, Domínguez J (2007) Histomorphometric study of femoral heads in hip osteoarthritis and osteoporosis. Histol Histopathol 22:1091–1097
Cox LG, van Donkelaar CC, van Rietbergen B, Emans PJ, Ito K (2012) Decreased bone tissue mineralization can partly explain subchondral sclerosis observed in osteoarthritis. Bone 50:1152–1161
Li G, Ma Y, Cheng TS, Landao-Bassonga E, Qin A, Pavlos NJ, Zhang C, Zheng Q, Zheng MH (2014) Identical subchondral bone microarchitecture pattern with increased bone resorption in rheumatoid arthritis as compared to osteoarthritis. Osteoarthr Cartil 22:2083–2092
Li G, Zheng Q, Landao-Bassonga E, Cheng TS, Pavlos NJ, Ma Y, Zhang C, Zheng MH (2015) Influence of age and gender on microarchitecture and bone remodeling in subchondral bone of the osteoarthritic femoral head. Bone 77:91–97
Burr DB, Gallant MA (2012) Bone remodelling in osteoarthritis. Nat Rev Rheumatol 8:665–673
Coughlin TR, Kennedy OD (2016) The role of subchondral bone damage in post-traumatic osteoarthritis. Ann NY Acad Sci 1383:58–66
Burr DB, Radin EL (2003) Microfractures and microcracks in subchondral bone: are they relevant to osteoarthrosis? Rheum Dis Clin North Am 29:675–685
Li ZC, Dai LY, Jiang LS, Qiu S (2012) Difference in subchondral cancellous bone between postmenopausal women with hip osteoarthritis and osteoporotic fracture: implication for fatigue microdamage, bone microarchitecture, and biomechanical properties. Arthritis Rheum 12:3955–3962
Ramme AJ, Lendhey M, Raya JG, Kirsch T, Kennedy OD (2016) A novel rat model for subchondral microdamage in acute knee injury: a potential mechanism in post-traumatic osteoarthritis. Osteoarthr Cartil 10:1776–1785
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502
Burr DB, Stafford T (1990) Validity of the bulk-staining technique to separate artifactual from in vivo bone microdamage. Clin Orthop Rel Res 260:305–308
Kuliwaba JS, Findlay DM, Atkins GJ, Forwood MR, Fazzalari NL (2000) Enhanced expression of osteocalcin mRNA in human osteoarthritic trabecular bone of the proximal femur is associated with decreased expression of interleukin-6 and interleukin-11 mRNA. J Bone Miner Res 15:332–341
Dempster DW (2006) Anatomy and functions on the adults skeleton. In: Favus MJ (ed) Primer on the metabolic bone diseases and disorder of mineral metabolism, 6th edn. American Society for Bone and Mineral Research, Washington, pp 7–11
Burr DB (1997) Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res 12:6–15
Mori S, Burr DB (1993) Increased intracortical remodeling following fatigue damage. Bone 14:103–109
Li J, Mashiba T, Burr DB (2001) Bisphosphonate treatment suppresses not only stochastic remodeling but also the targeted repair of microdamage. Calcif Tissue Int 69:281–286
Frost HM (1986) Bone microdamage: Factors that impair its repair. In: Uhthoff HK (ed) Current concepts of bone fragility. Springer, Berlin
Felton DT (2013) Osteoarthritis as a disease of mechanics. Osteoarthr Cartil 21:10–15
Acknowledgements
The authors thank Mika Higashihara and Yoshiko Agawa for preparing the histology specimens used in this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All the authors have contributed equally to this work. MS, KI, TM, TM, and TY designed the study, contributed to the experimental work and analysis, prepared the draft of the paper, reviewed it critically for intellectual content, and approved the final version. KI is the guarantor. All the authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Shimamura, M., Iwata, K., Mashiba, T. et al. Accumulation of microdamage in subchondral bone at the femoral head in patients with end-stage osteoarthritis of the hip. J Bone Miner Metab 37, 880–885 (2019). https://doi.org/10.1007/s00774-019-00988-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-019-00988-z