Abstract
Biofilm formation on abiotic surfaces has become a major public health concern because of the serious problems they can cause in various fields. Biofilm cells are extremely resistant to stressful conditions, because of their complex structure impedes antimicrobial penetration to deep-seated cells. The increased resistance of biofilm to currently applied control strategies underscores the urgent need for new alternative and/or supplemental eradication approaches. The combination of two or more methods, known as Hurdle technology, offers an excellent option for the highly effective control of biofilms. In this perspective, the use of functional enzymes combined with biosourced antimicrobial such as essential oil (EO) is a promising alternative anti-biofilm approach. However, these natural antibiofilm agents can be damaged by severe environmental conditions and lose their activity. The microencapsulation of enzymes and EOs is a promising new technology for enhancing their stability and improving their biological activity. This review article highlights the problems related to biofilm in various fields, and the use of encapsulated enzymes with essential oils as antibiofilm agents.
Key points
• Problems associated with biofilms in the food and medical sectors and their subsequent risks on health and food quality.
• Hurdle technology using enzymes and essential oils is a promising strategy for an efficient biofilms control.
• The microencapsulation of enzymes and essential oils ensures their stability and improves their biological activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pathogenic bacterial contaminations of abiotic surfaces in food and medical sectors represent a serious public health problem, as they can lead to severe human infections worldwide (Abdallah et al. 2014; Khelissa et al. 2017). Foodborne infections generally occur after consumption of food and drink contaminated with pathogens. These contaminations can occur during any step of food processing, through food handlers and contaminated food contact surfaces and equipment (Verraes et al. 2013). According to the annual report of the Centers for Disease Control and Prevention (CDC), 841 foodborne disease outbreaks were reported in the USA in 2017, resulting in 14,481 illnesses, 827 hospitalizations, 20 deaths, and 14 food product recalls (CDC 2019). The World Health Organization (WHO) reported that an estimated 600 million people – nearly one in ten people worldwide – become ill from consuming contaminated food and 420,000 die each year of which 30% occur among children under 5 years old. In addition, 110 billion US$ are lost annually in medical expenses and productivity due to unsafe food in low- and middle-income countries (WHO 2020). In 2019, 1,783 foodborne illnesses were reported in France, affecting 15,641 people, of which 609 (4%) were hospitalized (hospitalization or emergency room visit) and 12 (0.08%) died (SPF 2021). Healthcare-associated infections (HCAIs) are the most common adverse event in the healthcare field worldwide. These infections can occur in all types of healthcare settings through healthcare personnel’s hands and contaminated devices and surfaces (catheters, surgical instruments, endoscopes, respiratory systems, needles, etc.) (Weber et al. 2013; Ssekitoleko et al. 2020). According to the US Centers for Disease Control and Prevention, almost 1.7 million hospitalized patients contract HCAIs each year while treated for other health problems, and more than 98,000 patients (one in 17) die from them (Klevens et al. 2007). In 2017, the National Point Prevalence Survey (PPS) on HCAI in France showed that one in twenty patients hospitalized in a healthcare facility were infected. The four main sites of HCAI, accounting for 71.5% of documented infectious sites – urinary tract infections (28.5%), surgical site infections (15.9%), pneumonia (15.6%), and bloodstream infections (11.4%) – were identical in 2012 and 2017 (SPF 2019).
In natural and artificial environments, bacteria tend to live attached to the abiotic surfaces and to develop a complex structure called biofilm. It has been found that approximately 40–80% of the bacterial cells on earth are able to form biofilms (Flemming and Wuertz 2019). Biofilms, unlike planktonic cells, are a self-protected grown cluster of bacteria. They are defined as a structured microbial community, adhering to a surface, to interfaces and to each other, and embedded in a self-produced polymer matrix that offers a highly protective environment against biocide attack (Donlan and Costerton 2002; Karygianni et al. 2020). The formation of biofilms poses serious problems in many fields due to the potential increased resistance to chemical biocides, antibiotics, and UV radiation; increased secretion of secondary metabolic products; and high gene exchange rates (de Carvalho 2017; Xu et al. 2017; Gebreyohannes et al. 2019; Rodrigues and Černáková 2020). Several studies have shown that industrial ecosystems are favorable areas for bacterial growth and biofilm formation (Coughlan et al. 2016). In a hospital environment, biofilms have been found to survive and persist on many medical device surfaces and on the tissue of patients, resulting in several persistent infections (Dongari-Bagtzoglou 2008; Percival et al. 2015). Thus, the control of biofilms remains the most important task for many industries to reduce the microbiological risk associated with its persistence in these areas. Several strategies have recently been proposed to combat biofilms, which include chemical removal such as detergents, biocides, and surfactants; and mechanical removal such as thawing, freezing, sonication, and scraping (de Carvalho 2007; Zea et al. 2020). However, complete removal of biofilm by the single use of these methods has been shown to be difficult to achieve due to the high protection of biofilm cells by EPS that act as an initial protective barrier to the biofilm cells and make biofilm 10–1,000 times more resistant to antimicrobial agents than the planktonic cells (Singh et al. 2017; Tan et al. 2018). In addition, although the sanitation is one of the most widely used and essential techniques to control biofilm in the industries, it is important to note that the application of these sanitizers for many decades could be a major cause of the emergence of antibiotic resistance in bacteria and their spread to pathogens, which has led to the search for new natural antimicrobial agents to overcome these issues (Bayoumi et al. 2012; al Kassaa et al. 2021).
The combined use of two or more hurdle methods to control biofilm (Hurdle technology) is a potentially effective strategy for an efficient biofilm cell removal from abiotic surfaces, as they would attack microorganisms in different ways (Khan et al. 2017). The synergistic effect of reducing bacterial contamination from abiotic surfaces using Hurdle technology has been successfully demonstrated in numerous studies (Lequette et al. 2010; Pechaud et al. 2012; Ban and Kang 2016; Lim et al. 2017, 2019; Jung et al. 2018; Hussain et al. 2019). In this way, the combination of enzymes with bio-based antimicrobials will be a promising method for controlling biofilm in such a way that the enzymes would destabilize and destroy the biofilm matrix, so that bacteria protected by the matrix would be eliminated more effectively by the antimicrobials. It’s now established that enzymes control and eliminate biofilms owing to their ability to degrade major components of the biofilm matrix, promote cell lysis, induce biofilm disruption, and disrupt cell-to-cell signals that govern biofilm maintenance and formation (Mohamed et al. 2018). Essential oils (EOs), which are aromatic oily liquids derived from plant materials are known as natural and safe bio-based biocides for synthetic drugs and antiseptics and have been widely tested in vitro against a broad range of pathogenic bacteria (Oulkheir et al. 2017). In addition to their antibacterial activity, numerous studies have demonstrated their anti-biofilm activity by removing and preventing biofilm formation (Vázquez-Sánchez et al. 2015; Oh et al. 2017; Engel et al. 2017; Mohamed et al. 2018). The use of EOs and their application have to face many challenges such as their volatility, stability issues, and their poor water solubility which may decrease their activity. It is therefore necessary to find out a new strategy, such as encapsulating these molecules in different material supports, to effectively improve their activity. Microencapsulation of EOs is a good tool to increase their stability, decrease their water immiscibility, control their release, and limit the physicochemical interactions between these molecules and the biofilm matrix components. These interactions are often associated with a decrease in the efficacy of the antibacterial molecules (Cui et al. 2016a; El Asbahani et al. 2015; Engel et al. 2017).
In this regard, this review will focus on the various factors that influence the adhesion of bacteria and the formation of biofilms on abiotic surfaces, as well as the biofilm resistance towards disinfectants. Moreover, the problems associated with biofilms in food and medical sectors, and the different strategies for biofilm prevention and eradication will be highlighted, in addition to the effectiveness of hurdle technology in removing biofilms using different bio-based molecules. Finally, the place of the microencapsulation as a tool for the formulation of bio-based biocides to fight biofilm will be discussed.
What is a bacterial biofilm and how is it formed?
Biofilms are generally defined as microbial populations that are irreversibly associated (cannot be removed by mild rinsing) with a surface and wrapped in a self-produced EPS matrix (Donlan 2002; Hall-Stoodley and Stoodley 2009). Microorganisms structured within biofilm differ from their planktonic counterparts by the transcribed genes. Bacteria are able to form biofilms when the growth conditions are suitable on different abiotic surfaces encountered in healthcare, food industries, industrial or potable water systems, and natural aquatic systems (Abdallah et al. 2014).
Biofilm formation steps
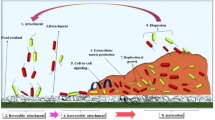
The process of biofilm formation is complex and several factors may be involved. Bacterial cell adhesion and biofilm formation are significantly linked to the substratum properties such as hydrophobicity, electric charge, and rugosity. In addition, cell surface and the presence of pili, flagella, glycocalyx, or fimbriae are important for cell adhesion and biofilm formation (Donlan 2001; Roy et al. 2017; Hage et al. 2021). A bacterial biofilm can be structured in four common stages (Fig. 1). In the first stage, the bacterial cells bind to a biotic or abiotic surface (1); then the cells cluster multiply and form microcolonies (2) followed by the formation mature biofilm (3). The last stage is the detachment and dispersion of bacterial cells in the surrounding environment (4) (Abdallah et al. 2014; Khelissa et al. 2017).
The initial stage is governed by the reversible interactions mediated by the non-specific Lifshitz-van der Waals, Lewis acid–base, and electrostatic forces (Kaplan 2010) and requires the presence of specific adhesins located on the host (e.g., fimbriae, flagella) (1) (Rosan and Lamont 2000; Abdallah et al. 2014). In the second step, the adherent bacteria synthesize exopolysaccharide proteins and other components of the polymer matrix that maintain the bacterial cells together in a mass and tightly fix the bacterial mass to the surface and contribute to the irreversible adhesion (2). In the third stage, the biofilm becomes mature and able to express different genes and contributes to the antimicrobial resistance of the biofilm (3) (Kaplan 2010; Mah and O’Toole 2001; Chakraborty and Kumar 2019). The final biofilm formation step is the dislocation of cells from the biofilm and their dispersion in the environment (4). Cell detachment can be triggered by a variety of factors such as mechanical disturbances, polymer matrix enzymatic degradation, surfactant production, and exopolysaccharide release (Kaplan 2010). These cells have the ability to adhere to new surfaces and re-form a biofilm and may contribute to biological dispersion, bacterial survival, and disease transmission that are known as biofilm lifecycle. As at other stages of biofilm development, bacteria respond to multiple environmental signals (e.g., nutrient concentrations), signal transduction pathways, effectors, and bacterial cell density, a phenomenon better known as quorum sensing (QS) (Karatan and Watnick 2009; Liu et al. 2019). QS is the regulation of gene expression in the response of cell density by the liberation of chemical signal molecules called autoinducers (acylated homoserine lactones as autoinducers of Gram-negative bacteria and oligo-peptides as autoinducers of Gram-positive bacteria) that allows the differentiation of bacterial biofilm. When these molecules attain a minimal threshold stimulatory concentration, the activation or repression of new genes occurs (Zhao et al. 2020). Thus, QS allows bacteria to display many responses that benefit the population, such as enhanced accessibility to nutrients and more favorable environment and promotes action against competing bacteria and environmental stresses (Zhao et al. 2020).
Extracellular polymeric substance, composition, and functions
A biofilm is composed of attached microbial cells encased within a matrix of EPS. EPS was initially designated as “extracellular polysaccharides,” but it has been renamed as “extracellular polymeric substance” because it can also contain many other substances (Flemming and Wingender 2010). EPS may represent 50 to 90% of the total organic carbon of biofilms (Evans 2014). The composition of the matrix varies according to the bacterial species, strains, and growing conditions; it is highly hydrated (can contain up to 97% water) and is mainly composed of proteins, polysaccharides, and extracellular DNA (eDNA) (Fulaz et al. 2019). Biofilm matrix can also contain surfactants, lipids, glycolipids, extracellular enzymes, and cations (Karatan and Watnick 2009; Flemming and Wingender 2010; Karygianni et al. 2020). Most of the today knowledge has been generated by using model organisms forming biofilms, in particular Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, Candida albicans, Streptococcus mutans, and Vibrio cholerae (Fig. 2). Several advanced reviews have detailed the functional role and composition of EPS in various matrices formed by these organisms as biofilms of a single species (Zarnowski et al. 2014; Peterson et al. 2015; Hobley et al. 2015; Flemming et al. 2016; Dragoš and Kovács 2017; Bowen et al. 2018).
Composition of extracellular polymeric substances (EPS) in the biofilms of some model organisms, epsA-epsO operon-encoded exopolysaccharide, poly-γ-glutamate (γ-PGA), polysaccharide intercellular adhesin (PIA), poly-β(1–6)-N-acetylglucosamine (PNAG), Vibrio cholerae Vibrio polysaccharide (VPS), biofilm surface layer protein (BslA), translocation-dependent antimicrobial spore component (TasA)/TasA anchoring and assembly protein (TapA), fibronectin-binding proteins (FnBPs), staphylococcal protein A (SpA), Staphylococcus aureus surface protein G (SasG), biofilm-associated protein (BAP) extracellular, phenol-soluble modulins (PSMs), glucosyltransferases (Gtf), fructosyltransferases (Ftf), glucan binding proteins (GbpA, GbpB, GbpC), type IV pilins (T4P), lectins (LecA/LecB), biofilm-associated protein (Bap1), rugosity and biofilm modulators (RbmA/RbmC), mannose-sensitive hemagglutinin (MSHA) pili, agglutinin-like sequence protein (Als), hyphal wall proteins (Hwp) cell wall, heat-shock proteins (Hsp70). Further details on EPS components, including those from other microbes (such as Escherichia coli), are available from the following references (Mann and Wozniak 2012; Vlamakis et al. 2013; Zarnowski et al. 2014; Teschler et al. 2015; Hobley et al. 2015; Ibáñez de Aldecoa et al. 2017; Dragoš and Kovács 2017; Cochet and Peri 2017; Bowen et al. 2018; Nett and Andes 2020)
EPS, which has been termed as the “dark matter of biofilms,” may provide a potential number of challenges and play a very crucial role in adhesion, aggregation, cohesion, structural integrity, and protective barrier of biofilm (Fulaz et al. 2019). The EPS matrix provides key architectural and protective support for microbial communities in the biofilm, blocking access of xenobiotic and antimicrobials to biofilm cells and providing protection against environmental stresses such as pH change, UV radiation, desiccation, and osmotic shock (Al Kassaa et al. 2019; Karygianni et al. 2020).
The polysaccharides play a fundamental role in the biofilm formation and antibiotic tolerance and as a virulence factor in opportunistic pathogens. Examples of the most common polysaccharides are alginate, cellulose, polysaccharide synthesis locus (PSL), and pellicle (PEL) polysaccharides Pel, Psl, and the staphylococcal polysaccharide intercellular adhesin (PIA) (Fulaz et al. 2019; Bundalovic-Torma et al. 2020). The proteins play also an essential role in biofilm adhesion and cohesion and, in some cases, are found in higher proportions than polysaccharides (Karygianni et al. 2020). The common proteins presented in the biofilm matrix are amyloid fibers (Fulaz et al. 2019). Furthermore, previous studies have shown that eDNA plays a significant role in the structural stability, formation, and integrity of the bacterial biofilms (Devaraj et al. 2019) (Table 1).
Biofilm architecture
Although some structural properties of biofilm can generally be regarded as universal, it has been reported that each biofilm community is unique (Tolker-Nielsen and Molin 2000). The morphology of biofilm can be rough smooth and flat or filamentous; furthermore, the biofilm can change on its degree of porosity, with mushroom-like macrocolonies surrounded by voids filled with water (Flemming and Wingender 2010). The concept of this diversity of structure is descriptive not only for mixed crop biofilms (environmental biofilms) but also for pure crop biofilms common to medical devices and those associated with infectious diseases (Donlan 2002). Several parameters can explain this heterogeneity, including the surface properties (e.g., hydrophobicity, roughness, electrochemical properties), hydrodynamic forces (e.g., mass transfer, shear forces, frictional drag, form drag), the presence of nutrients or inhibitors (e.g., concentration, antimicrobial properties, mass transfer properties, reactivity), and the consortia and ecological diversity of the biofilm (e.g., cell signal, presence of morphotypes, motility, food chains, trophic structure) (Stoodley et al. 1997). In addition, the structured communities of biofilm depend highly on the quantity, characteristic, and the three-dimensional structure (the dense areas, pores, and channels) of the EPS (Sutherland 2001).
Factors influencing bacterial cells adhesion
The attachment of a cell to a substrate is called adhesion, and the attachment of one cell to another is called cohesion (Garrett et al. 2008). For the first steps of adhesion, the interactions between the conditional layer and the substrate strongly influence the growth of cellular communities; this layer can be composed of many organic or inorganic particles and modifies the substrates which facilitate the accessibility to bacteria. The biofilm adheres in a reversible or irreversible manner. Factors such as available energy, surface functionality, bacterial orientation, temperature, and pressure conditions have a significant effect on the initial adhesion of bacterial cells. Then if the repellent forces are higher than the attraction forces, the bacteria detach from the surface; this is more apt to occur before a substrate is conditioned (Garrett et al. 2008). If the physical appendages of bacteria (flagella, fimbriae, and pili) overcome the repellent physical forces of the electric double layer, a number of reversibly adsorbed cells remain immobilized and become irreversibly adsorbed (Weger et al. 1987). Some research has shown that microbial adhesion is highly dependent on the hydrophobic-hydrophilic and topography properties of interacting surfaces (Liu et al. 2004; Hage et al. 2021).
Factors of biofilm resistance
There are different factors related to the physiological and structural characteristics of a biofilm that influence its resistance to disinfectants. Biofilm can be protected against antimicrobials by the limitation of diffusion or reaction of disinfectants; thus, due to the presence of EPS and a multiple layer of cells that can form a complex and dense structure, biocides have a difficult entering and achieving the inner layers, which affects their effectiveness (Bridier et al. 2011). The organic matter present in the matrix such as proteins, nucleic acids, or carbohydrates can deeply interfere with the efficiency of disinfectants (Banach et al. 2015). The phenotypic adaptation of biofilm cells to nonlethal doses of disinfectants can also lead to biofilm resistance toward biocides. In addition, due to the limited penetration of antimicrobials and the low levels of exposition of the biofilm deep layers to the antimicrobial agent, the biofilm can develop adaptive responses to sublethal concentrations of the disinfectant (Bridier et al. 2011). Moreover, the physiological adaptations of biofilm cells, such as the expression of specific genes according to the environmental conditions of biofilms, allow the increase of the biofilm resistance. Many studies confirmed this adaptation by the comparison of gene expression profiles, and proteomic analyses of planktonic and biofilm states in different species (Sauer 2003; Whiteley et al. 2001; Abdallah et al. 2014), such as the expression of specific genes encoding for changes in membrane composition (Wolska et al. 2016), efflux pump (Soto 2013), and enzyme production. It has been reported that in Pseudomonas aeruginosa biofilms, quorum sensing is involved in the expression of catalase and superoxide dismutase genes. These enzymes are involved in protection against oxidative stress (Ahmed et al. 2019). Biofilm can also develop resistance by mutations and gene transfers (plasmids, transposons, or integrons) that provide cell-specific characteristic such as metabolic capabilities, virulence expression, and antimicrobial resistance (Bridier et al. 2011). Moreover, multispecies biofilms can protect against antimicrobials when present in complex communities, where interactions between species can lead to the formation of a large complex matrix, protection of members of bacterial communities, and expanded gene pool with more efficient passive resistance, quorum sensing systems, DNA sharing, metabolic cooperation, and many other synergies (Elias and Banin 2012; Wolcott et al. 2013). For instance, in vitro studies conducted on polymicrobial biofilms including Staphylococcus epidermidis and Candida albicans showed an altered susceptibility of each species to antimicrobial drugs due to their mutual interactions: the EPS of Staphylococcus epidermidis inhibited the penetration of fluconazole, while Candida albicans appeared to protect Staphylococcus epidermidis from vancomycin (Adam et al. 2002).
Microbiological hazard associated with bacterial biofilms
Bacteria are capable of colonizing and forming biofilms on almost any type of surface, including synthetic and natural surfaces (Hall-Stoodley et al. 2004; Sweet et al. 2011). Bacterial structured biofilms improve the ability of bacteria to survive under stress and cause serious problems in many sectors such as industries, water systems, medical facilities, and public health (Khelissa et al. 2017; Jamal et al. 2018; Di Pippo et al. 2018; Avila-Novoa et al. 2018). The detrimental effects of biofilms on the human society are therefore multiple.
The high capacity of bacteria to adhere and form biofilms on abiotic surfaces is a main concern for industries that provide a suitable environment for their formation (Donlan 2002; Simões et al. 2010; Flemming et al. 2013). Biofilms provoke biofouling of the industrial equipment such as cooling towers and heat exchangers. The biofouling is defined operationally as the development of biofilm that exceeds a defined threshold of interference (Murthy and Venkatesan 2009). This problem leads to energy loss, effective heat transfer, increased fluid friction resistance, and accelerated corrosion, as well as reduced the quality of product and many process additives and chemicals (Xiong and Liu 2010).
For example, the formation of biofilm in water distribution systems leads to reduced water quality and increased health risks (Dewanti and Wong 1995; Rao et al. 1998; Barak 2006). In the paper industry, biofilms cause the breakdown of chemicals such as calcium carbonate sludge and starch that are added to pulp sludge in the wet-end processing (Barak 2006).
In addition, the presence of biofilms is widespread in the food industry. They can be present on all kinds of surfaces such as plastics, glass, metal, wood, and food products (Chmielewski and Frank 2003). Microbial cell adherence to food contact surfaces is a serious concern for the food service and food processing industries, as adhesion can lead to cell survival and biofilm growth, allowing cross- and post-processing contamination. This reduces the shelf life of food products and constitutes the major factor of foodborne diseases (Shi and Zhu 2009; Bridier et al. 2015). In general, abiotic surfaces in contact with product may be cleaned many times per day, whereas environmental surfaces like walls may be cleaned weekly. This provides a longer time for adherent cells to grow on environmental supports. Thus, the extensive colonization of surfaces and the formation of mature biofilm may occur on these environmental surfaces. However, most of food product contact surfaces can hold only the adherent bacteria cells and young biofilm (Gibson et al. 1999). These adherent cells and biofilms not only pose a hygiene hazard in the food industry but also contribute to the economical costs due to technological failures, impedance of heat transfer, mechanical blockage, and metal surfaces corrosion (Houdt and Michiels 2010; Téllez 2010). Thereby, the need for efficient cleaning techniques is necessary to prevent the hazardous and expensive damage that bacterial biofilms can cause (Chmielewski and Frank 2003). Although many species of bacteria are able to form biofilms in the food industry, among the major genera of foodborne bacteria that are biofilm producers are Pseudomonas, Listeria, Enterobacter, Flavobacterium, Alcaligenes, Staphylococcus, and Bacillus (Téllez 2010). Most importantly, Pseudomonas contributes to the formation of polymicrobial biofilms with other foodborne pathogens providing them shelter for persistence (Bai et al. 2021).
In healthcare environments, biofilms can be found on several biomedical device surfaces (e.g., pacemakers, catheters, prosthetic heart valves, contact lenses, breast implants, and cerebrospinal fluid shunts) and on patient’s tissues (dead tissues, e.g., bone sequestration and living tissues, e.g., tooth surfaces, lung tissue) (Hall-Stoodley et al. 2004; Wu et al. 2015; Alav et al. 2018). Several Gram-negative and Gram-positive bacteria can form biofilms on the biomedical devices, but the most commonly found are Pseudomonas aeruginosa, Staphylococcus epidermidis, Staphylococcus aureus, and Enterococcus faecalis. It has been reported that approximately two-thirds of infections related to medical devices are attributed to staphylococcal species (Hall-Stoodley et al. 2004; Shokouhfard et al. 2015; Pakharukova et al. 2018; Khatoon et al. 2018). Pseudomonas aeruginosa can also form biofilms on the interior surfaces in hospital water distribution systems (Loveday et al. 2014). In addition, infections caused by enterococci have become of particular concern in recent years because of their ability to develop resistance against a wide range of antimicrobial drugs used in medical practice. They are also involved in serious life-threatening infections in patients suffering from cancers or chronic diseases (Boccella et al. 2021). Furthermore, the emergence of polymicrobial infections has serious implications for patient care because of the difficulties associated with selecting the most appropriate antimicrobial therapy, particularly when multidrug-resistant pathogens are implicated (Francolini and Donelli 2010). Bacteria forming biofilms can cause several life-threatening human diseases and infections such as otitis media, infective endocarditis, osteomyelitis, periodontitis, cystic fibrosis, and chronic wounds (Southey-Pillig et al. 2005; Akyıldız et al. 2013; Masters et al. 2019). It has been reported that biofilm is involved for more than 65% of all microbial infections and has a high resistance to antimicrobials and host defense system components (Jamal et al. 2018; Ciofu and Tolker-Nielsen 2019). Hence, biofilms have a considerable impact on the human healthcare.
Biofilm control
The controlling of biofilm accumulation remains the most arduous task for the many industries for which it is very important that both the inactivation and removal of biofilms from surfaces have to be realized (Simões et al. 2003; Dzianach et al. 2019).
As discussed previously, bacteria structured in biofilms are more resistant, than planktonic cells, to physical and chemical methods used in cleaning and disinfection of abiotic surfaces (Martin and Feng 2009). Several methods and strategies can be used to control biofilm such as chemical treatment, mechanical removal, quorum sensing inhibition, nanotechnological method, enzymatic dispersion, biosurfactants, and biosourced compounds such as essential oils derived from plants, bacteriocins, and bacteriophages (Fig. 3).
Different methods for biofilm control, quorum sensing (QS), and reactive oxygen species (ROS) (de Carvalho 2007; García-Almendárez et al. 2008; Winkelströter et al. 2011, 2015; Torres et al. 2011; Bayoumi et al. 2012; Beyth et al. 2015; Silva 2015; Nobrega et al. 2015; Chopra et al. 2015; Scholtz et al. 2015; Coughlan et al. 2016; Gutiérrez et al. 2016; Coronel-León et al. 2016; Campana et al. 2017; Nica et al. 2017; Castellano et al. 2017)
It is necessary to first understand the difference between disinfectants and sanitizers used in the industry. Disinfection means irreversibly destroying or inactivating specific infectious fungi and bacteria, but not necessarily spores, on hard surfaces. However, sanitizing means reducing microorganisms to levels considered safe for humans (Allan Pfuntner 2012).
Several chemical disinfectants can be used to treat biofilms such as NaOCl, peracetic acid, NaOH, and H2O2. The efficiency of these disinfectants is related to their oxidation of cellular structures (Rosenberg et al. 2008; Bayoumi et al. 2012; Nam et al. 2014; Bang et al. 2014; Ban and Kang 2016; Møretrø et al. 2017; Yang et al. 2017; Alvarez-Ordóñez et al. 2019). However, previous studies have indicated that most of these disinfectants have little or no significant effect on the removal of established biofilms (Walker et al. 2007). It has been reported that disinfection with chlorine and chlorine dioxide can decrease the concentration of planktonic cells, but has no effect on biofilm biomass (Berry et al. 2006). Other studies show that treatment with sodium hypochlorite, the main commercial disinfectant does not significantly reduce the biomass of biofilms formed by Escherichia coli on the stainless steel surface (Lim et al. 2019). Chlorine is known as the most widespread artificial chemical disinfectant used because of its broad antimicrobial spectrum, easiness of application, and cost-effectiveness. Nevertheless, it is rapidly inactivated by organic matter. Moreover, chlorine activity is pH dependent and exhibits corrosion even to stainless steel and may combine with organic compounds to form toxic by-products (Chmielewski and Frank 2003; Guzel-Seydim et al. 2004; Houdt and Michiels 2010). In addition, it is important to note that the use of these sanitizers for decades could be one of the main causes of the development of antibiotic resistance in bacteria and their spread to pathogens (Capita and Alonso-Calleja 2013). These issues combined with the growing consumer concerns about their own health and environmental consciousness are leading to setup new alternative strategies to control biofilm such as the use of biosourced active molecules such as biosourced enzymes and essential oils (Knowles et al. 2005; Desai et al. 2012).
Enzymatic disruption
The use of enzymes is an effective tool for eradicating biofilm owing to its ability to degrade the physical integrity of the EPS by binding and breaking down the components of the EPS into smaller units that can be transferred across cell membranes and then metabolized, thus destroying the multi-structural biofilm (Xavier et al. 2005; Mohamed et al. 2018). By a pre-treatment using enzymes, the biocides can be substituted or their concentration can be considerably reduced since the enzymatic effect on the EPS matrix promotes the access of the chemical biocides to the cells (Meireles et al. 2016). A wide range of enzyme applications have been described (Table 2) with the aim of reducing microbiological biofilm risk and replacing hazardous and ineffective chemical biocides, as well as providing an alternative green solution against biofilm formation due to their high biodegradability and low toxicity (Cortés et al. 2011; Srey et al. 2013). These characteristics make enzymes as a high-performance tool for controlling biofilm; thus, they are commonly used in detergents used in many industries (Torres et al. 2011; Huang et al. 2014).
Nevertheless, the enzyme effectiveness in eradicating and destroying biofilm is highly dependent on the composition of the matrix (Walker et al. 2007). Due to the heterogeneous composition of this matrix, different types of enzymes can be used to destroy biofilms. These enzymes can be applied individually or in combination with a complementary treatment (Meireles et al. 2016). There are currently four types of enzymes of potential interest in biofilm removal: polysaccharide degrading enzymes, proteolytic enzymes, anti-QS, and oxidizing enzymes that belong to three main classes, hydrolase, lyases, and oxydoreductases (Boels 2011; Thallinger et al. 2013; Huang et al. 2014; Coughlan et al. 2016; Meireles et al. 2016).
Essential oils (EOs) as antibiofilm compounds
Essential oils (EOs) are volatile and aromatic liquids derived from plants. These compounds can be composed of complex mixtures and of low-weight molecules, whose typical main components are dependent on the plant source (Engel et al. 2017). The biological activities of EOs and their components are largely recognized, including antimicrobial activities against bacteria, yeasts, and molds (Burt 2004; Reyes-Jurado et al. 2015; Calo et al. 2015).
EOs or their purified antimicrobial components are natural alternative biocides that have recently attracted attention as potential cleaners for the following reasons. (i) Many studies suggest that the chemical antimicrobial agents, currently used, trigger the development of antimicrobial resistance in the target microorganisms (Boakye et al. 2019). However, the development of bacterial resistance to EOs is limited because each EO is composed of a mixture of various active antimicrobial agents (Wińska et al. 2019). (ii) The vapors emitted by EOs are highly bactericidal that can offer an additional advantage for the disinfection of hard-to-reach areas that need to be cleaned (López et al. 2005, 2007). (iii) Due to the ongoing trend towards green technology and changing consumer attitudes, there is a commercial advantage in antimicrobial agents that can be classified as “green,” such as EOs of plant origin (Soni et al. 2013).
The antimicrobial activity of EOs is caused mainly by their hydrophobic characteristic, which helps them to disperse into bacterial cell membrane lipids, causing disruption of the structure and increasing its permeability. This can lead to leakage of ions and other cell molecules and then lead to cell death (Rao et al. 2019). In general, EOs are slightly more effective against Gram-positive bacteria than Gram-negative ones (Ratledge and Wilkinson 1988; Davidson and Naidu 2000; Canillac and Mourey 2001; Cimanga et al. 2002; Delaquis et al. 2002). Gram-negative bacteria can be expected to be less sensitive to the action of EOs since they possess a lipopolysaccharide-coated outer membrane that surrounds the cell wall and limits the diffusion of hydrophobic compounds (Ratledge and Wilkinson 1988; Burt 2004). Nevertheless, not all research on antimicrobial activity of EOs has shown that Gram-positive bacteria are more susceptible (Wilkinson et al. 2003). Furthermore, the antimicrobial activity of EOs is linked to their interactions, chemical composition, and the volatile molecules proportions (Dhifi et al. 2016).
EOs extracted from different plants can be used for their antibacterial effect such as monoterpenoids (such as borneol, camphor, carvacrol, eucalyptol, limonene, pinene, thujone), sesquiterpenoids (such as caryophyllene, humulene), and flavonoids (such as cinnamaldehyde and other phenolic acids) (Campana et al. 2017). In general, EOs with a high level of phenolic compounds, such as eugenol, carvacrol, and thymol, have significant antibacterial activities. These components are primarily responsible for disruption of the cytoplasmic membrane, electron flow, active transport, proton motive force, and coagulation of the cell contents (Dhifi et al. 2016). Numerous studies prove the antimicrobial activity of EOs against one or more microorganisms (Sivropoulou et al. 1996; Lambert and Johnston 2001; Ooi et al. 2006; Rota et al. 2008; Xu et al. 2008). Moreover, EOs have been shown to be highly effective against the most serious foodborne pathogens such as Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella spp. (Braga et al. 2006). In addition, several studies (Table 3) demonstrate that essential oils have a significant antimicrobial activity against biofilm and biofilm formation (Soni et al. 2013; Neyret et al. 2014; Amaral et al. 2015).
Hurdle technology as an efficient strategy to control biofilms
Due to the complexity of biofilms, a single use of a disinfectant may be insufficient to remove the entire undesirable biofilm. Hurdle technology involves the combined intelligent use of hurdles such as physical–chemical, chemical-chemical, or biological–chemical disinfection methods to achieve effective control of undesirable monomicrobial and polymicrobial biofilms by striking different targets within bacterial cells at the same time (Fig. 4) (Yuan et al. 2021). The synergistic effect of hurdle technology in reducing biofilm contamination has been proven by numerous studies (Ban and Kang 2016; Jung et al. 2018; KIM et al. 2019; Lim et al. 2019; Hussain et al. 2019; Venkatesh et al. 2009; Francolini and Donelli 2010).
In healthcare and food ecosystems, disinfection must be carried out economically and safely, by reducing the frequency of disinfection, and in the shortest timeframe possible, with the lowest use of chemicals, labor costs, and energy, producing the least amount of waste and without damaging the equipment. Thus, Hurdle technology could be more effective in controlling biofilms compared to the single use of disinfectants. Potential solutions for combined disinfection procedures must therefore be carefully selected to achieve an effective disinfection effect.
Previous studies have shown that the combined use of physical and chemical disinfection strategies is very efficient against biofilms (Vankerckhoven et al. 2011; Kim et al. 2016; Jung et al. 2018). Indeed, the combined treatment of biofilms with ultraviolet irradiation (234 mJ/cm2) and hydrogen peroxide (5 ppm) proved to be 10 times more effective than treatment with hydrogen peroxide alone, which could lead to a more eco-friendly treatment (Vankerckhoven et al. 2011). In addition, treatment with biocide solutions that contain more than one bioactive agent was also found to be effective in removing biofilms from industrial surfaces (Ortega Morente et al. 2013). It has been also reported that the combination of different disinfectant compounds can facilitate their diffusion into the biofilm matrix and improve their oxidative activity, resulting in high bactericidal activity even at low concentrations (Ríos-Castillo et al. 2017). Dhowlaghar et al. (2018) demonstrated that the use of a mixture of hydrogen peroxide and quaternary ammonium disinfectants or hydrogen peroxide, octanoic acid, and peracetic acid was able to completely remove Listeria monocytogenes biofilm from stainless steel surface, whereas treatment with a single active component in the disinfection procedure could not eliminate biofilm cells completely. In addition, the combined use of EDTA, ethanol, N-acetylcysteine, and recombinant human talactoferrin with amphotericin B, fluconazole, nafcillin, and vancomycin has been successfully applied as catheter lock solutions to rescue colonized catheters. It was found that these combinations were effective in inhibiting both monomicrobial and polymicrobial biofilms of Staphylococcus epidermidis and Candida albicans (Venkatesh et al. 2009).
The use of enzymes for the removal of biofilms in the industrial settings generally misses biocidal activity, making them unsuitable for bactericidal applications. To solve this problem, a combined use of enzymatic and antibacterial control approaches is desirable, as the action of the enzyme would contribute positively to the antibacterial activity of the disinfectant (Table 4). This strategy has the potential advantage of avoiding the overuse of toxic antimicrobial agents.
Many studies demonstrate that the antimicrobial agent use after enzymatic treatment can significantly inactivate microbial cells in biofilms (Table 4). EOs are biosourced compounds used as alternative natural disinfectants suitable for biofilm control. In addition, it has been reported an increased activity of these natural antimicrobials to inactivate biofilms when combined with other methods (Table 4). Thus, the use of enzymes in combination with an EO as biological hurdles seems to be a promising strategy to control biofilms. The goal of this strategy is that enzymes disrupt and destroy the biofilm matrix, so that the biosourced antimicrobials can hit the target efficiently and easily.
Encapsulation as a tool to improve antibiofilm compound activities
Microencapsulation aims in protecting the bioactivity of solid, liquid, or gaseous materials by trapping within a surrounding matrix forming particles with a diameter of 1 to 1000 μm (Fu and Hu 2017). Microparticles can be in the form of microspheres or microcapsules. Microspheres are matrix systems in which the core is dispersed (heterogeneous microsphere) and/or dissolved in a polymer matrix (homogenous microsphere) (Silva et al. 2003), while microcapsules are particles consisting of an inner core surrounded by a material that is significantly different from that of the core. Mononuclear and polynuclear microcapsules can be classified according to the division of the core or not. However, the terms microcapsules and microspheres can be used synonymously (Singh et al. 2010).
Coating materials for microencapsulation
The coating serves as a protective film for isolating the core from inadequate exposure; the core can be released in the ideal place or at the ideal time, in various manners depending on the characteristics of the coatings material, such as physical pressure, friction, diffusion, dissolution of the wall, and biodegradation (Suave et al. 2006; Qin 2016). The appropriate selection of wall material is extremely critical as it has a significant impact on the effectiveness and stability of the microcapsule. The most appropriate wall material should have the same properties: controlled release under specific conditions; nonreactive with the core; capacity to hold and stabilize the core inside the capsule; ability to protect the core from unfavorable conditions; absence of disagreeable taste in case of food application; and economic feasibility (Nazzaro et al. 2012; Gharsallaoui et al. 2012). A variety of coating materials can be used in microencapsulation such as synthetic polymers such as nonbiodegradable polymers (e.g., poly methyl methacrylate (PMMA), acrolein, glycidyl methacrylate epoxy polymers) (Kreuter et al. 1983; Margel and Wiesel 1984) and biodegradable polymers (e.g., lactides, glycolides, and their copolymers) (Wakiyama et al. 1981); and natural polymers such as proteins (e.g., albumin, gelatin, collagen) (Toshio et al. 1981), carbohydrates (e.g., agarose, carrageenan, chitosan, starch) (Patel et al. 2011), and chemically modified carbohydrates (e.g., poly dextran, poly starch) (Jain 2000).
Control of the release of encapsulated molecules
Encapsulation should protect and isolate the core from the environment until the desired release at the appropriate time and place (Gouin 2004). Many factors affect the rate of releasing including the interactions between wall materiel and core, the volatility of core, the ratio of core to support material, the size, and viscosity of particle of wall material, among others (Roberts and Taylor 2000). The release of the core is conditioned by several mechanisms involved: degradation (enzymes such as lipases and proteases that degrade lipids and proteins, respectively) (Rosen 2005), diffusion (chemical properties of core and wall material and physical properties of wall determine the releasing of core from intact wall) (Choudhury et al. 2021), use of solvent (contact with solvent that dissolve wall material) (Frascareli et al. 2012), pH (solubility of membrane wall altered by changes of pH) (Toldrá and Reig 2011), pressure (applied pressure to the capsule wall cause releasing) (Wong et al. 2009), and temperature (expanding or collapsing of wall material in a critical temperature which name temperature-sensitive release or melting of wall material when the temperature increase which name fusion-activated release) (Park and Maga 2006). Moreover, the combination of two or more mechanisms can be used (Desai and Park 2005).
Microencapsulation methods
Many encapsulation methods such as spray drying, extrusion, and coacervation are currently used for the encapsulation of the antimicrobial substances (Fig. 5). The choice of the most appropriate method depends on the capsule application, the type of core, the chemical and physical characteristics of the core and wall, the particle size required, the mechanism of release required, the scale of production, and the cost (Suave et al. 2006). The spray drying technique is the most common encapsulation method that has been used for decades to encapsulate mainly flavors, lipids, and pigments (Gharsallaoui et al., 2007). Several applications of spray drying in industrial field range from encapsulation of fragrances and flavors in food industries to pigments in manufacture (Laohasongkram et al. 2011). In addition, this technique is widely used for the encapsulation of enzymes and antimicrobials substances such as EOs to ensure their activities (Schutyser et al. 2012; Dajic Stevanovic et al. 2020). However, the optimal choice of drying conditions and adapted matrix formulations is necessary to avoid serious thermal damage leading to a loss of enzymatic activity or volatility of the essential oil (Gharsallaoui et al. 2007; Schutyser et al. 2012). Spray drying is a relatively inexpensive and commercially feasible method of microencapsulation. Biomolecules used as carriers for this technique are starch, maltodextrins, chitosan, and gum Arabic (Dajic Stevanovic et al. 2020). Spray drying microencapsulation involves 4 steps as shown in Fig. 6: (1) preparation of the emulsion, (2) homogenization of the emulsion, (3) atomization of the dispersion, and (4) dehydration of the atomized particles (Bakry et al. 2016). This process consists of forming an emulsion, suspension, or solution containing the wall material and core; then pulverization in a drying chamber in which circulates hot air, upon contact with the hot air, the water evaporates immediately, and the core is encapsulated into the wall material (Laohasongkram et al. 2011).
Role of microencapsulation in biofilm control
Microencapsulation is widely used in the fields of medicine, food, cosmetics, pharmaceuticals, textiles, agriculture, and advanced materials, which make this technique widely used in the encapsulation of active constituents: enzymes, EOs, flavors, colors, sweeteners, microorganisms, etc. (Desai and Park 2005; Fu and Hu 2017). One of the recent use of microencapsulation is the control of biofilm on industrial equipment and materials and by the encapsulation of antimicrobial substances to ensure their stability and long-term activity, as well as to limit their interactions with the biofilm matrix components (Khelissa et al. 2021a, b).
As mentioned earlier, the use of enzymes is an important tool to remove biofilms through the enzyme ability to degrade the EPS and destroy biofilms (Xavier et al. 2005). Thus, extensive researches have been conducted to immobilize enzymes in mechanically resistant capsules, in most cases in dry microcapsules, in order to protect enzymes during storage and to control their release (Mohamad et al. 2015). Microencapsulation is a promising strategy to prevent and stabilize enzymes under severe reaction conditions from denaturation by proteolysis and dilution effects, and thus maintain high catalytic activity (Chaize et al. 2004; Tetter and Hilvert 2017; Zdarta et al. 2018). Orgaz et al. (2007) demonstrated that the combination of delayed-release encapsulated pronase with cellulase, pectin lyase, or esterase leads to three to four decimal reduction in cells and detach up to 90% of the biofilm of Pseudomonas fluorescens after 2 h at 25 °C. These data prove that these results are more favorable than those obtained with the same application of the equivalent soluble enzyme mixtures. Tan et al. (2020) show that the co-immobilization of deoxyribonuclease I (DNase) and cellobiose dehydrogenase (CDH) results in a bifunctional particle that targets both the microorganisms and the biofilm matrix. The assessment of the antibiofilm activities of these particles has shown a high ability to penetrate through the biofilm matrix and interfere with microbial cells, thus exhibiting a stronger activity to inhibit biofilm formation as well as to disrupt preformed biofilms.
Furthermore, the use of EOs to control biofilm has been extensively studied in recent years; a wide variety of EOs can be used as antibiofilm compounds. However, EOs are not stable and can be degraded in the presence of oxygen, light, and temperature. Thus, efforts have been attempted to protect them by encapsulation in various colloidal systems such as microspheres, microcapsules, liposomes, and nanoemulsions (Sherry et al. 2013). Many studies show that protecting the EOs with antimicrobial activity in a capsule can increase their bioactivity and efficiency to remove biofilm from surfaces (Dohare et al. 2014; Duncan et al. 2015; Cui et al. 2016a), as well as decreasing volatility and improving stability and water solubility (Bilia et al. 2014). For example, peppermint oil encapsulated in starch-based emulsions showed increased stability and bioavailability characteristics and improved activity against Staphylococcus aureus and Listeria monocytogenes relative to free EOs (Liang et al. 2012). The antibacterial activity of EOs after nanoencapsulation has been shown to very often exceed the efficacy of the current antibiotic (Zaman et al. 2017). Dohare et al. (2014) reported that the encapsulation of Eucalyptus globulus oil has increased its antibiofilm activity against Escherichia coli biofilm from 62 to 81% compared to the soluble one; thus, the use Eucalyptus globulus oil encapsulated into a nanoparticle is important for controlling biofilm associated with microbial infections and diseases. Other studies showed that the two antimicrobials, carvacrol and eugenol, encapsulated in micellar nonionic surfactant solutions, were significantly effective against two strains of E. coli O157:H7, reducing viable counts by 3.5 to 4.8 log CFU/cm2 within 20 min of exposure (Pérez-Conesa et al. 2011). Furthermore, Cui et al. (2016a, b) evaluated the anti-biofilm effect of cinnamon oil, encapsulated in liposomes, on methicillin-resistant Staphylococcus aureus (MRSA) biofilms. The results showed that the use of liposomes improves the stability of cinnamon oil, which has an effective antibacterial performance on MRSA and its biofilms and prolongs the time of action.
Conclusion
The prevention/eradication of biofilms in the industrial and medical sectors is one of their main concerns. This field can provide an appropriate environment for the development of biofilms that threaten public health and increase economic losses. A clear understanding of the mechanisms of biofilm formation and resistance to disinfecting agents is necessary to provide an effective strategy to prevent and destroy biofilms. Biofilm resistance to disinfectants appears to be multifactorial and involves several parameters. In addition, the side effects caused by these agents require the search for alternative natural antimicrobial agents to obtain the requested treatment and overcome the disadvantage of the conventional antimicrobial used. The biofilm matrix is the main physical barrier preventing the penetration of biocides into biofilms. Therefore, if one or more compounds capable of destroying the structural components of the matrix produced by biofilm as well as active against the microbial biofilm are found, then the “microbe city” (biofilm) would be permanently destroyed and eradicated. However, the activity and stability of the anti-biofilm agents used may be affected by several parameters. On this basis, the encapsulation of these compounds can be useful to protect and ensure their stability and activities against matrix biofilm as well as biofilm-producing microorganisms, in order to prevent the formation and/or eradicate the establishing biofilms.
References
Abdallah M, Benoliel C, Drider D, Dhulster P, Chihib N-E (2014) Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch Microbiol 196:453–472. https://doi.org/10.1007/s00203-014-0983-1
Adam B, Baillie GS, Douglas LJ (2002) Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J Med Microbiol 51:344–349. https://doi.org/10.1099/0022-1317-51-4-344
Ahmed MN, Porse A, Abdelsamad A, Sommer M, Høiby N, Ciofu O (2019) Lack of the major multifunctional catalase KatA in P. aeruginosa accelerates evolution of antibiotic resistance in ciprofloxacin-treated biofilms. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.00766-19
Akyıldız İ, Take G, Uygur K, Kızıl Y, Aydil U (2013) Bacterial biofilm formation in the middle-ear mucosa of chronic otitis media patients. Indian J Otolaryngol Head Neck Surg 65:557–561. https://doi.org/10.1007/s12070-012-0513-x
Al Kassaa I, Mechemchani S, Zaylaa M, Ismail MB, El Omari K, Dabboussi F, Hamze M (2019) Characterization of lactobacilli strains isolated from baby’s feces for their potential immunobiotic application. Iran J Microbiol 11:379–388
al Kassaa I, Mechmchani S, Zaylaa M, Ismail MB, El Omari K, Dabboussi F, Hamze M (2021) Enterococcus faecium CMUL1216 an immunobiotic strain with a potential application in animal sector. Biocontrol Sci 26:75–84. https://doi.org/10.4265/bio.26.75
Alav I, Sutton JM, Rahman KM (2018) Role of bacterial efflux pumps in biofilm formation. J Antimicrob Chemother 73:2003–2020. https://doi.org/10.1093/jac/dky042
Allan Pfuntner MA (2012) Sanitizers and disinfectants: the chemicals of prevention. In: Food Saf. Mag. https://www.foodsafetymagazine.com/magazine-archive1/augustseptember-2011/sanitizers-and-disinfectants-the-chemicals-of-prevention/. Accessed 18 Jun 2019
Alvarez-Ordóñez A, Coughlan LM, Briandet R, Cotter PD (2019) Biofilms in food processing environments: challenges and opportunities. Annu Rev Food Sci Technol 10
Amaral VCS, Santos PR, da Silva AF, dos Santos AR, Machinski M, Mikcha JMG (2015) Effect of carvacrol and thymol on Salmonella spp. biofilms on polypropylene. Int J Food Sci Technol 50:2639–2643. https://doi.org/10.1111/ijfs.12934
Avila-Novoa M-G, Iñíguez-Moreno M, Solís-Velázquez O-A, González-Gómez J-P, Guerrero-Medina P-J, Gutiérrez-Lomelí M (2018) Biofilm Formation by Staphylococcus aureus isolated from food contact surfaces in the dairy industry of Jalisco, Mexico. In: J. Food Qual. https://www.hindawi.com/journals/jfq/2018/1746139/. Accessed 6 Aug 2020
Bai X, Nakatsu CH, Bhunia AK (2021) Bacterial biofilms and their implications in pathogenesis and food safety. Foods 10:2117. https://doi.org/10.3390/foods10092117
Bakry AM, Abbas S, Ali B, Majeed H, Abouelwafa MY, Mousa A, Liang L (2016) Microencapsulation of oils: a comprehensive review of benefits, techniques, and applications. Compr Rev Food Sci Food Saf 15:143–182. https://doi.org/10.1111/1541-4337.12179
Ban G-H, Kang D-H (2016) Effect of sanitizer combined with steam heating on the inactivation of foodborne pathogens in a biofilm on stainless steel. Food Microbiol 55:47–54
Banach JL, Sampers I, Van Haute S, van der Fels-Klerx HJI (2015) Effect of disinfectants on preventing the cross-contamination of pathogens in fresh produce washing water. Int J Environ Res Public Health 12:8658–8677. https://doi.org/10.3390/ijerph120808658
Banar M, Emaneini M, Satarzadeh M, Abdellahi N, Beigverdi R, van Leeuwen WB, Jabalameli F (2016) Evaluation of mannosidase and trypsin enzymes effects on biofilm production of Pseudomonas aeruginosa isolated from burn wound infections. PLoS ONE 11:e0164622. https://doi.org/10.1371/journal.pone.0164622
Bang J, Hong A, Kim H, Beuchat LR, Rhee MS, Kim Y, Ryu J-H (2014) Inactivation of Escherichia coli O157:H7 in biofilm on food-contact surfaces by sequential treatments of aqueous chlorine dioxide and drying. Int J Food Microbiol 191:129–134. https://doi.org/10.1016/j.ijfoodmicro.2014.09.014
Barak A (2006) Control of development of biofilms in industrial process water
Bayoumi MA, Kamal RM, Abd El Aal SF, Awad EI (2012) Assessment of a regulatory sanitization process in Egyptian dairy plants in regard to the adherence of some food-borne pathogens and their biofilms. Int J Food Microbiol 158:225–231. https://doi.org/10.1016/j.ijfoodmicro.2012.07.021
Berry D, Xi C, Raskin L (2006) Microbial ecology of drinking water distribution systems. Curr Opin Biotechnol 17:297–302. https://doi.org/10.1016/j.copbio.2006.05.007
Bester E, Kroukamp O, Hausner M, Edwards EA, Wolfaardt GM (2011) Biofilm form and function: carbon availability affects biofilm architecture, metabolic activity and planktonic cell yield. J Appl Microbiol 110:387–398. https://doi.org/10.1111/j.1365-2672.2010.04894.x
Beyth N, Houri-Haddad Y, Domb A, Khan W, Hazan R (2015) Alternative antimicrobial approach: nano-antimicrobial materials. In: Evid. Based Complement. Alternat. Med. https://www.hindawi.com/journals/ecam/2015/246012/abs/. Accessed 19 Jun 2019
Bilia AR, Guccione C, Isacchi B, Righeschi C, Firenzuoli F, Bergonzi MC (2014) Essential oils loaded in nanosystems: a developing strategy for a successful therapeutic approach. Evid-Based Complement Altern Med ECAM 2014.https://doi.org/10.1155/2014/651593
Boakye Y, Osafo N, Amaning Danquah C, Adu F, Agyare C (2019) Antimicrobial agents: antibacterial agents, anti-biofilm agents, antibacterial natural compounds, and antibacterial chemicals. pp 1–24
Boccella M, Santella B, Pagliano P, De Filippis A, Casolaro V, Galdiero M, Borrelli A, Capunzo M, Boccia G, Franci G (2021) Prevalence and antimicrobial resistance of enterococcus species: a retrospective cohort study in Italy. Antibiotics 10:1552. https://doi.org/10.3390/antibiotics10121552
Boels G (2011) Enzymatic removal of biofilms: a report. Virulence 2:490–489. https://doi.org/10.4161/viru.2.5.17317
Bowen WH, Burne RA, Wu H, Koo H (2018) Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol 26:229–242. https://doi.org/10.1016/j.tim.2017.09.008
Braga PC, Dal Sasso M, Culici M, Galastri L, Marceca MT, Guffanti EE (2006) Antioxidant potential of thymol determined by chemiluminescence inhibition in human neutrophils and cell-free systems. Pharmacology 76:61–68. https://doi.org/10.1159/000089719
Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F (2011) Resistance of bacterial biofilms to disinfectants: a review. Biofouling 27:1017–1032. https://doi.org/10.1080/08927014.2011.626899
Bridier A, Sanchez-Vizuete P, Guilbaud M, Piard J-C, Naïtali M, Briandet R (2015) Biofilm-associated persistence of food-borne pathogens. Food Microbiol 45:167–178. https://doi.org/10.1016/j.fm.2014.04.015
Brindle ER, Miller DA, Stewart PS (2011) Hydrodynamic deformation and removal of Staphylococcus epidermidis biofilms treated with urea, chlorhexidine, iron chloride, or DispersinB. Biotechnol Bioeng 108:2968–2977. https://doi.org/10.1002/bit.23245
Bundalovic-Torma C, Whitfield GB, Marmont LS, Howell PL, Parkinson J (2020) A systematic pipeline for classifying bacterial operons reveals the evolutionary landscape of biofilm machineries. PLoS Comput Biol 16.https://doi.org/10.1371/journal.pcbi.1007721
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022
Calo JR, Crandall PG, O’Bryan CA, Ricke SC (2015) Essential oils as antimicrobials in food systems – a review. Food Control 54:111–119. https://doi.org/10.1016/j.foodcont.2014.12.040
Campana R, Casettari L, Fagioli L, Cespi M, Bonacucina G, Baffone W (2017) Activity of essential oil-based microemulsions against Staphylococcus aureus biofilms developed on stainless steel surface in different culture media and growth conditions. Int J Food Microbiol 241:132–140. https://doi.org/10.1016/j.ijfoodmicro.2016.10.021
Canillac N, Mourey A (2001) Antibacterial activity of the essential oil of Picea excelsa on Listeria, Staphylococcus aureus and coliform bacteria. Food Microbiol 18:261–268. https://doi.org/10.1006/fmic.2000.0397
Cao B, Shi L, Brown RN, Xiong Y, Fredrickson JK, Romine MF, Marshall MJ, Lipton MS, Beyenal H (2011) Extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms: characterization by infrared spectroscopy and proteomics. Environ Microbiol 13:1018–1031. https://doi.org/10.1111/j.1462-2920.2010.02407.x
Capita R, Alonso-Calleja C (2013) Antibiotic-resistant bacteria: a challenge for the food industry. Crit Rev Food Sci Nutr 53:11–48. https://doi.org/10.1080/10408398.2010.519837
Castellano P, Pérez Ibarreche M, Blanco Massani M, Fontana C, Vignolo GM (2017) Strategies for pathogen biocontrol using lactic acid bacteria and their metabolites: a focus on meat ecosystems and industrial environments. Microorganisms 5.https://doi.org/10.3390/microorganisms5030038
Centers for Disease Control and Prevention (CDC) (2019) surveillance for foodborne disease outbreaks, United States, annual report. Department of Health and Human Services. CDC, Atlanta, Georgia: U.S.
Chaize B, Colletier J-P, Winterhalter M, Fournier D (2004) Encapsulation of enzymes in liposomes: high encapsulation efficiency and control of substrate permeability. Artif Cells Blood Substit Biotechnol 32:67–75. https://doi.org/10.1081/BIO-120028669
Chakraborty P, Kumar A (2019) The extracellular matrix of mycobacterial biofilms: could we shorten the treatment of mycobacterial infections? Microb Cell 6:105–122. https://doi.org/10.15698/mic2019.02.667
Chmielewski RAN, Frank JF (2003) Biofilm formation and control in food processing facilities. Compr Rev Food Sci Food Saf 2:22–32. https://doi.org/10.1111/j.1541-4337.2003.tb00012.x
Chopra L, Singh G, Kumar Jena K, Sahoo DK (2015) Sonorensin: a new bacteriocin with potential of an anti-biofilm agent and a food biopreservative. Sci Rep 5:13412. https://doi.org/10.1038/srep13412
Choudhury N, Meghwal M, Das K (2021) Microencapsulation: an overview on concepts, methods, properties and applications in foods. Food Front 2:426–442. https://doi.org/10.1002/fft2.94
Cimanga K, Kambu K, Tona L, Apers S, De Bruyne T, Hermans N, Totté J, Pieters L, Vlietinck AJ (2002) Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J Ethnopharmacol 79:213–220. https://doi.org/10.1016/s0378-8741(01)00384-1
Ciofu O, Tolker-Nielsen T (2019) Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—how P. aeruginosa can escape antibiotics. Front Microbiol 10:913. https://doi.org/10.3389/fmicb.2019.00913
Cochet F, Peri F (2017) The role of carbohydrates in the lipopolysaccharide (lps)/toll-like receptor 4 (tlr4) signalling. Int J Mol Sci 18.https://doi.org/10.3390/ijms18112318
Coronel-León J, Marqués AM, Bastida J, Manresa A (2016) Optimizing the production of the biosurfactant lichenysin and its application in biofilm control. J Appl Microbiol 120:99–111. https://doi.org/10.1111/jam.12992
Cortés ME, Bonilla JC, Sinisterra RD (2011) Biofilm formation, control and novel strategies for eradication. 11
Coughlan LM, Cotter PD, Hill C, Alvarez-Ordóñez A (2016) New weapons to fight old enemies: novel strategies for the (bio)control of bacterial biofilms in the food industry. Front Microbiol 7.https://doi.org/10.3389/fmicb.2016.01641
Craigen B, Dashiff A, Kadouri DE (2011) The use of commercially available alpha-amylase compounds to inhibit and remove Staphylococcus aureus biofilms. Open Microbiol J 5:21–31. https://doi.org/10.2174/1874285801105010021
Cui H, Li W, Li C, Vittayapadung S, Lin L (2016a) Liposome containing cinnamon oil with antibacterial activity against methicillin-resistant Staphylococcus aureus biofilm. Biofouling 32:215–225. https://doi.org/10.1080/08927014.2015.1134516
Cui H, Zhou H, Lin L (2016b) The specific antibacterial effect of the Salvia oil nanoliposomes against Staphylococcus aureus biofilms on milk container.https://doi.org/10.1016/J.FOODCONT.2015.09.034
Dajic Stevanovic Z, Sieniawska E, Glowniak K, Obradovic N, Pajic-Lijakovic I (2020) Natural macromolecules as carriers for essential oils: from extraction to biomedical application. Front Bioeng Biotechnol 8.https://doi.org/10.3389/fbioe.2020.00563
Davidson PM, Naidu AS (2000) Natural food antimicrobial systems. In: Phytophenols. CRC Press, pp 265–293
de Carvalho CCCR (2007) Biofilms: recent developments on an old battle. Recent Pat Biotechnol 1:49–57. https://doi.org/10.2174/187220807779813965
de Carvalho CCCR (2017) Biofilms: microbial strategies for surviving uv exposure. In: Ahmad SI (ed) Ultraviolet light in human health, diseases and environment. Springer International Publishing, Cham, pp 233–239
Delaquis PJ, Stanich K, Girard B, Mazza G (2002) Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int J Food Microbiol 74:101–109. https://doi.org/10.1016/s0168-1605(01)00734-6
Desai KGH, Park HJ (2005) Recent developments in microencapsulation of food ingredients. Dry Technol 23:1361–1394. https://doi.org/10.1081/DRT-200063478
Desai MA, Soni KA, Nannapaneni R, Schilling MW, Silva JL (2012) Reduction of Listeria monocytogenes biofilms on stainless steel and polystyrene surfaces by essential oils. J Food Prot 75:1332–1337. https://doi.org/10.4315/0362-028X.JFP-11-517
Devaraj A, Buzzo JR, Mashburn-Warren L, Gloag ES, Novotny LA, Stoodley P, Bakaletz LO, Goodman SD (2019) The extracellular DNA lattice of bacterial biofilms is structurally related to Holliday junction recombination intermediates. Proc Natl Acad Sci 116:25068–25077. https://doi.org/10.1073/pnas.1909017116
Dewanti R, Wong AC (1995) Influence of culture conditions on biofilm formation by Escherichia coli O157:H7. Int J Food Microbiol 26:147–164. https://doi.org/10.1016/0168-1605(94)00103-d
Dewasthale S, Mani I, Vasdev K (2018) Microbial biofilm: current challenges in health care industry. J Appl Biotechnol Bioeng 5. https://doi.org/10.15406/jabb.2018.05.00132
Dhifi W, Bellili S, Jazi S, Bahloul N, Mnif W (2016) Essential oils’ chemical characterization and investigation of some biological activities: a critical review. Medicines 3.https://doi.org/10.3390/medicines3040025
Dhowlaghar N, Abeysundara PDA, Nannapaneni R, Schilling MW, Chang S, Cheng W-H, Sharma CS (2018) Growth and biofilm formation by Listeria monocytogenes in catfish mucus extract on four food contact surfaces at 22 and 10°c and their reduction by commercial disinfectants. J Food Prot 81:59–67. https://doi.org/10.4315/0362-028X.JFP-17-103
Di Pippo F, Di Gregorio L, Congestri R, Tandoi V, Rossetti S (2018) Biofilm growth and control in cooling water industrial systems.FEMS Microbiol Ecol 94.https://doi.org/10.1093/femsec/fiy044
Dohare S, Dubey SD, Kalia M, Verma P, Pandey HS, Nand, Singh KS, Agarwal VP (2014) Anti-biofilm activity of eucalyptus globulus oil encapsulated silica nanoparticles against E . coli biofilm
Dolçà C, Ferrándiz M, Capablanca L, Franco E, Mira E, López F, García D (2015) Microencapsulation of rosemary essential oil by co-extrusion/gelling using alginate as a wall material. J Encapsulation Adsorpt Sci 05:121–130. https://doi.org/10.4236/jeas.2015.53010
Dongari-Bagtzoglou A (2008) Pathogenesis of mucosal biofilm infections: challenges and progress. Expert Rev Anti Infect Ther 6:201–208. https://doi.org/10.1586/14787210.6.2.201
Donlan RM (2001) Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 33:1387–1392. https://doi.org/10.1086/322972
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890. https://doi.org/10.3201/eid0809.020063
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193. https://doi.org/10.1128/CMR.15.2.167-193.2002
Dragoš A, Kovács ÁT (2017) The peculiar functions of the bacterial extracellular matrix. Trends Microbiol 25:257–266. https://doi.org/10.1016/j.tim.2016.12.010
Duncan B, Li X, Landis RF, Kim ST, Gupta A, Wang L-S, Ramanathan R, Tang R, Boerth JA, Rotello VM (2015) Nanoparticle-stabilized capsules for the treatment of bacterial biofilms. ACS Nano 9:7775–7782. https://doi.org/10.1021/acsnano.5b01696
Dunne WM (2002) Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev 15:155–166. https://doi.org/10.1128/CMR.15.2.155-166.2002
Dzianach PA, Dykes GA, Strachan NJC, Forbes KJ, Pérez-Reche FJ (2019) Challenges of biofilm control and utilization: lessons from mathematical modelling. J R Soc Interface 16.https://doi.org/10.1098/rsif.2019.0042
El Asbahani A, Miladi K, Badri W, Sala M, Aït Addi EH, Casabianca H, El Mousadik A, Hartmann D, Jilale A, Renaud FNR, Elaissari A (2015) Essential oils: from extraction to encapsulation. Int J Pharm 483:220–243. https://doi.org/10.1016/j.ijpharm.2014.12.069
Elias S, Banin E (2012) Multi-species biofilms: living with friendly neighbors. FEMS Microbiol Rev 36:990–1004. https://doi.org/10.1111/j.1574-6976.2012.00325.x
Engel JB, Heckler C, Tondo EC, Daroit DJ, da Silva MP (2017) Antimicrobial activity of free and liposome-encapsulated thymol and carvacrol against Salmonella and Staphylococcus aureus adhered to stainless steel. Int J Food Microbiol 252:18–23. https://doi.org/10.1016/j.ijfoodmicro.2017.04.003
Evans LV (2014) Biofilms: recent advances in their study and control. CRC Press
Fagerlund A, Langsrud S, Heir E, Mikkelsen MI, Møretrø T (2016) Biofilm matrix composition affects the susceptibility of food associated staphylococci to cleaning and disinfection agents. Front Microbiol 7.https://doi.org/10.3389/fmicb.2016.00856
Flemming H-C (2016) EPS—Then and now. Microorganisms 4.https://doi.org/10.3390/microorganisms4040041
Flemming H-C, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633. https://doi.org/10.1038/nrmicro2415
Flemming H-C, Wuertz S (2019) Bacteria and archaea on Earth and their abundance in biofilms. Nat Rev Microbiol 17:247–260. https://doi.org/10.1038/s41579-019-0158-9
Flemming H-C, Neu TR, Wozniak DJ (2007) The EPS matrix: the “house of biofilm cells.” J Bacteriol 189:7945–7947. https://doi.org/10.1128/JB.00858-07
Flemming H-C, Meier M, Schild T (2013) Mini-review: microbial problems in paper production. Biofouling 29:683–696. https://doi.org/10.1080/08927014.2013.798865
Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14:563–575. https://doi.org/10.1038/nrmicro.2016.94
Francolini I, Donelli G (2010) Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol Med Microbiol 59:227–238. https://doi.org/10.1111/j.1574-695X.2010.00665.x
Frascareli EC, Silva VM, Tonon RV, Hubinger MD (2012) Effect of process conditions on the microencapsulation of coffee oil by spray drying. Food Bioprod Process 90:413–424. https://doi.org/10.1016/j.fbp.2011.12.002
Fu F, Hu L (2017) 15 - Temperature sensitive colour-changed composites. In: Fan M, Fu F (eds) Advanced high strength natural fibre composites in construction. Woodhead Publishing, pp 405–423
Fulaz S, Vitale S, Quinn L, Casey E (2019) Nanoparticle–biofilm interactions: the role of the EPS matrix. Trends Microbiol 27:915–926. https://doi.org/10.1016/j.tim.2019.07.004
García-Almendárez BE, Cann IKO, Martin SE, Guerrero-Legarreta I, Regalado C (2008) Effect of Lactococcus lactis UQ2 and its bacteriocin on listeria monocytogenes biofilms. Food Control 19:670–680. https://doi.org/10.1016/j.foodcont.2007.07.015
Garrett TR, Bhakoo M, Zhang Z (2008) Bacterial adhesion and biofilms on surfaces. Prog Nat Sci 18:1049–1056. https://doi.org/10.1016/j.pnsc.2008.04.001
Gebreyohannes G, Nyerere A, Bii C, Sbhatu DB (2019) Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 5:e02192. https://doi.org/10.1016/j.heliyon.2019.e02192
Gharsallaoui A, Roudaut G, Chambin O, Voilley A, Saurel R (2007) Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res Int 40:1107–1121. https://doi.org/10.1016/j.foodres.2007.07.004
Gharsallaoui A, Roudaut G, Beney L, Chambin O, Voilley A, Saurel R (2012) Properties of spray-dried food flavours microencapsulated with two-layered membranes: roles of interfacial interactions and water. Food Chem 132:1713–1720. https://doi.org/10.1016/j.foodchem.2011.03.028
Gibson H, Taylor JH, Hall KE, Holah JT (1999) Effectiveness of cleaning techniques used in the food industry in terms of the removal of bacterial biofilms. J Appl Microbiol 87:41–48. https://doi.org/10.1046/j.1365-2672.1999.00790.x
Gouin S (2004) Microencapsulation: industrial appraisal of existing technologies and trends. Trends Food Sci Technol 15:330–347
Grover N, Plaks JG, Summers SR, Chado GR, Schurr MJ, Kaar JL (2016) Acylase-containing polyurethane coatings with anti-biofilm activity. Biotechnol Bioeng 113:2535–2543. https://doi.org/10.1002/bit.26019
Gutiérrez D, Ruas-Madiedo P, Martínez B, Rodríguez A, García P (2014) Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS ONE 9:e107307. https://doi.org/10.1371/journal.pone.0107307
Gutiérrez D, Rodríguez-Rubio L, Martínez B, Rodríguez A, García P (2016) bacteriophages as weapons against bacterial biofilms in the food industry. Front Microbiol 7:825. https://doi.org/10.3389/fmicb.2016.00825
Guzel-Seydim ZB, Greene AK, Seydim AC (2004) Use of ozone in the food industry. LWT - Food Sci Technol 37:453–460. https://doi.org/10.1016/j.lwt.2003.10.014
Hage M, Khelissa S, Abdallah M, Akoum H, Chihib N-E, Jama C (2021) Cold plasma assisted deposition of organosilicon coatings on stainless steel for prevention of adhesion of Salmonella enterica serovar Enteritidis. Biofouling 0:1–13. https://doi.org/10.1080/08927014.2021.1877274
Hall-Stoodley L, Stoodley P (2009) Evolving concepts in biofilm infections. Cell Microbiol 11:1034–1043. https://doi.org/10.1111/j.1462-5822.2009.01323.x
Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108. https://doi.org/10.1038/nrmicro821
Hansen MF, Svenningsen SL, Røder HL, Middelboe M, Burmølle M (2019) Big impact of the tiny: bacteriophage–bacteria interactions in biofilms. Trends Microbiol 27:739–752. https://doi.org/10.1016/j.tim.2019.04.006
Hobley L, Harkins C, MacPhee CE, Stanley-Wall NR (2015) Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiol Rev 39:649–669. https://doi.org/10.1093/femsre/fuv015
Houdt RV, Michiels CW (2010) Biofilm formation and the food industry, a focus on the bacterial outer surface. J Appl Microbiol 109:1117–1131. https://doi.org/10.1111/j.1365-2672.2010.04756.x
Huang H, Ren H, Ding L, Geng J, Xu K, Zhang Y (2014) Aging biofilm from a full-scale moving bed biofilm reactor: characterization and enzymatic treatment study. Bioresour Technol 154:122–130. https://doi.org/10.1016/j.biortech.2013.12.031
Hussain MS, Kwon M, Park E, Seheli K, Huque R, Oh D-H (2019) Disinfection of Bacillus cereus biofilms on leafy green vegetables with slightly acidic electrolyzed water, ultrasound and mild heat. LWT 116:108582. https://doi.org/10.1016/j.lwt.2019.108582
Ibáñez de Aldecoa AL, Zafra O, González-Pastor JE (2017) Mechanisms and regulation of extracellular DNA release and its biological roles in microbial communities. Front Microbiol 8.https://doi.org/10.3389/fmicb.2017.01390
Jain RA (2000) The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 21:2475–2490. https://doi.org/10.1016/S0142-9612(00)00115-0
Jamal M, Ahmad W, Andleeb S, Jalil F, Imran M, Nawaz MA, Hussain T, Ali M, Rafiq M, Kamil MA (2018) Bacterial biofilm and associated infections. J Chin Med Assoc 81:7–11. https://doi.org/10.1016/j.jcma.2017.07.012
Jung S-J, Park SY, Ha S-D (2018) Synergistic effect of X-ray irradiation and sodium hypochlorite against Salmonella enterica serovar typhimurium biofilms on quail eggshells. Food Res Int 107:496–502. https://doi.org/10.1016/j.foodres.2018.02.063
Kaplan JB (2010) Biofilm Dispersal. J Dent Res 89:205–218. https://doi.org/10.1177/0022034509359403
Karatan E, Watnick P (2009) Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev MMBR 73:310–347. https://doi.org/10.1128/MMBR.00041-08
Karygianni L, Ren Z, Koo H, Thurnheer T (2020) Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol 28:668–681. https://doi.org/10.1016/j.tim.2020.03.016
Khairnar S, Kini R, Harwalkar M, Salunkhe K, Chaudhari S (2012) A review on freeze drying process of pharmaceuticals. Int J Res Pharm Sci IJRPS 2013:76–94
Khan I, Tango CN, Miskeen S, Lee BH, Oh D-H (2017) Hurdle technology: a novel approach for enhanced food quality and safety – a review. Food Control 73:1426–1444. https://doi.org/10.1016/j.foodcont.2016.11.010
Khatoon Z, McTiernan CD, Suuronen EJ, Mah T-F, Alarcon EI (2018) Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 4:e01067. https://doi.org/10.1016/j.heliyon.2018.e01067
Khelissa O, Abdallah M, Jama C, Faille C, Chihib N-E (2017) Bacterial contamination and biofilm formation on abiotic surfaces and strategies to overcome their persistence. J Mater Environ Sci 8:3326–3346
Khelissa S, El Fannassi Y, Mechmechani S, Alhuthali S, El Amrani MA, Gharsallaoui A, Barras A, Chihib N-E (2021a) Water-soluble ruthenium (ii) complex derived from optically pure limonene and its microencapsulation are efficient tools against bacterial food pathogen biofilms: Escherichia coli, Staphylococcus aureus, Enteroccocus faecalis, and Listeria monocytogenes. Front Microbiol 12:3388. https://doi.org/10.3389/fmicb.2021.711326
Khelissa S, Gharsallaoui A, Wang J, Dumas E, Barras A, Jama C, Jbilou F, Loukili N, Chihib N-E (2021b) Anti-biofilm activity of dodecyltrimethylammonium chloride microcapsules against Salmonella enterica serovar Enteritidis and Staphylococcus aureus. Biofouling 37:49–60. https://doi.org/10.1080/08927014.2021.1873958
Kim M, Park S, Ha S-D (2016) Synergistic effect of a combination of ultraviolet–C irradiation and sodium hypochlorite to reduce Listeria monocytogenes biofilms on stainless steel and eggshell surfaces. Food Control 70.https://doi.org/10.1016/j.foodcont.2016.05.003
Kim M-J, Lim ES, Kim J-S (2019) Enzymatic inactivation of pathogenic and nonpathogenic bacteria in biofilms in combination with chlorine. J Food Prot 82:605–614. https://doi.org/10.4315/0362-028X.JFP-18-244
Kiran S, Sharma P, Harjai K, Capalash N (2011) Enzymatic quorum quenching increases antibiotic susceptibility of multidrug resistant Pseudomonas aeruginosa. Iran J Microbiol 3:1–12
Klevens RM, Edwards JR, Richards CL, Horan TC, Gaynes RP, Pollock DA (1974) Cardo DM (2007) Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep Wash DC 122:160–166. https://doi.org/10.1177/003335490712200205
Knowles JR, Roller S, Murray DB, Naidu AS (2005) Antimicrobial action of carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica Serovar typhimurium. Appl Environ Microbiol 71:797–803. https://doi.org/10.1128/AEM.71.2.797-803.2005
Kreuter J, Nefzger M, Liehl E, Czok R, Voges R (1983) Distribution and elimination of poly (methyl methacrylate) nanoparticles after subcutaneous administration to rats. J Pharm Sci 72:1146–1149. https://doi.org/10.1002/jps.2600721009
Lambert RJW, Johnston MD (2001) The effect of interfering substances on the disinfection process: a mathematical model. J Appl Microbiol 91:548–555. https://doi.org/10.1046/j.1365-2672.2001.01422.x
Laohasongkram K, Mahamaktudsanee T, Chaiwanichsiri S (2011) Microencapsulation of Macadamia oil by spray drying. Procedia Food Sci 1:1660–1665. https://doi.org/10.1016/j.profoo.2011.09.245
Leid JG (2009) Bacterial Biofilms Resist Key Host Defenses. Microbe 4:66–70
Lens P, O’Flaherty V, Moran AP, Stoodley P, Mahony T (2003) Biofilms in medicine, industry and environmental biotechnology. IWA Publishing
Lequette Y, Boels G, Clarisse M, Faille C (2010) Using enzymes to remove biofilms of bacterial isolates sampled in the food-industry. Biofouling 26:421–431. https://doi.org/10.1080/08927011003699535
Leroy C, Delbarre C, Ghillebaert F, Compere C, Combes D (2008) Effects of commercial enzymes on the adhesion of a marine biofilm-forming bacterium. Biofouling 24:11–22. https://doi.org/10.1080/08927010701784912
Liang R, Xu S, Shoemaker CF, Li Y, Zhong F, Huang Q (2012) Physical and antimicrobial properties of peppermint oil nanoemulsions. J Agric Food Chem 60:7548–7555. https://doi.org/10.1021/jf301129k
Lim ES, Lee JE, Kim J-S, Koo OK (2017) Isolation of indigenous bacteria from a cafeteria kitchen and their biofilm formation and disinfectant susceptibility. LWT 77:376–382. https://doi.org/10.1016/j.lwt.2016.11.060
Lim ES, Koo OK, Kim M-J, Kim J-S (2019) Bio-enzymes for inhibition and elimination of Escherichia coli O157:H7 biofilm and their synergistic effect with sodium hypochlorite. Sci Rep 9:9920. https://doi.org/10.1038/s41598-019-46363-w
Liu Y, Yang S-F, Li Y, Xu H, Qin L, Tay J-H (2004) The influence of cell and substratum surface hydrophobicities on microbial attachment. J Biotechnol 110:251–256. https://doi.org/10.1016/j.jbiotec.2004.02.012
Liu C, Sun D, Zhu J, Liu W (2019) Two-component signal transduction systems: a major strategy for connecting input stimuli to biofilm formation. Front Microbiol 9.https://doi.org/10.3389/fmicb.2018.03279
López P, Sánchez C, Batlle R, Nerín C (2005) Solid- and vapor-phase antimicrobial activities of six essential oils: susceptibility of selected foodborne bacterial and fungal strains. J Agric Food Chem 53:6939–6946. https://doi.org/10.1021/jf050709v
López P, Sanchez C, Batlle R, Nerín C (2007) Vapor-phase activities of cinnamon, thyme, and oregano essential oils and key constituents against foodborne microorganisms. J Agric Food Chem 55:4348–4356. https://doi.org/10.1021/jf063295u
Loveday HP, Wilson JA, Kerr K, Pitchers R, Walker JT, Browne J (2014) Association between healthcare water systems and Pseudomonas aeruginosa infections: a rapid systematic review. J Hosp Infect 86:7–15. https://doi.org/10.1016/j.jhin.2013.09.010
Madsen J, Burmølle M, Hansen L, Sørensen S (2012) The interconnection between biofilm formation and horizontal gene transfer
Mah T-FC, O’Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39. https://doi.org/10.1016/S0966-842X(00)01913-2
Mann EE, Wozniak DJ (2012) Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol Rev 36:893–916. https://doi.org/10.1111/j.1574-6976.2011.00322.x
Margel S, Wiesel E (1984) Acrolein polymerization: monodisperse, homo, and hybrido microspheres, synthesis, mechanism, and reactions. J Polym Sci Polym Chem Ed 22:145–158. https://doi.org/10.1002/pol.1984.170220115
Martin S, Feng H (2009) 13 - Novel methods for biofilm control and removal from food processing equipment. In: Fratamico PM, Annous BA, Gunther NW (eds) Biofilms in the Food and Beverage Industries. Woodhead Publishing, pp 359–372
Masters EA, Trombetta RP, de Mesy Bentley KL, Boyce BF, Gill AL, Gill SR, Nishitani K, Ishikawa M, Morita Y, Ito H, Bello-Irizarry SN, Ninomiya M, Brodell JD, Lee CC, Hao SP, Oh I, Xie C, Awad HA, Daiss JL, Owen JR, Kates SL, Schwarz EM, Muthukrishnan G (2019) Evolving concepts in bone infection: redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy.” Bone Res 7:1–18. https://doi.org/10.1038/s41413-019-0061-z
Meireles A, Borges A, Giaouris E, Simões M (2016) The current knowledge on the application of anti-biofilm enzymes in the food industry. Food Res Int 86:140–146. https://doi.org/10.1016/j.foodres.2016.06.006
Mohamad NR, Marzuki NHC, Buang NA, Huyop F, Wahab RA (2015) An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol Biotechnol Equip 29:205–220. https://doi.org/10.1080/13102818.2015.1008192
Mohamed SH, Mohamed MSM, Khalil MS, Azmy M, Mabrouk MI (2018) Combination of essential oil and ciprofloxacin to inhibit/eradicate biofilms in multidrug-resistant Klebsiella pneumoniae. J Appl Microbiol 125:84–95. https://doi.org/10.1111/jam.13755
Molobela IP, Cloete TE (Thomas E, Beukes M (2010) Protease and amylase enzymes for biofilm removal and degradation of extracellular polymeric substances (EPS) produced by Pseudomonas fluorescens bacteria
Møretrø T, Schirmer BC, Heir E, Fagerlund A, Hjemli P, Langsrud S (2017) Tolerance to quaternary ammonium compound disinfectants may enhance growth of Listeria monocytogenes in the food industry. Int J Food Microbiol 241:215–224
Murthy PS, Venkatesan R (2009) Industrial biofilms and their control. In: Flemming H-C, Murthy PS, Venkatesan R, Cooksey K (eds) Marine and Industrial Biofouling. Springer, Berlin, Heidelberg, pp 65–101
Nam H, Seo H-S, Bang J, Kim H, Beuchat LR, Ryu J-H (2014) Efficacy of gaseous chlorine dioxide in inactivating Bacillus cereus spores attached to and in a biofilm on stainless steel. Int J Food Microbiol 188:122–127. https://doi.org/10.1016/j.ijfoodmicro.2014.07.009
Nazzaro F, Orlando P, Fratianni F, Coppola R (2012) Microencapsulation in food science and biotechnology. Curr Opin Biotechnol 23:182–186. https://doi.org/10.1016/j.copbio.2011.10.001
Nett JE, Andes DR (2020) Contributions of the biofilm matrix to candida pathogenesis.J Fungi 6.https://doi.org/10.3390/jof6010021
Neyret C, Herry J-M, Meylheuc T, Dubois-Brissonnet F (2014) Plant-derived compounds as natural antimicrobials to control paper mill biofilms. J Ind Microbiol Biotechnol 41:87–96. https://doi.org/10.1007/s10295-013-1365-4
Nguyen UT, Burrows LL (2014) DNase I and proteinase K impair Listeria monocytogenes biofilm formation and induce dispersal of pre-existing biofilms. Int J Food Microbiol 187:26–32. https://doi.org/10.1016/j.ijfoodmicro.2014.06.025
Nica IC, Stan MS, Popa M, Chifiriuc MC, Lazar V, Pircalabioru GG, Dumitrescu I, Ignat M, Feder M, Tanase LC, Mercioniu I, Diamandescu L, Dinischiotu A (2017) Interaction of new-developed TiO2-Based photocatalytic nanoparticles with pathogenic microorganisms and human dermal and pulmonary fibroblasts. Int J Mol Sci 18.https://doi.org/10.3390/ijms18020249
Nobrega FL, Costa AR, Kluskens LD, Azeredo J (2015) Revisiting phage therapy: new applications for old resources. Trends Microbiol 23:185–191. https://doi.org/10.1016/j.tim.2015.01.006
Oh SY, Yun W, Lee JH, Lee CH, Kwak WK, Cho JH (2017) Effects of essential oil (blended and single essential oils) on anti-biofilm formation of Salmonella and Escherichia coli. J Anim Sci Technol 59.https://doi.org/10.1186/s40781-017-0127-7
Ooi LSM, Li Y, Kam S-L, Wang H, Wong EYL, Ooi VEC (2006) Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb cinnamomum cassia Blume. Am J Chin Med 34:511–522. https://doi.org/10.1142/S0192415X06004041
Orgaz B, Neufeld RJ, Sanjose C (2007) Single-step biofilm removal with delayed release encapsulated Pronase mixed with soluble enzymes. Enzyme Microb Technol 40:1045–1051. https://doi.org/10.1016/j.enzmictec.2006.08.003
Ortega Morente E, Fernández-Fuentes MA, Grande Burgos MJ, Abriouel H, Pérez Pulido R, Gálvez A (2013) Biocide tolerance in bacteria. Int J Food Microbiol 162:13–25. https://doi.org/10.1016/j.ijfoodmicro.2012.12.028
Oulahal-Lagsir N, Martial-Gros A, Bonneau M, Blum LJ (2003) “Escherichia coli-milk” biofilm removal from stainless steel surfaces: synergism between ultrasonic waves and enzymes. Biofouling 19:159–168. https://doi.org/10.1080/08927014.2003.10382978
Oulkheir S, Aghrouch M, Mourabit FE, Dalha F, Graich H, Amouch F, Ouzaid K, Moukale A, Chadli S (2017) Antibacterial activity of essential oils extracts from cinnamon, thyme, clove and geranium against a gram negative and gram positive pathogenic bacteria. J Dis Med Plants Spec Issue New Vistas Res Ayurveda Syst Med 3:1–5. https://doi.org/10.11648/j.jdmp.s.2017030201.11
Pakharukova N, Tuittila M, Paavilainen S, Malmi H, Parilova O, Teneberg S, Knight SD, Zavialov AV (2018) Structural basis for Acinetobacter baumannii biofilm formation. Proc Natl Acad Sci 115:5558–5563. https://doi.org/10.1073/pnas.1800961115
Park D, Maga JA (2006) Identification of key volatiles responsible for odour quality differences in popped popcorn of selected hybrids. Food Chem 99:538–545. https://doi.org/10.1016/j.foodchem.2005.08.019
Patel N, Patel D, Bharadia P, Pandya V, Modi D (2011) Microsphere as a novel drug delivery. Int J Pharm LIFE Sci 2:992–997
Pechaud Y, Marcato-Romain CE, Girbal-Neuhauser E, Queinnec I, Bessiere Y, Paul E (2012) Combining hydrodynamic and enzymatic treatments to improve multi-species thick biofilm removal. Chem Eng Sci
Percival SL, Suleman L, Vuotto C, Donelli G (2015) (2015) Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol 64:323–334. https://doi.org/10.1099/jmm.0.000032
Pérez-Conesa D, Cao J, Chen L, McLandsborough L, Weiss J (2011) Inactivation of Listeria monocytogenes and Escherichia coli O157:H7 biofilms by micelle-encapsulated eugenol and carvacrol. J Food Prot 74:55–62. https://doi.org/10.4315/0362-028X.JFP-08-403
Peterson BW, He Y, Ren Y, Zerdoum A, Libera MR, Sharma PK, van Winkelhoff A-J, Neut D, Stoodley P, van der Mei HC, Busscher HJ (2015) Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol Rev 39:234–245. https://doi.org/10.1093/femsre/fuu008
Qin Y (2016) 5 - Applications of advanced technologies in the development of functional medical textile materials. In: Qin Y (ed) Medical Textile Materials. Woodhead Publishing, pp 55–70
Rao TS, Nancharaiah YV, Nair KVK (1998) Biocidal efficacy of monochloramine against biofilm bacteria. Biofouling 12:321–332. https://doi.org/10.1080/08927019809378363
Rao J, Chen B, McClements DJ (2019) Improving the efficacy of essential oils as antimicrobials in foods: mechanisms of action. Annu Rev Food Sci Technol 10:365–387. https://doi.org/10.1146/annurev-food-032818-121727
Ratledge C, Wilkinson SG (1988) An overview of microbial lipids. In: Microbial Lipids, Ratledge C., Wilkinson S.G. Academic Press Limited, London, UK, pp 3–22
Reyes-Jurado F, Franco-Vega A, Ramírez-Corona N, Palou E, López-Malo A (2015) Essential oils: antimicrobial activities, extraction methods, and their modeling. Food Eng Rev 3:275–297. https://doi.org/10.1007/s12393-014-9099-2
Ríos-Castillo AG, González-Rivas F, Rodríguez-Jerez JJ (2017) Bactericidal efficacy of hydrogen peroxide-based disinfectants against gram-positive and gram-negative bacteria on stainless steel surfaces. J Food Sci 82:2351–2356. https://doi.org/10.1111/1750-3841.13790
Roberts DD, Taylor AJ (eds) (2000) Flavor release. American Chemical Society, Washington, DC
Rodrigues CF, Černáková L (2020) Farnesol and tyrosol: secondary metabolites with a crucial quorum-sensing role in candida biofilm development. Genes 11:444. https://doi.org/10.3390/genes11040444
Rodríguez-López P, Puga CH, Orgaz B, Cabo ML (2017) Quantifying the combined effects of pronase and benzalkonium chloride in removing late-stage Listeria monocytogenes–Escherichia coli dual-species biofilms. Biofouling 33:690–702. https://doi.org/10.1080/08927014.2017.1356290
Rosan B, Lamont RJ (2000) Dental plaque formation. Microbes Infect 2:1599–1607. https://doi.org/10.1016/S1286-4579(00)01316-2
Rosen M (2005) Delivery system handbook for personal care and cosmetic products: technology, applications and formulations. Elsevier Science
Rosenberg LE, Carbone AL, Römling U, Uhrich KE, Chikindas ML (2008) Salicylic acid-based poly (anhydride esters) for control of biofilm formation in Salmonella enterica serovar Typhimurium. Lett Appl Microbiol 46:593–599
Rota MC, Herrera A, Martínez RM, Sotomayor JA, Jordán MJ (2008) Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 19:681–687. https://doi.org/10.1016/j.foodcont.2007.07.007
Roy R, Tiwari M, Donelli G, Tiwari V (2017) Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence 9:522–554. https://doi.org/10.1080/21505594.2017.1313372
Sauer K (2003) The genomics and proteomics of biofilm formation. Genome Biol 4:219. https://doi.org/10.1186/gb-2003-4-6-219
Scholtz V, Pazlarova J, Souskova H, Khun J, Julak J (2015) Nonthermal plasma–A tool for decontamination and disinfection. Biotechnol Adv 33:1108–1119. https://doi.org/10.1016/j.biotechadv.2015.01.002
Schutyser MAI, Perdana J, Boom RM (2012) Single droplet drying for optimal spray drying of enzymes and probiotics. Trends Food Sci Technol 27:73–82. https://doi.org/10.1016/j.tifs.2012.05.006
Sherry M, Charcosset C, Fessi H, Greige-Gerges H (2013) Essential oils encapsulated in liposomes: a review. J Liposome Res 23:268–275. https://doi.org/10.3109/08982104.2013.819888
Shi X, Zhu X (2009) Biofilm formation and food safety in food industries. Trends Food Sci Technol 20:407–413. https://doi.org/10.1016/j.tifs.2009.01.054
Shokouhfard M, Kermanshahi RK, Shahandashti RV, Feizabadi MM, Teimourian S (2015) The inhibitory effect of a Lactobacillus acidophilus derived biosurfactant on biofilm producer Serratia marcescens. Iran J Basic Med Sci 18:1001–1007
Silva FV (2015) High pressure processing of milk: modeling the inactivation of psychrotrophic Bacillus cereus spores at 38–70° C. J Food Eng 165:141–148
Silva C, Ribeiro A, Ferreira D, Veiga F (2003) Administração oral de peptídeos e proteínas: II. Aplicação de métodos de microencapsulação. Rev Bras Ciênc Farm 39:1–20
Simões M, Simões LC, Vieira MJ (2010). A Review of Current and Emergent Biofilm Control Strategies. https://doi.org/10.1016/j.lwt.2009.12.008
Simões M, Carvalho H, Pereira MO, Vieira MJ (2003) Studies on the behaviour of Pseudomonas fluorescens biofilms after Ortho-phthalaldehyde treatment. - Abstract - Europe PMC. 19:151–157
Singh MN, Hemant KSY, Ram M, Shivakumar HG (2010) Microencapsulation: a promising technique for controlled drug delivery. Res Pharm Sci 5:65–77
Singh S, Singh SK, Chowdhury I, Singh R (2017) Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol J 11:53–62. https://doi.org/10.2174/1874285801711010053
Sivropoulou A, Papanikolaou E, Nikolaou C, Kokkini S, Lanaras T, Arsenakis M (1996) Antimicrobial and cytotoxic activities of Origanum essential oils. J Agric Food Chem 44:1202–1205
Soni KA, Oladunjoye A, Nannapaneni R, Schilling MW, Silva JL, Mikel B, Bailey RH (2013) Inhibition and inactivation of Salmonella typhimurium biofilms from polystyrene and stainless steel surfaces by essential oils and phenolic constituent carvacrol. J Food Prot 76:205–212. https://doi.org/10.4315/0362-028X.JFP-12-196
Soto SM (2013) Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 4:223–229. https://doi.org/10.4161/viru.23724
Southey-Pillig CJ, Davies DG, Sauer K (2005) Characterization of temporal protein production in Pseudomonas aeruginosa biofilms. J Bacteriol 187:8114–8126. https://doi.org/10.1128/JB.187.23.8114-8126.2005
SPF (2019) Enquête nationale de prévalence des infections nosocomiales et des traitements anti-infectieux en établissements de santé, France, mai-juin 2017. https://www.santepubliquefrance.fr/maladies-et-traumatismes/infections-associees-aux-soins-et-resistance-aux-antibiotiques/infections-associees-aux-soins/enquete-nationale-de-prevalence-des-infections-nosocomiales-et-des-traitements-anti-infectieux-en-etablissements-de-sante-france-mai.
SPF (2021) Surveillance des toxi-infections alimentaires collectives. Données de la déclaration obligatoire, 2019. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-infectieuses-d-origine-alimentaire/toxi-infections-alimentaires-collectives/documents/bulletin-national/surveillance-des-toxi-infections-alimentaires-collectives.-donnees-de-la-declaration-obligatoire-2019.
Srey S, Jahid IK, Ha S-D (2013) Biofilm formation in food industries: a food safety concern. Food Control 31:572–585. https://doi.org/10.1016/j.foodcont.2012.12.001
Ssekitoleko RT, Oshabaheebwa S, Munabi IG, Tusabe MS, Namayega C, Ngabirano BA, Matovu B, Mugaga J, Reichert WM, Joloba ML (2020) The role of medical equipment in the spread of nosocomial infections: a cross-sectional study in four tertiary public health facilities in Uganda. BMC Public Health 20:1561. https://doi.org/10.1186/s12889-020-09662-w
Stoodley P, Boyle JD, Dodds I, Lappin-Scott HM (1997) Consensus model of biofilm structure. In: Biofilms: community interactions and control, Wimpenny JWT, Gilbert PS, Lappin-Scott HM, Jones M, editors. Cardiff, UK: Bioline, pp 1–9
Suave J, Dall’Agnol EC, Pezzin APT, Silva DAK, Meier MM, Soldi V, (2006) Microencapsulação: Inovação em diferentes áreas. Rev Saúde E Ambient Environ J 7:12–20
Sutherland IW (2001) The biofilm matrix – an immobilized but dynamic microbial environment. Trends Microbiol 9:222–227. https://doi.org/10.1016/S0966-842X(01)02012-1
Sweet MJ, Croquer A, Bythell JC (2011) Development of bacterial biofilms on artificial corals in comparison to surface-associated microbes of hard corals. PLoS ONE 6:e21195. https://doi.org/10.1371/journal.pone.0021195
Tan Y, Ma S, Leonhard M, Moser D, Haselmann GM, Wang J, Eder D, Schneider-Stickler B (2018) Enhancing antibiofilm activity with functional chitosan nanoparticles targeting biofilm cells and biofilm matrix. Carbohydr Polym 200:35–42. https://doi.org/10.1016/j.carbpol.2018.07.072
Tan Y, Ma S, Leonhard M, Moser D, Ludwig R, Schneider-Stickler B (2020) Co-immobilization of cellobiose dehydrogenase and deoxyribonuclease I on chitosan nanoparticles against fungal/bacterial polymicrobial biofilms targeting both biofilm matrix and microorganisms. Mater Sci Eng C 108:110499. https://doi.org/10.1016/j.msec.2019.110499
Tasia W, Lei C, Cao Y, Ye Q, He Y, Xu C (2020) Enhanced eradication of bacterial biofilms with DNase I-loaded silver-doped mesoporous silica nanoparticles. Nanoscale 12:2328–2332. https://doi.org/10.1039/C9NR08467C
Téllez S (2010) Biofilms and their impact on food industry. VISAVET Outreach J
Teschler JK, Zamorano-Sánchez D, Utada AS, Warner CJA, Wong GCL, Linington RG, Yildiz FH (2015) Living in the matrix: assembly and control of Vibrio cholerae biofilms. Nat Rev Microbiol 13:255–268. https://doi.org/10.1038/nrmicro3433
Tetter S, Hilvert D (2017) Enzyme encapsulation by a ferritin cage. Angew Chem Int Ed 56:14933–14936. https://doi.org/10.1002/anie.201708530
Thallinger B, Prasetyo EN, Nyanhongo GS, Guebitz GM (2013) Antimicrobial enzymes: an emerging strategy to fight microbes and microbial biofilms. Biotechnol J 8:97–109. https://doi.org/10.1002/biot.201200313
Toldrá F, Reig M (2011) Innovations for healthier processed meats. Trends Food Sci Technol 22:517–522. https://doi.org/10.1016/j.tifs.2011.08.007
Tolker-Nielsen N, Molin N (2000) Spatial organization of microbial biofilm communities. Microb Ecol 40:75–84
Torres CE, Lenon G, Craperi D, Wilting R, Blanco A (2011) Enzymatic treatment for preventing biofilm formation in the paper industry. Appl Microbiol Biotechnol 92:95–103. https://doi.org/10.1007/s00253-011-3305-4
Toshio Y, Mitsuru H, Shozo M, Hitoshi S (1981) Specific delivery of mitomycin c to the liver, spleen and lung: Nano- and m1crospherical carriers of gelatin. Int J Pharm 8:131–141. https://doi.org/10.1016/0378-5173(81)90017-X
Vankerckhoven E, Verbessem B, Crauwels S, Declerck P, Muylaert K, Willems KA, Rediers H (2011) Exploring the potential synergistic effects of chemical disinfectants and UV on the inactivation of free-living bacteria and treatment of biofilms in a pilot-scale system. Water Sci Technol 64:1247–1253. https://doi.org/10.2166/wst.2011.718
Vázquez-Sánchez D, Cabo ML, Rodríguez-Herrera JJ (2015) Antimicrobial activity of essential oils against Staphylococcus aureus biofilms. Food Sci Technol Int Cienc Tecnol Los Aliment Int 21:559–570. https://doi.org/10.1177/1082013214553996
Vázquez-Sánchez D, Galvão JA, Ambrosio CMS, Gloria EM, Oetterer M (2018) Single and binary applications of essential oils effectively control Listeria monocytogenes biofilms. Ind Crops Prod 121:452–460. https://doi.org/10.1016/j.indcrop.2018.05.045
Venkatesh M, Rong L, Raad I, Versalovic J (2009) Novel synergistic antibiofilm combinations for salvage of infected catheters. J Med Microbiol 58:936–944. https://doi.org/10.1099/jmm.0.009761-0
Verraes C, Van Boxstael S, Van Meervenne E, Van Coillie E, Butaye P, Catry B, de Schaetzen M-A, Van Huffel X, Imberechts H, Dierick K, Daube G, Saegerman C, De Block J, Dewulf J, Herman L (2013) Antimicrobial resistance in the food chain: a review. Int J Environ Res Public Health 10:2643–2669. https://doi.org/10.3390/ijerph10072643
Vlamakis H, Chai Y, Beauregard P, Losick R, Kolter R (2013) Sticking together: building a biofilm the Bacillus subtilis way. Nat Rev Microbiol 11:157–168. https://doi.org/10.1038/nrmicro2960
Wakiyama N, Juni K, Nakano M (1981) Preparation and evaluation in vitro of polylactic acid microspheres containing local anesthetics. Chem Pharm Bull (tokyo) 29:3363–3368
Walker SL, Fourgialakis M, Cerezo B, Livens S (2007) Removal of microbial biofilms from dispense equipment: the effect of enzymatic Pre-digestion and detergent treatment. J Inst Brew 113:61–66. https://doi.org/10.1002/j.2050-0416.2007.tb00257.x
Wang H, Wang H, Xing T, Wu N, Xu X, Zhou G (2016) Removal of Salmonella biofilm formed under meat processing environment by surfactant in combination with bio-enzyme. LWT - Food Sci Technol 66:298–304. https://doi.org/10.1016/j.lwt.2015.10.049
Wang B, Akanbi TO, Agyei D, Holland BJ, Barrow CJ (2018) Chapter 7 - Coacervation technique as an encapsulation and delivery tool for hydrophobic biofunctional compounds. In: Grumezescu AM, Holban AM (eds) Role of Materials Science in Food Bioengineering. Academic Press, pp 235–261
Weber DJ, Anderson D, Rutala WA (2013) The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis 26:338–344. https://doi.org/10.1097/QCO.0b013e3283630f04
Weger LAD, van der Vlugt CI, Wijfjes AH, Bakker PA, Schippers B, Lugtenberg B (1987) Flagella of a plant-growth-stimulating Pseudomonas fluorescens strain are required for colonization of potato roots. J Bacteriol 169:2769–2773. https://doi.org/10.1128/jb.169.6.2769-2773.1987
Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, Greenberg EP (2001) Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860. https://doi.org/10.1038/35101627
WHO (2020) Food safety. https://www.who.int/news-room/fact-sheets/detail/food-safety. Accessed 14 Oct 2021
Wilkinson JM, Hipwell M, Ryan T, Cavanagh HMA (2003) Bioactivity of Backhousia citriodora: antibacterial and antifungal activity. J Agric Food Chem 51:76–81. https://doi.org/10.1021/jf0258003
Winkelströter LK, Gomes BC, Thomaz MRS, Souza VM, De Martinis ECP (2011) Lactobacillus sakei 1 and its bacteriocin influence adhesion of Listeria monocytogenes on stainless steel surface. Food Control 22:1404–1407. https://doi.org/10.1016/j.foodcont.2011.02.021
Winkelströter LK, Tulini FL, De Martinis ECP (2015) Identification of the bacteriocin produced by cheese isolate Lactobacillus paraplantarum FT259 and its potential influence on Listeria monocytogenes biofilm formation. LWT - Food Sci Technol 64:586–592. https://doi.org/10.1016/j.lwt.2015.06.014
Wińska K, Mączka W, Łyczko J, Grabarczyk M, Czubaszek A, Szumny A (2019) Essential oils as antimicrobial agents—myth or real alternative? Molecules 24:2130. https://doi.org/10.3390/molecules24112130
Wolcott R, Costerton JW, Raoult D, Cutler SJ (2013) The polymicrobial nature of biofilm infection. Clin Microbiol Infect 19:107–112. https://doi.org/10.1111/j.1469-0691.2012.04001.x
Wolska KI, Grudniak AM, Rudnicka Z, Markowska K (2016) Genetic control of bacterial biofilms. J Appl Genet 57:225–238. https://doi.org/10.1007/s13353-015-0309-2
Wong SW, Yu B, Curran P, Zhou W (2009) Characterising the release of flavour compounds from chewing gum through HS-SPME analysis and mathematical modelling. Food Chem 114:852–858. https://doi.org/10.1016/j.foodchem.2008.10.030
Wu H, Moser C, Wang H-Z, Høiby N, Song Z-J (2015) Strategies for combating bacterial biofilm infections. Int J Oral Sci 7:1–7. https://doi.org/10.1038/ijos.2014.65
Xavier JB, Picioreanu C, Rani SA, van Loosdrecht MCM, Stewart PS (2005) Biofilm-control strategies based on enzymic disruption of the extracellular polymeric substance matrix–a modelling study. Microbiol Read Engl 151:3817–3832. https://doi.org/10.1099/mic.0.28165-0
Xiong Y, Liu Y (2010) Biological control of microbial attachment: a promising alternative for mitigating membrane biofouling. Appl Microbiol Biotechnol 86:825–837. https://doi.org/10.1007/s00253-010-2463-0
Xu J, Zhou F, Ji B-P, Pei R-S, Xu N (2008) The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett Appl Microbiol 47:174–179. https://doi.org/10.1111/j.1472-765X.2008.02407.x
Xu D, Jia R, Li Y, Gu T (2017) Advances in the treatment of problematic industrial biofilms. World J Microbiol Biotechnol 33:97. https://doi.org/10.1007/s11274-016-2203-4
Yang S-C, Lin C-H, Aljuffali IA, Fang J-Y (2017) Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch Microbiol 199:811–825
Yuan L, Sadiq FA, Wang N, Yang Z, He G (2021) Recent advances in understanding the control of disinfectant-resistant biofilms by hurdle technology in the food industry. Crit Rev Food Sci Nutr 61:3876–3891. https://doi.org/10.1080/10408398.2020.1809345
Zaman SB, Hussain MA, Nye R, Mehta V, Mamun KT, Hossain N (2017) A review on antibiotic resistance: alarm bells are ringing. Cureus 9.https://doi.org/10.7759/cureus.1403
Zarnowski R, Westler WM, Lacmbouh GA, Marita JM, Bothe JR, Bernhardt J, Sahraoui AL-H, Fontaine J, Sanchez H, Hatfield RD, Ntambi JM, Nett JE, Mitchell AP, Andes DR (2014) Novel Entries in a Fungal Biofilm Matrix Encyclopedia. mBio 5. https://doi.org/10.1128/mBio.01333-14
Zdarta J, Meyer AS, Jesionowski T, Pinelo M (2018) A general overview of support materials for enzyme immobilization: characteristics, properties, practical utility. Catalysts 8:92. https://doi.org/10.3390/catal8020092
Zea L, McLean RJC, Rook TA, Angle G, Carter DL, Delegard A, Denvir A, Gerlach R, Gorti S, McIlwaine D, Nur M, Peyton BM, Stewart PS, Sturman P, Velez Justiniano YA (2020) Potential biofilm control strategies for extended spaceflight missions. Biofilm 2.https://doi.org/10.1016/j.bioflm.2020.100026
Zhao X, Yu Z, Ding T (2020) Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms 8.https://doi.org/10.3390/microorganisms8030425
Author information
Authors and Affiliations
Contributions
SM and AG and KO and MH and NEC conceived the outline of this review. SM contributed the figures. All authors contributed to literature searches and the writing of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable, since the work does not involve any study with human participants or animals.
Consent to participate
Not applicable.
Consent for publication
All co-authors have given their consent to publish this manuscript.
Competing interests
The authors declare that there are no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mechmechani, S., Khelissa, S., Gharsallaoui, A. et al. Hurdle technology using encapsulated enzymes and essential oils to fight bacterial biofilms. Appl Microbiol Biotechnol 106, 2311–2335 (2022). https://doi.org/10.1007/s00253-022-11875-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-11875-5