Abstract
Polyamines (PAs) are ubiquitous low-molecular-weight, aliphatic compounds with wide as well as complex application in fundamental areas of plant growth and development. PAs are mediator of basic metabolism of organisms which include cell division and differentiation, biotic and abiotic stress tolerance, reversal of oxidative damage, stabilization of nucleic acids, and protein and phospholipid binding. In plants, it attributes in direct and indirect organogenesis, endogenous phytohormone regulation, cellular compartmentalization, fruit and flower development, senescence, and secondary metabolite production which are highly tuned as first line of defense response. There are several aspects of polyamine-directed mechanism that regulate overall plant growth in vitro and in vivo. In the present review, we have critically discussed the role played by polyamine on the enhanced production of bioactive natural products and how the same polyamines are functioning against different environmental stress conditions, i.e., salinity, drought, high CO2 content, herbivory, and physical wounding. The role of polyamines on elicitation process has been highlighted previously, but it is important to note that its activity as growth regulator under in vitro condition is correlated with an array of intertwined mechanism and physiological tuning. Medicinal plants under different developmental stages of micropropagation are characterized with different functional aspects and regulatory changes during embryogenesis and organogenesis. The effect of precursor molecules as well as additives and biosynthetic inhibitors of polyamines in rhizogenesis, callogenesis, tuberization, embryogenesis, callus formation, and metabolite production has been discussed thoroughly. The beneficial effect of exogenous application of PAs in elicitation of secondary metabolite production, plant growth and morphogenesis and overall stress tolerance are summarized in this present work.
Key points
• Polyamines (PAs) play crucial roles in in vitro organogenesis.
• PAs elicitate bioactive secondary metabolites (SMs).
• Transgenic studies elucidate and optimize PA biosynthetic genes coding SMs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
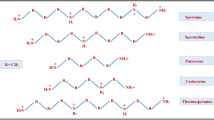
Polyamines (PAs) are low-molecular-weight, ubiquitous polycationic compounds, with aliphatic nitrogenous bases. In both prokaryotes and eukaryotes, cell growth, differentiation, maturation, and cell death are known to be mediated by PAs. However, in plants, PAs play a crucial modulatory role in growth and development and in response to biotic and abiotic stressors (Chen et al. 2019). Alongside PAs, there are many plant biostimulants such as biopolymers, inorganic compounds, and microbial metabolites but the mode of action and the effects of PAs on secondary metabolite production are unique. The aliphatic PAs putrescine (Put), spermidine (Spd), and spermine (Spm) are ubiquitous in almost all living cells. They are known to modulate an array of physiological, molecular, and developmental responses, viz., DNA synthesis, cell division, organogenesis, and embryogenesis. They are also known to prevent protein denaturation and facilitate protein folding, activate stress response, maintain cationic and pH levels, promote defense reactions, induce light-induced growth and developmental processes, initiate secondary metabolite biosynthesis as precursors, etc. (Santanen 2000). Earlier, the functional role of PAs has been extensively investigated on physiological alterations in plants, plant hypersensitivity against abiotic stresses, regulation of cell cycle, gene expression in relation to altered DNA and protein synthesis, amelioration of tissue damage, influence on ion uptake and cellular membrane permeability, and antioxidant capacity in conjunction with other signaling molecules such as proline (Pro), nitric oxide (NO), and γ-aminobutyric acid (GABA) (Mustafavi et al. 2018).
Medicinal plants are the baseline of healthcare in many developing and poor countries due to extreme poverty, prevalence of age-old traditions, and absence of government-aided basic healthcare facilities. Moreover, they have acquired their special position in cosmetic, herbal repellant manufacturing, and food processing industries. Some of the ingredient plants are disappearing fast from their natural habitat due to overexploitation; some plants have huge demand but limited geographical distribution (the orchids especially used in traditional Chinese medicine or TCM); some medicinal plants are difficult to cultivate naturally due to low seed setting or slow growth, and for some expensive aromatic spice like saffron, one needs the full plants and flower just to harvest the stigmas! Rapid decline in forest and agricultural lands is another factor that triggered the search for alternative route of medicinal plant cultivation. In the present days, tissue cultures of endangered plant species and medicinal and agriculturally important plants have attained wide popularity among researchers. Sometime, direct and indirect organogenesis of plants are aiding in the selection of elite genotypes in terms of production of novel, natural pharmaceuticals and nutraceuticals. Many plant-based transgenic studies have also been performed through these methods. Tissue culture technique is solely dependent on totipotency of plant cells, and regeneration of plantlets and plant biomass is largely dependent on culture medium, growth hormones, culture conditions, and some other biotic and abiotic factors. To ascertain successful outcome of finely tuned cell division and differentiation steps, plant tissues (explants) were cultured under aseptic and controlled condition and different concentration and combination of auxin-cytokinin ratio aids the process. There are reports of using additives like charcoal, adenine sulfate, and coconut water to enhance the tissue differentiation. However, according to some researchers, use of PAs along with plant growth regulators accelerates the whole process manifold. There are several reports of PA supplementation in the culture medium enriched with auxin and/or cytokinin, and it positively influences stages of callogenesis, rhizogenesis, somatic embryogenesis, and callus production. In some plants, in vitro cultures, aided with PAs, actually enhance secondary metabolite content as PAs are great mediator of stress tolerance and regulator of many physiological and metabolic conditions.
Methodology
To understand the role of polyamines on growth, reproduction, and secondary metabolite production of medicinal plants, we have retrieved polyamine-related data from various reliable scientific search engines like Google Scholar, PubMed, Scopus, and ResearchGate using relevant key strings like “polyamine elicitation”, “plant hormone polyamine “, “medicinal plant polyamine”, “polyamine mode of action”, “polyamine secondary metabolite”, and “polyamine stress reversal”; from a large number of cited materials, we have categorized the effect of exogenously applied polyamines. Moreover, we have discussed the role of polyamines in abiotic stress reversal, antioxidant defense, hormonal regulation, ion channel metastasis, photosynthetic energy utilization, and carbon and nitrogen assimilation and highlighted secondary metabolite production via the application of metabolic engineering involving polyamines or polyamine biosynthetic genes. In Tables 1 and 2, we have elucidated how polyamine application could modify the growth stimulation as well as metabolite elicitation in vitro; Table 3 details the studies conducted on polyamine-regulated hairy root culture; Tables 4 and 5 represent the details of transgenic studies conducted to investigate the role of PAs in alkaloid, endogenous polyamine, and conjugated polyamine accumulation; and in Table 6, we have discussed the status of transgenic studies on polyamine-mediated stress reversal. Figure 1 presents a schematic representation of polyamine-induced alkaloid production in medicinal plants.
Polyamines: origin, biosynthesis, and role in stress response
PAs are ubiquitous in nature, and inside plant cells, they are present in their highly reactive polycationic form. Their low-molecular-weight, ability to form ionic and electrostatic linkages, and conformational stability help in inter- and intra-cellular translocation and compartmentalization; maintain the level of free and conjugated PAs via regulated inter-conversion; and help to directly influence enzymatic action, protein synthesis, and cellular permeability that collectively contribute in developmental processes as well as activate secondary metabolite pathways in response to external and internal stresses (Mustafavi et al. 2018). The diamine putrescine functions as the substrate for Spd production which is aided by spermidine synthase, and subsequently spermine synthase converts Spd to Spm. It is important to note that apart from vacuole, cell membrane, and other organelle, Spm is located in the plant nucleus also. However, a parallel pathway that involves amino acid methionine aids both in conversion of Put to Spd and in synthesizing cadaverine (cad). The action of S-adenosylmethionine synthase (SAMS) and S-adenosylmethionine decarboxylase (SAMDC) limits the conversion rate and Spm synthesis. Put itself is the product of urea cycle that involves ornithine (mammals, fungi) and arginine (plants, bacteria). In plants, conversion of arginine to Put is controlled by ADC or arginine decarboxylase which is the limiting and crucial stage of polyamine biosynthesis in plants as this enzyme is reported with nuclear or chloroplastic localization. However, polyamine oxidases or PAOs are responsible for reverse reaction which converts Spd from Spm and Put from Spd and they also trigger the formation of H2O2 (Mustafavi et al. 2018).
The rise in PA level in plant cell is considered as part of the stress (salinity, drought, pollution) reversing mechanism. The involvement of polyamine oxidases (PAOs) and the production of H2O2 in guard cells are other important factors in ethylene-induced stomatal closure (Hou et al. 2013). H2O2 is actually a key component in abscisic acid (ABA)–induced stomatal closure and can be synthesized by copper amine oxidase (CuAO). This enzyme may enhance [Ca2+]cyt levels in guard cell cytoplasm (as observed in Vicia faba) which in turn causes stomatal closure. Another interesting fact is that Put functions as the substrate of the enzyme and reproduces similar effects as ABA (apoplastic CuAO activity enhanced) with the help of intermediate activity of calcium messenger. ABA is the prime upstream signal that boosts H2O2 production via amine oxidases as well as activates the polyamine catabolic pathway in Vitis vinifera (Konstantinos et al. 2010). Generation of oxidative damage response or significant increase in reactive oxygen species (ROS) level in guard cells induces stomatal closure which is triggered by Put, Spd, and Spm in connection with the presence of higher amount of nitric oxide (NO). In this process, both NADPH oxidase and amine oxidase are involved. A comprehensive study conducted on Arabidopsis thaliana revealed the reversal of stomata closure could be achieved by the application of compounds like cPTIO (NO scavenger), catalase (ROS scavenger), L-NAME (NOS-like enzyme inhibitor), diphenylene iodonium (NADPH oxidase inhibitor), 2-bromoethylamine (CuAO inhibitor), or 1,12 diaminododecane (PAO inhibitor) (Agurla et al. 2018). It was also observed in Arabidopsis thaliana that under the ethylene-induced stress response hydrogen peroxide (H2O2) functions as the signaling molecule, a phenomenon related with NADPH oxidases as well as peroxidases. However, the polyamine oxidases (PAOs) are also involved in the process as the application of PAO blockers prevent ethylene-induced stomatal closure and subsequent H2O2 production in Arabidopsis thaliana (Hou et al. 2013). The modulation in cellular polyamine content may trigger hypersensitivity in plants in response to particular form of abiotic stresses. In Arabidopsis mutant (acl5/spms), absence of Spm enhanced drought sensitivity and could only be reversed by Spm pretreatment. Moreover, it can modulate the stomatal movements as observed in mutant plant that kept stomatal apertures open, while in wild plants all stomata were closed (Yamaguchi et al. 2007). In Citrus reticulata Blanco (red tangerine), pre-treating in vitro–grown plantlets with 1 mM Spm causes less wilting, enhances the activities of antioxidant enzymes like peroxidase (POD) and superoxide dismutase (SOD), and prevents electrolyte leakage or water loss when compared to other PAs. Interestingly, Spm lowers the level of ROS by triggering the overexpression of arginine decarboxylase which in turn boosts Put production from arginine (Shi et al. 2010).
Polyamines in plant growth and development
PAs are mostly applied exogenously under in vitro condition as an additive to PGR-enriched culture media to check its role in different modes of direct and indirect organogenesis and to compare the outcome with the normal untreated plants. However, exogenous application may include seed priming (to check the effect of PAs on seed germination) and foliar spray (to screen the role of PAs on biomass enhancement or stress tolerance). In addition to that, many papers are available where in vitro application of PAs has been investigated in amelioration of stress response or enhancement in stress tolerance. Metabolic engineering, study of transgenic mutants, and PA-induced elicitation of secondary metabolite production from important medicinal plants are also an intrinsic part of polyamine-related research. In the following section, we have discussed different aspects of PA-related research in the context of high-value medicinal plants and their phyto-constituents. In Table 1, we have highlighted the role played by polyamine in micropropagation of medicinal plants, whereas in Table 2, we have discussed elicitation of secondary metabolites via polyamine treatment.
Polyamines in seed germination, biomass increase, flowering induction, and plant growth
Polyamine plays a critical role in seed germination and maturation. Actually, the growth phase regulation is triggered by increase and decrease in cellular polyamine content. The concentration of Put increases during the primary cell division phase or imbibitions and decreases during the cell elongation phase when radicle and plumule formation takes place following the transport of Spm and Spd from megagametophyte (Pieruzzi et al. 2011). The effect of seed priming technique has been screened in many medicinal plants where the PAs not only aided in dormancy breaking but also induced stress tolerance and antioxidant damage reversal. Attenuation of salt stress was observed in Borago officinalis L. (Shekari et al. 2015), Vigna sinensis L. (Alsokari 2011), and Matricaria chamomilla L., drought stress was countered in Valeriana officinalis L. (Mustafavi et al. 2016), and the temperature sensitivity was improved in Foeniculum vulgare Mill. after PA treatment (Mustafavi et al. 2015a). Among all the PAs, the sole application of Put, via seed priming or foliar spray method, was found to be much more beneficial in terms of increasing nutritional values, plant yield, and overall growth in many commercially valuable plants. In Nicotiana tabacum L., Pelargonium graveolens L. Hér., Matthiola fruticulosa L., Dianthus caryophyllus L. Maire, and Catharanthus roseus L., it improved seedling growth, leaf diameter, dry weight, and photosynthetic pigment content (Ayad et al. 2010). Exogenous polyamine application was found to activate metabolic cascades in relation to flowering in some ornamental flowering plants like Chrysanthemum indicum L., Gladiolus grandiflorus Andrews, and Dahlia pinnata L., where the application of foliar spray, composed of 50–300 mg/ml Put, increased the flower diameter and plant yield (number of flower per plant) significantly (Mahgoub et al. 2011; Mahros et al. 2011). Similarly in Allium cepa L., the bub yield improved after the application of Put (25, 50, and 100 mg/l) as foliar spray (Amin et al. 2011). So, when we want to emphasize on in vitro elicitation and polyamine-induced enhanced production of SMs, we need to remember, even the exogenous application of foliar spray may come handy for this aspect which may be of great help to the farmers or horticulturists. Interestingly the rise in antioxidant enzyme activity was observed in Valeriana officinalis L. after applying foliar spray containing 0.5 and 1 mM Spd (Mustafavi et al. 2015b). In Nicotiana tabacum L., application of foliar spray with 0.5 mM Put, Spd, and Spm increased ATP synthesis and utilization of light energy (Ioannidis and Kotzabasis 2007). Exogenous application of Spd improved seed germination, water stress reversal, starch metabolism, antioxidant defense, metallothionein, and other relevant gene expression in white clover (Li et al. 2016).
Shoot formation and multiplication
It was observed under both in vivo and in vitro studies that PAs are important for the growth and development of plants as they influence critical processes like cell elongation, proliferation, and differentiation; promote germination as well as embryogenesis; and regulate flowering response and senescence (Tiburcio et al. 2014). In meristematic tissues, PA concentration is reported to be high, and mainly Spd promotes the stem elongation (Takahashi and Kakehi 2010) and pollen development (Tiburcio et al. 2014) by improving the cell division rate. Plant growth regulators (PGRs) like gibberellic acid (GA) or auxins are directly influenced by presence of PAs, and in many instances, presence of polyamine in PGR-fortified tissue culture media actually increases the outcome manifold (Pieruzzi et al. 2011). Optimization of in vitro organogenesis is important to elucidate stages of shoot development and other physiological processes (Aragão et al. 2017). Ajithan et al. (2019) reported profuse multiplication (65.1 shoots/explants) after supplementing MS media with 20 mg/l Spd and 1.5 mg/l BAP. Moreover, shoot elongation rate was also increased after PA treatment in shoots. According to previous reports, Put treatment could alter protein metabolism included in different developmental processes, stress tolerance, and synthesis of bioactive secondary metabolites (Aragão et al. 2017). The effective role of Put was recorded to be 70 mg/l that improved shoot elongation and regeneration, and in Momordica charantia L. (Thiruvengadam et al. 2012), similar results were obtained. Khalil et al. (2016) suggested synergistic interaction between exogenous PGRs and PAs that ultimately triggers shoot proliferation. In case of in vitro culture of Achras sapota L., shoot bud proliferation and multiplication could be suppressed by inhibitors like α-difluoromethyl ornithine or α-difluoromethyl arginine (1 mM). However, combined application of Put (31.04 μM), Spm (49.42 μM), and Spd (103.27 μM) resulted in higher number of shoot/explant in S. officinarum culture (Sathish et al. 2018). In general higher cytokinin and low auxin ratio is preferred for induction of shoot proliferation. In Gloriosa superba, a similar type of combination of BAP (1.5 mg/l) and NAA (0.6 mg/l) induced multiple shoot formation but Put (15 mg/l) supplementation increased the phenomenon manifold (Sivakumar et al. 2019). However, exogenous supplementation of PAs (Put and Spd), or its precursors (arginine and ornithine), could revert hyperhydric conditions in apple cultivars. Culture medium fortified with Spd, ornithine, or arginine (10–5 M) reduced hyperhydricity percentage by 50%. Exogenous supplementation actually aids in the maintenance of high endogenous levels of PAs (Tabart et al. 2015). In Yam, both PAs and precursors (Arg and Orn) could accelerate tuber development and significantly increased tuber size (OndoOvono et al. 2010).
Tuberization
The promotive effect of PAs on yam tuber formation and development is well established. In case of Dioscorea sp., application of PAs and their precursors and inhibition of some metabolic pathways actually stimulate fast and effective microtuber formation. Moreover, exogenous Put could increase endogenous PA and auxin level which is not observed in case of Spd and Spm (OndoOvono et al. 2010). In Tulip, high bulbing competence is associated with higher endogenous Spd concentration, and shoot proliferation is triggered by higher level of endogenous Put, but this phenomenon differs from cultivar to cultivar. Spd-rich varieties generally show high bulbing ability. However, exogenous addition of all these Pas (50 and 100 µM), their precursors (arginine, ornithine, and methyl jasmonate) (500 and 1000 µM) exhibited positive effect on bulb formation during successive micropropagation stages (Podwyszyńska et al. 2015). Formation of protocorm like bodies from fractioned explants (tuber part) was observed by adding PAs (0.2, 0.4, and 1.0 mM) in MS medium supplemented with 4.44 µM BAP. It was observed that 0.4 mM Put caused highest conversion, whereas results of Spd and Spm treatments were not significant. The natural conversion of protocorm to shoot requires higher amount of endogenous Spd and Put. In Dendrobium huoshanense, this process could be manipulated by exogenous supplementation of PAs. PA translocation played a major role in increasing biomass in case of turmeric (Ferreira et al. 2016). However, among PAs, Spm (100 µM) was found to be the most effective.
Rhizogenesis
Polyamines play an important role in primary root development and trigger the adventitious root formation which in turn is beneficial for in vitro root culture for those medicinal plants which are reported with root-specific bioactive marker compounds like Rauwolfia serpentina (reserpine) and Hemidesmus indicus (2-hydroxy-4-methoxy benzaldehyde). Among the PAs, Put is more efficient in promoting rhizogenesis. In Pisum sativum L., 33.66 roots/ shoot was achieved after application of 30 mg/l Put and 0.6 mg NAA (Ajithan et al. 2019). Moreover, photosynthetic pigment, antioxidant enzyme level and profile, and chloroplast content increased remarkably. Aragão et al. (2017) reported exogenous Put mediated balancing of endogenous level of total free and bound Put. Different metabolic cascade, endogenous PAs, and biochemical markers (peroxidases) are involved with complex process of morphological changes specially rhizogenesis. Similar reports were retrieved from W. somnifera (Sivanandhan et al. 2011). Put (93.12 μM), Spd (68.84 μM), and Spm (24.71 μM) triggered profuse rooting (average 57 roots/shoot) (Sathish et al. 2018). Occurrence of rooting in medicinal plants and vegetables under in vitro condition is often not so cost-effective, but in Cynara scolymus L. (artichoke), root formation was promoted manifold after adding Put into culture medium (Guen-Le and Hourmant 2001).
Callus formation
Spermidine (2 mM) combined with ethylene inhibitor aminoethoxyvinylglycine (0.5 µM) could significantly enhance shoot regeneration. Moreover, the same concentration of Spm with additive silver thiosulfate (60 µM) and 9 µM 2,4-D increased stable Agrobacterium-mediated transformation in woody Apricot plant and produced large amount of GFP-expressing callus mass. Put is also effective in maintaining transformation efficiency (Petri et al. 2005). Momordica charantia (bitter melon) cultured in Gamborg’s (B5) medium showed profuse callus formation when cultured with 3.0 μM NAA, 1.0 μM TDZ, and 1.0 μM Put (Thiruvengadam et al. 2012).
Somatic embryogenesis
Somatic embryogenesis is dependent on several internal and external factors, totipotency of the plant cell, frequency and intensity of embryogenesis, stability and proper maturation or conversion of heart, and globular and torpedo stages into mature embryo. During different stages of embryogenesis, different PAs get activated. In the initial stage of high cell division, Put plays the fundamental role while during rapid cellular elongation phase at the end of embryo growth, synchronized role of Spm and Spd is important. PAs play a stimulatory role in this conversion process either singly or along with precursor molecules or ethylene. According to Santa-Catarina et al. (2007), Put is responsible for the somatic embryo generation and Spd as well as Spm promotes maturation by regulating different developmental cascades. In fact, PAs work together with ABA, IAA, and other amino acids in the development and maturation of embryo as determined from their respective concentration via HPLC study of Ocotea catharinensis zygotic embryo (Santa-Catarina et al. 2006). In guava, exogenous supply of PAs, viz., putrescine, spermidine, and spermine, could modulate this phenomenon (Akhtar 2013). In Daucus carota L., supplementation of Spm enhanced somatic embryogenesis (Takeda et al. 2002). During embryogenesis, additives like aminoethoxyvinyl glycine reduce ethylene production, or some additives like silver thiosulfate or PAs provide protection against ethylene-induced stress. In Saccharum officinarum L., 500 µM Put reportedly increased somatic embryo formation (Reis et al. 2016).
Effect of polyamines on photosynthesis and energy utilization
The application of foliar spray in Nicotiana tabacum L. improved the utilization of energy derived from photophosphorylation and enhanced ATP synthesis up to 70% (Ioannidis and Kotzabasis 2007). Later, it was reported that the application of nutrient solution enriched with polyamine combination (Put + Spd + Spm) increased NPR (net assimilation rate) in another species (Nicotiana rustica L. cv. Basmas) of Tobacco (Hajiboland and Ebrahimi 2011). In Dahlia pinnata L. and Catharanthus roseus (L.), Put-enriched foliar spray improved the total chlorophyll and carotenoid contents (Mahgoub et al. 2011), whereas in Valeriana officinalis L., Put along with Spd and Spm enhanced pigment content (Mustafavi et al. 2016). Exogenous application of Spm promotes the rate of photosynthesis and accumulation of carbohydrates in roots and leaf tissues of Cucumis sativus L. (Chen et al. 2011). On the other hand, Chen et al. (2011) reported the positive role of Spd in influencing the Rubisco activity which includes the reduction of the carbohydrate storage in leaves. Application of Put and uniconazole influenced the flower characteristics and total content of photosynthetic pigments in Chrysanthemum indicum L.
Polyamine-triggered biotic and abiotic stress reversal in medicinal plants: underlying mechanism
The detailed study of in vitro culture of medicinal plants and different aspects of micropropagation has elucidated how PAs are influencing the role of PGRs and what are the outcomes and how these are relevant for medicinal plant harvesting. Though it is true that almost all PAs work as the growth stimulant, in some cases PAs mimic the activity of plant hormone. Moreover, the complex PA biosynthesis is able to up- and down-regulate different metabolic pathways of plant hormones (Yan-hua et al. 2016). However, most interesting part of ABA-regulated stress response mediation includes stomatal opening and closure in which the level of ROS triggers the biosynthesis of signaling molecules. Interestingly, polyamine biosynthesis pathway is quite complex and its metabolism is capable to induce the synthesis of nitric oxide (NO) as well as H2O2; integrate with ROS signaling; and modulate ion channel activities and Ca2+ homeostasis, potentially by intermediate reaction or by unexplored route of direct conversion (Yamasaki and Cohen 2006). To decipher the underlying molecular mechanisms, one needs to understand the loss and gain of function mutations linked with polyamine metabolism.
Hormonal regulation and stress reversal
The detailed study of in vitro culture of medicinal plants and different aspects of micropropagation has elucidated how PAs are influencing the role of PGRs and what are the outcomes and how these are relevant for medicinal plant harvesting. Though it is true that almost all PAs work as the growth stimulant but in some cases PAs mimic the activity of plant hormone. Moreover, the complex PA biosynthesis is able to up- and down-regulate different metabolic pathways of plant hormones (Yan-hua et al. 2016). In many instances, PAs and plant hormones induce similar activity in plant but work independently. In Lycopersicon esculentum, reversal of cold stress is the result of independent action of Put and ABA (Kim et al. 2002). However, most interesting part of ABA-regulated stress response mediation includes stomatal opening and closure in which the level of ROS triggers the biosynthesis of signaling molecules. Interestingly, polyamine biosynthesis pathway is quite complex and its metabolism is capable to induce synthesis of nitric oxide (NO) as well as H2O2, potentially by intermediate reaction or by unexplored route of direct conversion (Yamasaki and Cohen 2006). The release of PAs is both plant’s response to stress and subsequent protective mechanism triggered by it which may include activation or inhibition of many enzyme-generated metabolic cycles. ABA, which reduces water loss, by inducing stomatal closure, is subjected to over-synthesis in response to drought condition, and in guard cells, endogenous polyamine level as well as exogenous polyamine application could manifest the desired outcome. This procedure involves the interplay between guard cell turgor pressure, transcription factors, ROS generation via apoplastic amino oxidases, production of H2O2 NO signaling, Ca2+ influx, and polyamine synthesis, which are intrinsically entangled with the ABA-induced stress responses (Yamasaki and Cohen 2006).
Interplay of polyamines and plant growth regulators
PAs reportedly mimic the activity of plant hormones, and the physiological processes or the metabolic synthesis steps are quite similar in many cases (Yan-hua et al. 2016). The activity of PAs is mostly correlated with the activity of plant hormone abscisic acid or ABA. Like PAs, ABA is involved with the integral part of stress signaling, abiotic stress reversal, and growth and development of plants. Elucidation of metabolic pathways has revealed the polyamine and ABA metabolism actually support each other as these two components work in sync with each other to regulate stress mechanism and plant defense. The enhanced production of ABA after exogenous application of Put indicates these two work together to determine the utilization of photo-assimilated carbon and control source to sink and vice versa transport of macro- and micro-molecules, enzymatic proteins, and other metabolites. Basically PAs and ABA activity and metabolism enable them to function as stress-modulating molecules in plant system where they control the production of other metabolites; regulate up- and down-regulation of growth-promoting transcription factors; activate antioxidant enzymes; trigger secondary metabolite accumulation in specific tissues; and increase or decrease cell division and DNA replication (Mattoo et al. 2015). They actively participate in free radical scavenging, prevent tissue damage (necrosis), and regulate senescence and fruit ripening (Sheteiwy et al. 2017). Furthermore, they assist in different transcriptional and translational signaling system; maintain inter- and intra-cellular cation stoichiometry; ensure ion channel homeostasis; and balance cytosolic and vacuolar Ca2+ content which in turn regulate the turgor pressure and stomatal opening and closure in response to drought stress (Pottosin and Shabala 2014). In ABA-, NO-, GABA-, ethylene-, and H2O2-regulated stress response networks, PAs play a critical role. In many cases, it has been reported the scavenging activity of PAs, ROS generation, regulation of nitrogen metabolism, and carbon assimilation may be directly linked to endogenous phytohormone level (Li et al. 2016). Interestingly, activity of polyamine is correlated with another important plant growth regulator, ethylene as both for putrescine and ethylene, SAM acts as the precursor molecule. The competition between these two compounds for SAM indirectly affects membrane stability. The combination of ethylene and PAs is effective in initiating somatic embryogenesis in vitro. Jasmonic acid signaling pathway and conjugated PAs also contribute in polyamine-phytohormone cross-talk. In some cereals, polyamine application improves the seed germination percentage which may be attributed to complex co-activity of PAs and other phytohormones. Enhanced concentration of ABA, GA, and IAA increases soluble sugar content in germinating seeds and prevents starch degradation in seeds which could be achieved by Spd and Spm pretreatments (Yang et al. 2016).
In previous sections, we have discussed how PAs contribute in growth and development of in vitro–grown plants when applied exogenously with plant growth regulator (PGR)–fortified culture media. It has been found that PAs and gibberellic acid (GA) synergistically elicit cell division and DNA replication. GA reportedly influences flowering and fruit ripening, but during seed germination, the degradation of seed starch aids in polyamine-triggered seed germination and dormancy breaking (Yang et al. 2016). In Cynara scolymus, GA stimulated Put biosynthesis via activation of ornithine decarboxylase pathway during in vitro rooting (Guen-Le and Hourmant 2001). Furthermore, plantlets or plant tissues harvested in auxin-rich medium has demonstrated higher accumulation of PAs which significantly boosted cell division and morphological diversification as a part of maturation and development (Pieruzzi et al. 2011). Synergistic application of kinetin and spermine induced positive physiological changes in Vigna sinensis, under salt stress (Alsokari 2011).
Effect of polyamine on cell growth and division
PAs actively participate in cell division, tissue diversification, and morphological differentiation in different stages of growth and development. There are several reports where polyamine treatment mediates protective role in DNA damage reversal caused by oxidative stress. Furthermore, they regulate genetic shuffling on expression of protein, transcription factors, and enzymes. The polyamine-induced regulation of post-translational modification alters cell cycle pattern, membrane stability, and conformation changes of DNA (Dey et al. 2014). Due to polycationic nature, PAs are effective in maintaining ion channel homeostasis, and again due to the same nature, PAs easily bind with anionic phosphate groups present abundantly in DNA, RNA, and translational proteins. Polyamine-triggered production of H2O2 participates in biotic and abiotic stress signaling and membrane permeabilization (Mattoo et al. 2015). Many regulatory signal transduction pathways present in plant do intersect with polyamine biosynthetic pathways which modulate the intra- and inter-cellular biochemical pool. PAs could bind directly with DNA and are able to alter the DNA–protein interactions which may be the prime molecular mechanisms involved in polyamine-induced cell proliferation. Consistent results have shown protective anti-apoptotic nature of PAs, but the catabolism of H2O2 is essentially linked with cell death response.
Ion channel homeostasis
In root epidermal and cortical cells of Hordeum vulgare seedlings, PAs (1 mM) are reported for involvement in K + /Na + ion channel homeostasis which in turn improves the salinity tolerance (Zhao et al. 2007). Actually, higher level of cytoplasmic Ca2+ and vacuolar influx of Na + /K + prevents their entry into the cytoplasm (Yamaguchi et al. 2006). It has been found that Spm plays an important role in suppression of the salt sensitivity (Yamaguchi et al. 2006). The activation of phospholipase C could alter the cytosolic Ca2+ level as it causes the hydrolysis of PIP2 to IP3 and intracellular Ca2+ release which as a result triggers stomatal closure in guard cells. Some polyamine-binding proteins have been reported in cytoplasm and plasma membrane in which phosphorylation and dephosphorylation is regulated by polyamine metabolism activated by plant stress stimuli (Michard et al. 2005). Zhao et al. (2007) suggested the identification of structural elements of ion channels involved with polyamine action could act as “extraordinary relevance” in elucidation of polyamine action. Though similar studies are not available on medicinal plants, in barley, high salinity tolerance was achieved by improving the homeostasis of K + /Na + which includes the repression of Na + influx into roots and the prevention of K + loss from shoots (Zhao et al. 2007). In pea mesophyll protoplasts, K + and Na + channels were blocked through NSCC or nonselective cation channels where plant stress stimulated the reduction of Na + uptake and K + leakage from the mesophyll cells (Pottosin and Shabala 2014). The polycationic nature of PAs is helpful in the regulation of cellular homeostasis as unlike the toxic metal and metalloids, Al3+, Gd3+, La3+, and PAs are non-toxic in nature and their accumulation in cytosol is always regulated by other metabolic pathways (Liu et al. 2000).
Regulation of other metabolic pathways
Polyamine biosynthesis network is closely interconnected with many other important metabolic pathways where the PAs actually dominate the production or enzymatic inhibition or compound-mediated feedback inhibition which cumulatively contributes in plant stress responses. Arginine which is the polyamine precursor molecule in plants could produce citrulline and NO with the help of nitric oxide synthase. Moreover, it could get converted into L-ornithine via the urea cycle which could increase the level of amino acid proline. As the action of ADC and conversion of arginine to Put are the limiting steps, the content of arginine may influence concentration of these molecules. The conversion of Spd to Spm may involve the production of another polyamine, thermospermine, which is generated by the enzyme thermospermine synthase. Spm on the other hand controls the production of pyrroline, β-alanine, and H2O2. The formation of proline from ornithine involves intermediate step of glutamate production. Glutamate with the action of glutamate decarboxylase produces ϒ-amino butyric acid (GABA) which may also be generated with the action of diamine oxidases or DAOs. DAOs, with the help of pyrroline, produce GABA from Put. In plants senescence and programmed cell death could be triggered by trans-glutaminase that alters polyamine activity (Del Duca et al. 2014). Ethylene which functions as a stress signaling molecule has a common precursor, S-adenosyl methionine or SAM, with PAs. This feature is responsible for antagonistic effects as observed during leaf maturation, flower wilting, fruit ripening, and senescence. Glutamate to glutamine inter-conversion is also another common chemical reaction. However, glutamine along with Put may trigger physiological stress tolerance response in Allium cepa (Amin et al. 2011). On the other hand, PAs induce the production of NO, GABA, and agmatine. Moreover, the PAOs and DAOs are involved in H2O2 generation and biotic and abiotic stress signaling. Actually, polyamine metabolism has strong metabolic connection with stress response. Some compounds which are involved in TCA cycle and take part in proper nitrogen assimilation have reportedly increased during water scarcity; proline accumulation increases during salt stress and other abiotic stresses. Interestingly, increased level of GABA and D1-pyrolline indicates enhanced utilization of Put as substrate by DAOs. On the other hand, enhanced proline content triggers utilization of L-ornithine as precursor for diamine putrescine (Mohapatra et al. 2009). Nevertheless, the PAs are interconnected with numerous metabolic pathways which are related with hormones, precursors for stress signaling molecules, substrates, etc.
Effect on nitrogen metabolism
PAs have many protective and regulatory role interlinked with the primary and secondary metabolite-producing pathways which affect cell division, DNA synthesis, tissue maturation, developmental cascade, hormone function, membrane integrity, ion channel homeostasis, carbon and nitrogen (C/N) balance, reproductive success, and overall survival (Handa and Mattoo 2010). Furthermore, along with these complex physiochemical networks, PAs regulate the nitrogen metabolism and photosynthetic carbon assimilation which are crucial for energy-driven catabolic and anabolic processes as well as maintenance of proper C:N ratio (Majumdar et al. 2016). Spd influences the photosynthesis and efficient energy utilization which in turn maintains carbon skeleton of the organism. It has been assumed that Spd enhances the activity of rate-limiting enzyme, nitrate reductase, which significantly influences the nitrogen metabolism (Miura 2013). The role played by PAs in regulating other metabolic pathways is relevant here as excess ammonium derived from restricted photosynthesis, nitrate reduction, and/or photorespiration were converted to glutamate which acts as precursor molecule for both proline and Put (Mattoo et al. 2010). Glutamate is essential in the supply of succinate used in TCA cycle. α-Ketoglutarate synthesizes lysine which acts as the precursor molecule of cadaverine responsible for the production of different N-rich secondary metabolites (Moschou et al. 2012). PAOs are responsible for interconversion of Put, Spd, and Spm, and DAOs trigger formation of D1-pyrroline, H2O2, and NH3 from Put. Moreover, the intermediates like GABA, NO, H2O2, and D1-pyrolline also play an important role in nitrogen assimilation. H2O2 functions as the signaling molecule, regulates cell wall development, ABA-induced stomata movement, cell cycle regulation anti-oxidation, etc., whereas GABA acts as a key signal transduction molecule involved in oxidative stress reversal (Angelini et al. 2010). Conjugated polyamines, which are more stable due to their conjugation with different phenolic acids, end in the storage tissues as accumulated products which in turn regulates polyamine concentration and C:N ratio in intra- and inter-cellular compartments. Furthermore, they are mostly accumulated in seeds and plays an important role in germination and growth and development of the young seedling which also aids in maintaining the nitrogen cycle of the plant system (Bassard et al. 2010).
Role of polyamines in stress reversal and enhanced secondary metabolite production
PAs promote the secondary metabolite production as elicitors which regulate different phases of metabolite biosynthesis; or they help in higher accumulation of active phyto-constituents by increasing the biomass, or they indirectly activate the metabolite production by stimulating different signaling pathways as a part of stress reversal process. There are numerous examples where PAs played a decisive role in production of bioactive natural products. This feature has two broad applications, first it aids in plant defense and second it multiplies commercial acceptance of elite chemotypes of medicinal plants which are showing better yield of metabolites. The micropropagation of medicinal plants which takes place under controlled environment often imposes many stress stimuli to regenerated plants. With growth, the decrease in macro- and micro-elements in culture medium often triggers higher release of phenolics to culture medium; often the growth gets limited due to space in culture vessel, decline in water content, competition for survival (in case of multiplication), and wilting, some common phenomenon observed in tissue culture condition. Moreover, during acclimatization and field transfer phase, rapid and acute changes in almost all climatic parameters, sudden transition from hetero-trophism to complete auto-trophism often triggers high level of stress responses in plants. In wild plants also, different environmental stress signals in the form of water scarcity, changes in rain pattern, salinity, freezing cold, polluted air, heavy metal–accumulated soil, and absence of pollinators often delimit their normal growth and development and stress reversal system gets activated to reverse the physiological, cellular, and chemical damages. Like wild plants, in vitro plants also demonstrate their own combative mechanism which includes higher production of stress-modulating phytochemicals (secondary metabolites, proteins, signaling molecules, or polyamines). However, when PAs are applied to the culture medium exogenously with PGRs or as seed priming agent or foliar spray, they trigger a cascade of metabolic action exhibited by many positive responses (Pal et al. 2015). The accumulation of PAs for the regulation of stress (drought, temperature variation, oxidative damage, salinity, or insect attack) also applicable for medicinal plant where exogenous application of mainly Spm, Spd, and Put has significantly transformed response of plants to different environmental stresses (Takahashi and Kakehi 2010). In Valeriana officinalis L., foliar application of PAs is effective in combating the water stress (Mustafavi et al. 2016); the activation of antioxidant defense system was noted in Panax ginseng CA Meyer after Spd treatment (Parvin et al. 2014), and the Spd priming significantly improved α-amylase activity under chilling stress (Sheteiwy et al. 2017). Moreover, the overexpression of PA-related biosynthetic genes (SPDS, ADC, ODC, and SAMDC) increased stress tolerance by regulating many metabolic responses (Liu et al. 2017). The elicitation triggered by polyamine often increases total biomass in stressed plants. The use of natural compounds to improve agronomical traits in stress-resistant varieties is a key feature of sustainable agriculture, and in Table 6, we have provided the details how PAs are improving commercial value of many agricultural crops and cereals. Hence, use of exogenous application of polyamine could be an eccentric tool to revalorize modern agricultural industry. The polyamine-triggered enhanced production of secondary metabolite is the result of already operative “direct stress combative” mechanism, but under in vitro condition, increased production of biomass or application of hairy root culture for root-specific metabolites could also be achieved. Put signaling enhanced ginsenoside biosynthesis in Panax quinquefolius roots during bioreactor culture (Yu et al. 2016). In another experiment, exogenous application of ABA and PAs enhanced salvianolic acid contents in Salvia miltiorrhiza bge. F. alba hairy root culture (Hao et al. 2012). Synergistic interaction between endogenous PAs with various plant growth regulators could stimulate different metabolic pathway, exhibit stress tolerance mechanism, and regulate array of signal transduction networks in plants (Gupta et al. 2013a,b). All these regulatory mechanism could attribute in modulation of in vitro regeneration pathways, could raise single cell or haploid culture, and even alter secondary metabolite production rate (Casas 2014). Such in vitro manipulations are critical to understand the role of PA mutants on growth and development. Moreover, the effective role of PAs in stress-triggered accumulation of bioactive metabolites could pave an economic way to boost industrial production of valuable and expensive metabolites (Diwan and Malpathak 2012; Dey et al. 2019). Addition of PAs significantly enhanced concentration of pharmaceutically important metabolites like psoralen, bergapten, and xanthotoxin in Ruta graveolens shoot cultures (Diwan and Malpathak 2012). Similarly in Stevia rebaudiana, PA treatment increased stevioside production (10.2 mg/g dry shoot biomass), total phenolic and flavonoid content, and shoot biomass (Khalil et al. 2016). Increased phenolic and flavonoid concentration, correlation between antioxidant property, and total phenolic and flavonoid count, as well as enhanced production of bacoside in vitro, potentiate the abiotic stress–ameliorating property of PAs (Dey et al. 2019). In Trachyspermum ammi L., foliar application of Put increases stress tolerance and essential oil accumulation (Zeid et al. 2014). A similar observation was reported in lemongrass after application of 100 mg/l Spm and pyridoxine (Orabi et al. 2015) (Table 2, 4).
Polyamines and plant biotechnology: Role of microbes
PAs are also known to be involved in plant biotechnological interventions where microbes are involved in many tissue culture experiments involving transgenic plants raised by the transformation of the soil microbe Agrobacterium. Spm (2 mM), silver thiosulfate (60 µM), and 9 µM 2,4-D enhanced the rate of stability in woody Apricot producing profuse amount of of GFP-expressing callus mass (Petri et al. 2005).
The hairy root culture is a significant biotechnological approach, utilizes the soil bacterium Agrobacterium rhizogenes responsible for production of profuse adventitious roots from the inoculated tissue. Scientists have utilized the plant-Agrobacterium system for the expression of desired genes which may involve stress tolerance, higher crop productivity, enhanced secondary metabolite synthesis, or simply higher accumulation of root biomass.
Hairy root culture and the application of polyamine as well as overexpression of polyamine biosynthetic genes (ODC, ADC, LDC, PMT, H6H, etc.) have proved that these types of experiments may lead to commercial production of many high-value alkaloids specially the tropane alkaloids. Furthermore, recent biochemical discoveries have elucidated the intrinsic relationship between agrobacterial infection, polyamine biosynthesis, and subsequent alkaloid production. The rolD genes present in Agrobacterium sp. encodes ornithine cyclodeaminase (OCD) responsible for the ornithine to proline conversion which also harbors some salinity tolerance. However, higher proline content hampers hydroxyproline biosynthesis which in turn reduces production of cell wall structural protein and relevant compounds. On the other hand, rolD expression is correlated with ornithine depletion which essentially affects polyamine metabolism. Apart from rolD, rolA gene is also responsible for altering the polyamine conjugation process (Pistelli et al. 2010) (Table 3). Besides, mutualism and symbiosis between plants and microbes like plant growth-promoting rhizobacteria (PGPR) and mycorrhizae relies on PA metabolism (Jiménez-Bremont et al. 2014). PGPR are considered as important elicitor molecules in plant secondary metabolite production (Asghari et al. 2020). Moreover, exogenously applied PAs promoted mycorrhizal development in vitro as well as in greenhouse conditions (Rezvanypour et al. 2015). In addition, PAs and biofilm formation by PGPR were also found to be interlinked which plays a major role in plant protection (Karatan and Michael 2013).
Role of polyamines in alkaloid production
The diamine Put is the precursor molecule of several alkaloids (nornicotine, nicotine, pyrrolizidine, and tropane alkaloids). In this conversion, PMT or putrescine N methyltransferase plays a key role as it could form the methylated version of Put, N-methylputrescine, which may form tropane or nicotine-type alkaloids with the help of DAOs. PMT shares close sequence similarity with spermidine synthase (SPDS) and spermine synthase (SPMS) which indicates occurrence of duplication and diversification of SPDS gene sequence with varied functional evolution. In another method, homospermidine synthase which works on Spd produces homospermidine that functions as the precursor compound of pyrrolizidine alkaloids. In homospermidine, Spd provides the aminobutyl group that gets combined with Put (Graser and Hartmann 2000). Sivanandhan et al. (2011) reported in Withania somnifera (L.) Dunal that polyamine-infused multiplication and rooting could trigger better withanolide accumulation. Interestingly, lysine decarboxylase or LDC that works on lysine (as ADC works on arginine and ODC acts on ornithine) produces cadaverine which is an important intermediate compound in biosynthesis of quinolizidine and piperidine alkaloids. Nicotinic acid can induce cadaverine to produce anabasine, and on the other hand, it produces one of the highly toxic tobacco alkaloid nicotine, by reacting with N-methylpyrrolinium. Alkaloids are one of the most diversified secondary metabolite groups with extensive range of bioactivities and commercial as well as medicinal application. Many of the alkaloids are structurally mimicked during the formulation of many life-saving drugs. However, in some plant families, this alkaloids act as insect repellant defense molecule which clearly suggests different direct and indirect contributions of PAs in reversal of biotic and abiotic stresses.
One of the diverse chemical groups with extensive distribution and most investigated (clinically and preclinically) tag are the alkaloids, highly bioactive nitrogen-containing natural products which are basically a kind of secondary metabolite which has a significant correlation with the “stress reversing, multi-functional” primary metabolite, polyamines. Especially the biosyntheses of tropane alkaloids and pyrrolizidine alkaloids are directly linked with polyamine metabolic pathway. Putrescine-derived alkaloids are very common in plant family of Solanaceae, some of which are categorically called tobacco alkaloids. Moreover, the source to sink translocation of these alkaloids and evolutionary pattern of homospermidine synthase, responsible for homospermidine production using Put and Spd, in different plant families and role of these compounds in plant defense (which is a prime area of polyamine activity) have widened the research based on polyamine-alkaloid cross-connection (Shoji and Hashimoto 2015) (Table 4).
Putrescine N-methyltransferase (PMT) converts putrescine to N-methylputrescine which is the primary step in the synthesis of tobacco alkaloids. Interestingly, PMT evolved from spermidine synthase (SPDS) and diversified its role and presence in different alkaloid-producing plants. N-methylputrescine gives rise to N-methylpyrrolinium cation which is an important reactive intermediate of tropane alkaloid pathway. The transgenic expression of PMT genes has revealed higher accumulation of tropane alkaloids in many plants (Table 5). Nicotine, hyoscyamine, scopolamine, etc. demonstrating many medicinal properties (most famous as anticholinergic drugs) are dependent on polyamine biosynthetic pathway for production (Junker et al. 2013). Nicotine itself is a toxic chemical, and it participates actively in plant defense against insect attacks which is again correlated with jasmonate signaling pathway (Shoji and Hashimoto 2015).
Pyrrolizidine alkaloids are important defense molecules of plants which are constitutively over-produced under acute threat of herbivory, and they cause mainly genotoxicity to insect predators. The biosynthesis of pyrrolizidine alkaloids starts with the synthesis of homospermidine which is generated via putrescine to spermine conversion catalyzed by homospermidine synthase (HSS). In the whole biosynthetic pathway, another enzyme, deoxyhypusine synthase (DHS), plays another regulatory role. It has been found that post-translational activation of eukaryotic initiation factor 5A (eIF5A) and its binding to HSS which is a subject of gene duplication are important for homospermidine production. However, under certain situation where HSS-eIF5A binding is not favored, DHS protein uses putrescine as the substrate for homospermidine generation (Ober and Kaltenegger 2009).
Metabolic engineering of polyamine biosynthetic genes: a novel approach for alkaloid biosynthesis
Exogenous application of PAs as seed priming, foliar spray, or additive in plant tissue culture media may enhance the production of secondary metabolites, but the introduction of metabolic engineering though different modes of in vitro culture has widened the scope of harvesting natural products in the most sustainable way. A number of alkaloids have been produced by introducing different transgenes in hairy root culture of the source plants. The formation of transgenic plants and overexpression of inserted gene and final identification of the desired plant products in the culture are the main theme of such experiments. Ornithine decarboxylase (ODC) and arginine decarboxylase (ADC) are the prime important enzyme in polyamine biosynthetic pathway as ornithine and arginine function as precursor molecule. However, a transgenic line of Datura innoxia Mill was created where the overexpression of these enzyme-coding genes enhanced polyamine metabolism which in turn enhanced the production of hyoscyamine (Narula et al. 2004). Putrescine N-methyltransferase or PMT is responsible for the Put methylation which uses SAM as methyl donor. However, PMT induced over-production of hyoscyamine in hairy root culture of Atropa belladonna L (Yang et al. 2011), Hyoscyamus muticus L (Moyano et al. 2003) and both hyoscyamine and scopolamine in Datura metel L. (Moyano et al. 2003) and Scopolia parviflora (Dunn) Nakai hairy root culture (Lee et al. 2005). Apart from these transgenes, hyoscyamine 6β-hydroxylase enhanced the in vitro production of scopolamine in Atropa belladonna L, Hyoscyamus muticus L., and Duboisia hybrida (Palazón et al. 2003). In hairy root cultures of Nepeta cataria L., application of auxins and PAs enhanced overall growth and rosmarinic acid production (Yang et al. 2010).
Role of ornithine enantiomers on metabolite biosynthesis
The implication of the activity of L- and D-amino acids on diversified metabolic pathways in plant regulating various modes of metabolism, growth, development, and reproductive success is immense. In plants ornithine is not only a precursor molecule for polyamine synthesis, but it is responsible for the production of nicotinic alkaloids in tobacco. However, in other plants, ornithine enantiomers significantly regulate the distribution and content of free, bound, and conjugated polyamines. In L-ornithine-enriched tobacco cells, the level of Put was significantly higher, whereas Spm and Spd productions were unregulated in D-ornithine-infused plant cells. Moreover, L-ornithine positively influenced nicotine production. Genetic studies have revealed the activity of SAMDC is highly enhanced in cells fortified with D-ornithine which catalyzes Spd/Spm biosynthesis. It has been concluded that D-ornithine regulates polyamine pool, whereas L-ornithine enhances secondary metabolite production (Gholami et al. 2013).
Conjugated polyamines and their role in plant reproduction
Polyamines not only occur in their free form in plants, but a large section of polyamines are available as hydroxycinnamic acid amides (HCCAs) or phenolamides which are the conjugated version of PAs where they conjugate with different phenolic acids (Tiburcio et al. 2014). The binding of PAs with phenolic acids alters their hydrophobicity, polarity, and other ionic characters and helps faster translocation, storage, inter-conversion, decomposition, and overall regulation of polyamine pool in plant cells (Bassard et al. 2010). Some common polyamine-hydroxycinnamic acid conjugates are caffeoyl-putrescine, coumaroyl-putrescine, feruloyl-putrescine, diferuloyl-spermidine, dicoumaroyl-spermidine, diferuloylspermine, feruloyltyramine, and coumaroyl-agamatine which have been identified in a large number of plants where caffeic acids, p-coumaric acids, and ferulic acids are the conjugation partner. However, the conjugated PAs are involved mostly in certain developmental processes which regulate the reproductive success of the plants. In flower buds of A. thaliana, hydroxycinnamic acid conjugates of Spd have been detected, and in anther tapetum, scientists have detected the presence of tricoumaroyl, tricaffeoyl, and triferuloyl conjugates (Grienenberger et al. 2009). However, under tissue culture condition, methyl jasmonate induced the accumulation of polyamine conjugates in tobacco roots, and it is concluded that the transport of this conjugates in floral initiation segments aids in breaking apical bud dormancy and initiates flower formation, maturation, pollen development, and fruit setting (Aloisi et al. 2016). Phenolamides, which are synthesized by the phenylpropanoid pathway, plays an important role in stress reversal, plant ontogeny, growth and development, fertility, and survival. During floral initiation, high concentration of phenolamides was detected in many plant species. Commercially, like secondary metabolites, these conjugated PAs are also important as some of them are enriched with medicinal property. N-feruloyltyramine, a key compound of garlic, is an effective medication against cardiovascular diseases (Park 2009). Elicitors like jasmonic acid and methyl jasmonate reportedly elicit the content of conjugated PAs (Table 4).
Constraints of polyamine action
It was observed in general that the application of exogenous PAs could improve total regeneration potential and secondary metabolite production capacity of medicinal plants under in vitro culture. The present review is a comprehensive effort to cast particularly the role played by PAs under in vitro condition. Exogenous supply of PAs facilitated regeneration and improved vigor, direct and indirect organogenesis, and acclimatization rate due to significant increase of anti-oxidation and photosynthesis (Aragão et al. 2017; Ajithan et al. 2019). However, regeneration protocol and composition of media are highly important as they determine the endogenous metabolism of chemical compounds which triggers important organogenic responses, critical for plant’s survival (Aragão et al. 2016). Endogenous PA level is another critical factor which is further dependent on enzymes like S-adenosylmethionine decarboxylase, diamine oxidase, and polyamine oxidase. In developing embryos, Put synthesis advanced by arginine decarboxylase plays an important developmental role in protein metabolism (Santanen 2000). In experiments with orchids, it was observed production of protocorm-like bodies was mostly showed by Put, and Spm and Spd were not as effective as Put. Lower Spm (0.2 and 0.4 mM) produced dark green callus, and higher concentration produced deformed protocorm. That may be due to the fact that tested concentrations of Spm and Spd were metabolically toxic to the plant growth that altered conversion rate of explants, and BAP supplementation could not reverse such fate. Red light is reported to increase level of plant growth regulators like auxins and PA concentration endogenously. The close association between Spm level and auxin-cytokinin signaling plays a key role during embryogenesis. Introduction of rhizogenesis potential in regenerated plantlets is mostly triggered by application of different concentration and combination of PAs, and high level of PAs actually controls the auxin-mediated activities. So, exogenous supplementation actually aids in maintaining endogenous level of PAs during different differential phases (Yu et al. 2019). The metabolic and physiological cascade associated with tuberization indicated co-dependence of PAs and their precursors on genotype, concentration, developmental stage, and application methods. Tissue culture techniques like somatic embryogenesis are advantageous for both in vitro propagation and gene transformation studies. The initiation, gradual development and final conversion into microshoot are critically regulated by successive changes in level of phytohormones and PAs. In Picea abies and Norway spruce cell line, AFO 541, such studies were performed and analyzed (Malá et al. 2012). In Table 6, we have denoted some examples where higher accumulation of PAs reversing the stress induced damages.
According to reports, PAs could interfere into growth and developmental process in plants under both in vitro and wild condition, and they also play immense regulatory role in stress physiology. Different transgenic approaches have revealed different responses to stress when putrescine and other higher polyamines (Spd and Spm) were used (Mattoo et al. 2010). Mainly the conserved ornithine decarboxylase pathway is responsible for Put biosynthesis, and some other arginine-dependent pathways based on arginine decarboxylase, agmatine iminohydrolase, and N-carbamoylputrescineamino hydrolase are also reported for synthesis of Put. The spermidine synthase and spermine synthase are two important key enzymes which catalyze conversion of Put to Spm or Spd. Exogenous application of PAs could enhance in vitro propagation, somatic embryogenesis, and callus formation (Takeda et al. 2002).
The exposure of plants to myriads of environmental stress factors (fluctuating climatic temperature, humidity, CO2 concentration, air quality, salinity, drought, UV-rays, heavy metal and metalloid toxicity, mechanical wounding, chemical treatment, allelopathy, pathogenic and insect attack) has diversified its defense response which is a complex metabolic plethora simultaneously ensuring survival and stress reversal (Mustafavi et al. 2018). The developmental process, reproductive cycles, and production of valuable botanical function together with stress-resistant mechanism facilitate morphological, physiochemical, and molecular defense. The optimization of productivity as well as rate of net product assimilation could be accomplished only by absolute utilization of photosynthesis derived energy. So, in plants, both types of reaction take place simultaneously where stress-resistant metabolites, solutes, proteins, and transcription factors work together to cope up with the dwelling stress with minimum expense of growth and reproduction. In medicinal plants, the secondary metabolites act as pro-defense-resistant molecules which may exhibit bioactive properties in other organisms, but what ensures the timely production and accumulation of secondary metabolites? Which metabolic reaction or what group of compounds are involved in these highly classified mechanism? It is true that a bunch of regulatory mechanisms involving the central dogma works at molecular, physiological, and morphological level, but in this, whole complex system, polyamines play a significant protective part.
The role of polyamines in increasing stress tolerance against biotic and abiotic stress factors has been studied extensively, but the elucidation of mode of action which is functional in polyamine-mediated secondary metabolite production has not been investigated widely. There are reports of polyamine-induced elicitation of natural products or how over-production of polyamines selectively improved stress tolerance in transgenic plants in vitro, but here we have focused on the prospects of exogenous polyamine application on secondary metabolite production. The most important feature of the whole research subject is to highlight specific areas operating in medicinal plants and how the action is different than the crop plants. Instead of specific reaction or biochemical pathway, the whole metabolic system does operate against any form of stress, and in cereals, fruit, and vegetables, maybe the physical manifestation is productivity, but in medicinal plants, the productivity could be pointed out in terms of enhanced production of natural products. The critical insight is that apart from biomass, primary and secondary metabolite pathways get boosted in all types of plants, but as the medicinal plants house commercially useful natural products, researchers have tried to detect the fold increase in metabolite synthesis by applying many analytical tools and techniques. Second, one needs to understand the polyamine activity involves cell cycle regulation, cellular differentiation, protein synthesis, genetic modulation, activation or inactivation of phytohormones, ion channel regulation, stress reversal, and triggering secondary metabolite production which comes synergistically with regulation of physiological, developmental, and reproductive processes.
In the review, a detailed analysis has been presented on how exogenous application of PAs significantly aids in growth, development, productivity, and reproductive success of the medicinal plants which are operating completely with simultaneous elicitation of natural products. Polyamines may act as plant biostimulants which is a novel strategy that could improve various biological responses of medicinal plants (Rafiee et al. 2016). The idea of using polyamines as elicitor molecules in vitro has yielded a positive result. In many cases where secondary metabolite accumulation takes place naturally in a particular plant part, harvesting of the particular tissues or plant part by applying selective target-oriented culture media, hormones, and/or growth regulators is quite commercially viable specially when many medicinal plants are threatened by over-exploitation. The third insight has come with the idea of improving the agronomical trait by applying polyamine exogenously. Agronomical traits not only include the better metabolite-yielding chemotypes but the plants with better survival potential, higher vigor and fertility, higher biomass, plenty occurrence of fruit and flower setting, delayed senescence, and productivity. In different medicinal plants, application of PAs reinforced these valuable traits in a positive way, and such type of metabolic regulation works at par with natural polyamine-regulating mechanism already present in plants. Basically use of polyamines is the most sustainable way of agronomic trait development with already existing mechanism. Polyamines are known for their role in enhancing stress tolerance, ion channel modulation, and nitrogen metabolism, so exogenously applied polyamines easily improve the desired traits in medicinal plants along with secondary metabolite elicitation. In recent years, the application of transgenic plants using the genes promoting different intermediate products of polyamine biosynthesis or application of transgenes that activates polyamine inter-conversion has also gathered huge interest as metabolic engineering has been successfully applied to synthesize a number of medicinal alkaloids (hyosciamine, scopolamine, anabasine, etc.). Although complete identification and activity-wise elucidation of structural proteins involved in polyamine action were not unfolded yet, quantitative trait locus mapping, cloning, traditional breeding, formation of transgenics with loss and gain function mutation (a lot studied in A. thaliana in this regard), and application of transcriptomics and metabolomics along with genome-wide association mapping may pave the crucial path futuristic research on polyamine. The fourth finding indicates polyamines work together with a number of biosynthetic pathways, but their individual role is specific. Even under stress condition, not all these polyamines show similar pattern of content-wise increase or decrease. Moreover, they combine with hormone, enzymes, growth regulators, or signaling molecules but the phase of plant development and the dosage are two very important factors which need to be customized plant-wise. Tissue specificity is another factor that needs to be considered to optimize the outcome of polyamine application in terms of secondary metabolite production.
Future perspectives
The complex metabolic pathway of polyamines, their vast significance in modulating a number of developmental processes, their active role in regulation of many toxic by-products, antioxidant compounds, growth hormones, and stress inducers have complicated the whole research aspect. Stress reversal, defense modulation, growth promotion, altering signaling pathways in metabolism, regulation of enzyme action, feedback inhibition of key compounds, enhanced biomass production, stimulation of flowering and aging response, elicitation of secondary metabolites, and genetic control along with polyamine inter-conversion and conjugation to selectively control different aspects of plants survival have been so interconnected that it is impossible to separate one point from the other. So, the fundamental focus must be on the outcome coming at the surface, the end result and their commercial application, and underlying modus operandi. The biosynthesis network, catabolic pathways, compartmentalization, and hormonal regulation are integral parts of polyamine research, and one needs to consider all these aspects while highlighting a particular functional aspect of PAs. PAs are involved in integrated stress reversal response of plants that includes all modes of biotic and abiotic stresses which in turn work as a metabolic switch to over-produce valuable alkaloids, iridoids, sterols, flavones, glycosides, or volatile oils. Endogenous expression or suppression of PA biosynthesis (studied in transgenics) as well as the exogenous application of PAs in different forms and combination and concentration yielded promising result in medicinal plant–based research where the enhanced production of natural products, faster growth and development, and bioactivity are the main objects of screening. However, the detailed structural studies, physiochemical analysis, deep-rooted investigation on genetic control, transcriptional targets, and metabolomics are essential to decipher the complex code of polyamine action in all categories of plants. The global climatic fluctuation, acute pressure on agriculture, and emergence of newer forms of threats, both biotic and abiotic, have triggered the need to understand polyamine action at its base. Nevertheless, in medicinal plants, the reversal of environmental stresses is quite intrinsic and lesser studies are there in comparison to cereal or model plant A. thaliana, but the prospects of polyamine research are immense. Polyamine may act as the most sustainable option for most cost-effective elicitation of bioactive phyto-constituents under in planta system. The future is exceptionally fruit-bearing, but intensive research is required to garner further insight on polyamine action which is necessary to manipulate its application as per the industrial demand.
References
Agurla S, Gayatri G, Raghavendra AS (2018) Polyamines increase nitric oxide and reactive oxygen species in guard cells of Arabidopsis thaliana during stomatal closure. Protoplasma 255:153–162. https://doi.org/10.1007/s00709-017-1139-3
Ajithan C, Vasudevan V, Sathish D, Sathish S, Krishnan V, Manickavasagam M (2019) The influential role of polyamines on the in vitro regeneration of pea (Pisum sativum L.) and genetic fidelity assessment by SCoT and RAPD markers. Plant Cell Tissue Organ Cult 2019:1–5. https://doi.org/10.1007/s11240-019-01699-z
Akhtar N (2013) Endogenous polyamines: a temporal cellular modulator of somatic embryogenesis in guava (Psidium guajava L.) cv. Allahabad safeda. Res Plant Sci 1:4–14. https://doi.org/10.12691/plant-1-2-1
Alet AI, Sanchez DH, Cuevas JC, del Valle S, Altabella T, Tiburcio AF, Marco F, Ferrando A, Espasandín FD, González ME, Ruiz OA (2011) Putrescine accumulation in Arabidopsis thaliana transgenic lines enhances tolerance to dehydration and freezing stress. Plant Signal Behav 6:278–286. https://doi.org/10.4161/psb.6.2.14702
Aloisi I, Cai G, Serafini-Fracassini D, Del Duca S (2016) Polyamines in pollen: from microsporogenesis to fertilization. Front Plant Sci 7:155. https://doi.org/10.3389/fpls.2016.00155
Alsokari SS (2011) Synergistic effect of kinetin and spermine on some physiological aspects of seawater stressed Vigna sinensis plants. Saudi J Biol Sci 18:37–44. https://doi.org/10.1016/j.sjbs.2010.07.002
Amin AA, Fatma AE, Ghrib M, El-awadi M, Rashed ESM (2011) Physiological response of onion plants to foliar application of putrescine and glutamine. Sci Hort 129(3):353–360. https://doi.org/10.1016/j.scienta.2011.03.052
Angelini R, Cona A, Federico R, Fincato P, Tavladoraki P, Tisi A (2010) Plant amine oxidases ‘on the move’: an update. Plant Physiol Biochem 48:560–564. https://doi.org/10.1016/j.plaphy.2010.02.001
Aragão VP, de Souza Ribeiro YR, Reis RS, Macedo AF, Floh EI, Silveira V, Santa-Catarina C (2016) In vitro organogenesis of Cedrela fissilis Vell. (Meliaceae): the involvement of endogenous polyamines and carbohydrates on shoot development. Plant Cell Tissue Organ Cult 124:611–620. https://doi.org/10.1007/s11240-015-0919-8
Aragão VP, Reis RS, Silveira V, Santa-Catarina C (2017) Putrescine promotes changes in the endogenous polyamine levels and proteomic profiles to regulate organogenesis in Cedrela fissilis Vellozo (Meliaceae). Plant Cell Tissue Organ Cult 130:495–505. https://doi.org/10.1007/s11240-017-1239-y
Asghari B, Khademian R, Sedaghati B (2020) Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Scientia Horticulturae 263:109132. https://doi.org/10.1016/j.scienta.2019.109132
Ayad HS, Reda F, Abdalla MSA (2010) Effect of putrescine and zinc on vegetative growth, photosynthetic pigments, lipid peroxidation and essential oil content of geranium (Pelargonium graveolens L.). World J Agri Sci 6:601–608
Bassard J-E, Ullmann P, Bernier F, Werck-Reichhart D (2010) Phenolamides: Bridging polyamines to the phenolic metabolism. Phytochem 71:1808–1824. https://doi.org/10.1016/j.phytochem.2010.08.003
Bhattacharyya P, Kumaria S, Tandon P (2016) High frequency regeneration protocol for Dendrobium nobile: a model tissue culture approach for propagation of medicinally important orchid species. South Afr J Bot 104:232–243. https://doi.org/10.1016/j.sajb.2015.11.013
Boobalan S, Kamalanathan D (2019) Spermidine influences enhanced micropropagation and antibacterial activity in Aerva javanica (Burm. F.) Shult. Industr Crops Prod 137:187–196. https://doi.org/10.1016/j.indcrop.2019.05.003
Cardillo AB, Giulietti AM, Palazón J, Bonfill M (2013) Influence of hairy root ecotypes on production of tropane alkaloids in Brugmansia candida. Plant Cell Tiss Org 114:305–312. https://doi.org/10.1007/s11240-013-0326-y
Casas JL (2014) Polyamines in plant in vitro culture. In: Anjum NA, Gill SS, Gill R (Eds.), Plant adaptation to environmental change: significance of amino acids and their derivatives. CABI, pp 266–280
Chen LF, Lu W, Sun J, Guo SR, Zhang ZX, Yang YJ (2011) Effects of exogenous spermidine on photosynthesis and carbohydrate accumulation in roots and leaves of cucumber (Cucumis sativus L.) seedlings under salt stress. J Nanjing Agri Univ 34:31–36
Chen D, Shao Q, Yin L, Younis A, Zheng B (2019) Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses. Front in Plant Sci 9:1–13. https://doi.org/10.3389/fpls.2018.01945
Diwan R, Malpathak N (2012) Effect of polyamines on shoot multiplication and furanocoumarin production in Ruta graveolens cultures. Nat Prod Commun 7:895–898. https://doi.org/10.1177/1934578X1200700723
Del Duca S, Serafini-Fracassini D, Cai G (2014) Senescence and programmed cell death in plants: polyamine action mediated by trans-glutaminase. Front Plant Sci 5:1–17. https://doi.org/10.3389/fpls.2014.00120
Dey A, Gupta K, Gupta B (2014) Role of polyamines in plant–pathogen interactions. In: Anjum NA, Gill SS, Gil R (eds) Plant adaptation to environmental change. CAB International, USA, pp 222–244
Dey A, Hazra AK, Nongdam P, Nandy S, Tikendra L, Mukherjee A, Banerjee S, Mukherjee S, Pandey DK (2019) Enhanced bacoside content in polyamine treated in-vitro raised Bacopa monnieri (L.) Wettst. South Afr J Bot 123:259–269. https://doi.org/10.1016/j.sajb.2019.03.012
Dey A, Nongdam P, Nandy S, Mukherjee S, Mukherjee A, Tikendra L, Hazra AK, Pandey, DK (2021) Polyamine elicited aristolochic acid production in in vitro clonally fidel Aristolochia indica L.: An ISSR and RAPD markers and HPTLC based study. South Afr J Bot 140:326–335
Gholami M, Fakhari AR, Ghanati F (2013) Selective regulation of nicotine and polyamines biosynthesis in tobacco cells by enantiomers of ornithine. Chirality 25:22–27. https://doi.org/10.1002/chir.22107
Graser G, Hartmann T (2000) Biosynthesis of spermidine, a direct precursor of pyrrolizidine alkaloids in root cultures of Senecio vulgaris L. Planta 211:239–245. https://doi.org/10.1007/s004250000260
Grienenberger E, Besseau S, GeoVroy P, Debayle D, Heintz D, Lapierre C, Pollet B, Heitz T, Legrand M (2009) A BAHD acyltransferase is expressed in the tapetum of Arabidopsis anthers and is involved in the synthesis of hydroxycinnamoyl spermidines. Plant J 58:246–259. https://doi.org/10.1111/j.1365-313X.2008.03773.x
Gupta B, Dey A, Gupta K (2013a) Plant polyamines in abiotic stress responses. Acta Physiol Plantarum 35:2015–2036. https://doi.org/10.1007/s11738-013-1239-4
Gupta B, Dey A, Gupta K (2013b) Polyamines and their role in plant osmotic stress tolerance. In: Tuteja N, Gill SS (eds) Climate Change and Plant Abiotic Stress Tolerance. Wiley-VCH Verlag GmbH & Co KGaA, Weinheim, pp 1053–1071
Hajiboland R, Ebrahimi N (2011) Effect of exposure to UV radiation on growth, photosynthesis and antioxidant defense system in tobacco (Nicotiana rustica L. cv. Basmas) plants treated with exogenous polyamines. Genet Plant Physiol 1:76–90
Handa AK, Mattoo AK (2010) Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol Biochem 48:540–546. https://doi.org/10.1016/j.plaphy.2010.02.009
Hao G, Ji H, Li Y, Shi R, Wang J, Feng L, Huang L (2012) Exogenous ABA and polyamines enhanced salvianolic acids contents in hairy root cultures of Salvia miltiorrhiza Bge. F. alba. Plant Omics 5:446
Hazarika P, Rajam MV (2011) Biotic and abiotic stress tolerance in transgenic tomatoes by constitutive expression of S-adenosylmethionine decarboxylase gene. Physiol Mol Biol Plants 17:115–128. https://doi.org/10.1007/s12298-011-0053-y
Hou ZH, Liu GH, Hou LX, Wang LX, Xin LIU (2013) Regulatory function of polyamine oxidase-generated hydrogen peroxide in ethylene-induced stomatal closure in Arabidopsis thaliana. J Integr Agric 12:251–262. https://doi.org/10.1016/S2095-3119(13)60224-5
Ioannidis NE, Kotzabasis K (2007) Effects of polyamines on the functionality of photosynthetic membrane in vivo and in vitro. Biochim Biophys Acta 1767:1372–1382. https://doi.org/10.1016/j.bbabio.2007.10.002
Jiménez-Bremont JF, Marina M, Guerrero-González ML, Rossi FR, Sánchez-Rangel D, Rodríguez-Kessler M, Ruiz OA, Gárriz A (2014) Physiological and molecular implications of plant polyamine metabolism during biotic interactions. Front Plant Sci 5:95. https://doi.org/10.3389/fpls.2014.00095
Junker A, Fischer J, Sichhart Y, Brandt W, Draeger BM (2013) Evolution of the key alkaloid enzyme putrescine N-methyltransferase from spermidine synthase. Front Plant Sci 4:260. https://doi.org/10.3389/fpls.2013.00260
Karatan E, Michael AJ (2013) A wider role for polyamines in biofilm formation. Biotechnol Lett 35:1715–1717. https://doi.org/10.1007/s10529-013-1286-3
Khalil SA, Kamal N, Sajid M, Ahmad N, Zamir R, Ahmad N, Ali S (2016) Synergism of polyamines and plant growth regulators enhanced morphogenesis, stevioside content, and production of commercially important natural antioxidants in Stevia rebaudiana Bert. In Vitro Cellular DevBiol-Plant 52:174–184. https://doi.org/10.1007/s11627-016-9749-6
Kim TE, Kim SK, Han TJ, Lee JS, Chang SC (2002) ABA and polyamines act independently in primary leaves of cold-stressed tomato (Lycopersicon esculentum). Physiol Plant 115(3):370–376. https://doi.org/10.1034/j.1399-3054.2002.1150306.x
Kim JN, Jang HG, Park SU, Ryu HW (2006) Effect of Polyamines on Indigo Biosynthesis in Hairy Root Cultures of Polygonum tinctorium Lour. Korean J Crop Sci 51:247–250
Kim SH, Kim SH, Palaniyandi SA, Yang SH, Suh JW (2015) Expression of potato S-adenosyl-L-methionine synthase (SbSAMS) gene altered developmental characteristics and stress responses in transgenic Arabidopsis plants. Plant Physiol Biochem 87:84–91. https://doi.org/10.1016/j.plaphy.2014.12.020
Konstantinos PA, Imene T, Panagiotis MN, Roubelakis-Angelakis KA (2010) ABA-dependent amine oxidases-derived H2O2 affects stomata conductance. Plant Signal Behav 5:1153–1156. https://doi.org/10.4161/psb.5.9.12679
Kovács L, Mendel Á, Szentgyörgyi A, Fekete S, Söre F, Posta K, Kiss E (2020) Comparative analysis of overexpressed Fragariavesca S-adenosyl-l-methionine synthase (FvSAMS) and decarboxylase (FvSAMDC) during salt stress in transgenic Nicotiana benthamiana. Plant Growth Regul 91:53–73. https://doi.org/10.1007/s10725-020-00587-3
Kumari S, Trivedi M, Shukla N, Mishra M (2018) Polyamine mediated genotype independent somatic embryogenesis in Papaya (Carica papaya L.). Plant Archiv 18:581–589
Kumria R, Rajam MV (2002) Ornithine decarboxylase transgene in tobacco affects polyamines, in vitro-morphogenesis and response to salt stress. J Plant Physiol 159:983–990. https://doi.org/10.1078/0176-1617-00822
Le Guen-Le SF, Hourmant A (2001) Stimulation of putrescine biosynthesis via the ornithine decarboxylase pathway by gibberellic acid in the in vitro rooting of globe artichoke (Cynara scolymus). Plant Growth Regul 35(3):277–284. https://doi.org/10.1023/A:1014494013416
Lee OS, Kang YM, Jung HY (2005) Enhanced production of tropane alkaloids in Scopolia parviflora by introducing the PMT (putrescine N-methyltransferase) gene. In Vitro Cell Dev Biol 41:167–172. https://doi.org/10.1079/IVP2004621
Lee SY, Baskar TB, Kim JK, Park SU (2016) Enhanced shoot organogenesis in Aloe saponaria following treatment with ethylene inhibitors and polyamines. Biosci Biotechnol Res Asia 13:17–21. https://doi.org/10.13005/bbra/1997
Li Z, Zhang Y, Zhang X, Peng Y, Merewitz E, Ma X, Huang L (2016) The alterations of endogenous polyamines and phytohormones induced by exogenous application of spermidine regulate antioxidant metabolism, metallothionein and relevant genes conferring drought tolerance in white clover. Environ Exp Bot 124:22–38. https://doi.org/10.1016/j.envexpbot.2015.12.004
Liu K, Fu H, Bei Q, Luan S (2000) Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol 124(3):1315–1326. https://doi.org/10.1104/pp.124.3.1315
Liu Z, Liu P, Qi D, Peng X, Liu G (2017) Enhancement of cold and salt tolerance of Arabidopsis by transgenic expression of the S-adenosylmethionine decarboxylase gene from Leymus chinensis. J Plant Physiol 211:90–99. https://doi.org/10.1016/j.jplph.2016.12.014
Mahgoub MH, Abdel ANG, Mazhar AMA (2011) Response of Dahlia pinnata L plant to foliar spray with putrescine and thiamine ongrowth, flowering and photosynthetic pigments. Am Eurasian JAgric Environ Sci 10(5):769–775
Mahros KM, Badawy EM, Mahgoub MH, Habib AM, Sayed IME (2011) Effect of putrescine and uniconazole treatments on flower characters and photosynthetic pigments of Chrysanthemum indicum L. plant. J Am Sci 7(3):399–408
Majumdar R, Barchi B, Turlapati SA, Gagne M, Minocha R, Long S, Minocha SC (2016) Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: the pathway is regulated at the post-transcriptional level. Front Plant Sci 7:78. https://doi.org/10.3389/fpls.2016.00078
Malá J, Gemperlová L, Cvikrová M, Máchová P (2012) Role of Polyamines in Efficiency of Norway Spruce (Hurst Ecotype) Somatic Embryogenesis. Rijeka: INTECH Open Access Publisher
Mattoo AK, Minoscha SC, Minocha R, Handa A (2010) Polyamines and cellular metabolism in plants: transgenic approaches reveal different responses to diamine putrescine versus higher polyamines spermidine and spermine. Amino Acids 38:405–413. https://doi.org/10.1007/s00726-009-0399-4
Mattoo AK, Fatima T, Upadhyay RK, Handa AK (2015) Polyamines in plants: biosynthesis from arginine, and metabolic, physiological and stress-response roles. In: D’Mello JPF (ed) Amino acids in higher plants. CAB International, USA, pp 177–194
Michard E, Dreyer I, Lacombe B, Sentenac H, Thibaud JB (2005) Inward rectification of the AKT2 channel abolished by voltage dependent phosphorylation. Plant J 44:783–797. https://doi.org/10.1111/j.1365-313X.2005.02566.x
Miura K (2013) Nitrogen and phosphorus nutrition under salinity stress. In: Ahmad P, Prasad MNV, Azooz MM (eds) Ecophysiology and responses of plants under salt stress. Springer, New York, pp 425–441
Mohapatra S, Minocha R, Long S, Minocha SC (2009) Transgenic manipulation of a single polyamine in poplar cells affects the accumulation of all amino acids. Amino Acids 38:1117–1129. https://doi.org/10.1007/s00726-009-0322-z
Moschou PN, Wu J, Cona A, Tavladoraki P, Angelini R, Roubelakis-Angelakis KA (2012) The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J Exp Bot 63:5003–5015. https://doi.org/10.1093/jxb/ers202
Moyano E, Fornalé S, Palazón J, Cusidó RM, Bagni N, Piñol MT (2002) Alkaloid production in Duboisia hybrid hairy root cultures overexpressing the pmt gene. Phytochemistry 59:697–702. https://doi.org/10.1016/S0031-9422(02)00044-4
Moyano E, Jouhikainen K, Tammela P, Palazón J, Cusidó RM, Piñol MT, Teeri TH, Oksman-Caldentey KM (2003) Effect of pmt gene overexpression on tropane alkaloid production in transformed root cultures of Datura metel and Hyoscyamus muticus. J Exp Bot 54:203–211. https://doi.org/10.1093/jxb/erg014
Mustafavi SH (2016) Effect of plant growth regulators on growth and productive characteristics of valerian at different environmental conditions. Dissertation. University of Maragheh
Mustafavi SH, Shekari F, Abbasi A (2015) Putrescine improve low temperature tolerance of fennel (Foeniculum vulgare Mill.) seeds. Cercet Agron Mold 48(1):69–76. https://doi.org/10.1515/cerce-2015-0018
Mustafavi SH, Shekari F, Nasiri Y, Hatami-Maleki H (2015b) Nutritional and biochemical response of water-stressed valerian plants to foliar application of spermidine. Biol Forum 7(1):1811–1815
Mustafavi SH, Shekari F, Hatami Maleki H (2016) Influence of exogenous polyamines on antioxidant defence and essential oil production in valerian (Valeriana officinalis L.) plants under drought stress. Acta Agri Slov 107(1):81–91. https://doi.org/10.14720/aas.2016.107.1.09
Mustafavi SH, Badi HN, Sękara A, Mehrafarin A, Janda T, Ghorbanpour M, Rafiee H (2018) Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol Plant 40:102. https://doi.org/10.1007/s11738-018-2671-2
Narula A, Kumar SV, Pande D, Rajam MV, Srivastava PS (2004) Agrobacterium-mediated transfer of arginine decarboxylase and ornithine decarboxylase genes to Datura innoxia enhances shoot regeneration and hyoscyamine biosynthesis. J Plant Biochem Biot 13:127–130
Ober D, Kaltenegger E (2009) Pyrrolizine alkaloid biosynthesis, evolution of a pathway in plant secondary metabolism. Phytochemistry 70:1687–1695. https://doi.org/10.1016/j.phytochem.2009.05.017
OndoOvono PO, Kevers C, Dommes J (2010) Tuber formation and development of Dioscorea cayenensis–Dioscorea rotundata complex in vitro effect of polyamines. In Vitro Cell DevBiol-Plant 46:81–88. https://doi.org/10.1007/s11627-009-9256-0
Orabi SA, Talaat IM, Balbaa LK, Abdalla AE (2015) Influence of pyridoxine and spermine on lemongrass (Cymbopogon citratus) plants. Nusantara Bioscie 7:139–143
Pal M, Szalai G, Janda T (2015) Speculation: polyamines are important in abiotic stress signaling. Plant Sci 237:16–23. https://doi.org/10.1016/j.plantsci.2015.05.003
Palazon J, Moyano E, Cusido RM, Bonfill M, Oksman-Caldentey KM, Piñol MT (2003) Alkaloid production in Duboisia hybrid hairy roots and plants overexpressing the h6h gene. Plant Sci 165:1289–1295. https://doi.org/10.1016/S0168-9452(03)00340-6
Park JB (2009) Isolation and characterization of N-feruloyltyramine as the P-selectin expression suppressor from garlic (Allium sativum). J Agric Food Chem 57:8868–8872. https://doi.org/10.1021/jf9018382
Parvin S, Lee OR, Sathiyaraj G, Khorolragchaa A, Kim YJ, Miah MG, Yang DC (2012) Modulation of polyamine levels in ginseng hairy root cultures subjected to salt stress. Russ J Plant Physiol 59:757–765. https://doi.org/10.1134/S102144371206012X
Parvin S, Lee OR, Sathiyaraj G, Khorolragchaa A, Kim YJ, Yang DC (2014) Spermidine alleviates the growth of saline-stressed ginseng seedlings through antioxidative defense system. Genetics 53:70–78. https://doi.org/10.1016/j.gene.2013.12.021
Petri C, Alburquerque N, Pérez-Tornero O, Burgos L (2005) Auxin pulses and a synergistic interaction between polyamines and ethylene inhibitors improve adventitious regeneration from apricot leaves and Agrobacterium-mediated transformation of leaf tissues. Plant Cell Tissue Organ Cult 82:105–111. https://doi.org/10.1007/s11240-004-7013-y
Pieruzzi FP, Dias LLC, Balbuena TS, Santa-Catarina C, Dos Santos ALW, Floh EIS (2011) Polyamines, IAA and ABA during germination in two recalcitrant seeds: Araucaria angustifolia (Gymnosperm) and Ocotea odorifera (Angiosperm). Ann Bot 108:337–345. https://doi.org/10.1093/aob/mcr133
Pistelli L, Giovannini A, Ruffoni B, Bertoli, Pistelli L (2010) Hairy root cultures for secondary metabolites production. Adv Exp Med Biol 698:167–184. https://doi.org/10.1007/978-1-4419-7347-4_13
Podwyszyńska M, Kosson R, Treder J (2015) Polyamines and methyl jasmonate in bulb formation of in vitro propagated tulips. Plant Cell Tissue Organ Cult 123:591–605. https://doi.org/10.1007/s11240-015-0863-7
Pottosin I, Shabala S (2014) Polyamines control of cation transport across plant membranes: implications for ion homeostasis and abiotic stress signaling. Front Plant Sci 5:1–16. https://doi.org/10.3389/fpls.2014.00154
Rafiee H, Naghdi Badi H, Mehrafarin A, Qaderi A, Zarinpanjeh N, Sekara A, Zand E (2016) Application of plant biostimulants as new approach to improve the biological responses of medicinal plants- a critical review. J Med Plants 15(59):6–39
Rezvanypour S, Hatamzadeh A, Elahinia SA, Asghari HR (2015) Exogenous polyamines improve mycorrhizal development and growth and flowering of Freesia hybrida. J Hort Res 23(2):17–25. https://doi.org/10.2478/johr-2015-0013
Reis RS, de Moura VE, Heringer AS, Santa-Catarina C, Silveira V (2016) Putrescine induces somatic embryo development and proteomic changes in embryogenic callus of sugarcane. J Proteome 130:170–179. https://doi.org/10.1016/j.jprot.2015.09.029
Santa-Catarina C, Silveira V, Balbuena TS, Viana AM, Estelita MEM, Handro W, Floh EI (2006) IAA, ABA, polyamines and free amino acids associated with zygotic embryo development of Ocotea catharinensis. Plant Growth Regul 49(2–3):237–247. https://doi.org/10.1007/s10725-006-9129-z
Santa-Catarina C, Silveira V, Scherer GFE, Floh EIS (2007) Polyamine and nitric oxide levels relate with morphogenetic evolution in somatic embryogenesis of Ocotea catharinensis. Plant Cell Tissue Organ Cult 90:93–101. https://doi.org/10.1007/s11240-007-9259-7
Santanen A (2000) Polyamine metabolism during development of somatic and zygotic embryos of Picea abies (Norway spruce). PhD dissertation, Dept Biosci, University of Helsinki
Sathish D, Vasudevan V, Theboral J, Elayaraja D, Appunu C, Siva R, Manickavasagam M (2018) Efficient direct plant regeneration from immature leaf roll explants of sugarcane (Saccharum officinarum L.) using polyamines and assessment of genetic fidelity by SCoT markers. In Vitro Cell DevBiol-Plant 54:399–412. https://doi.org/10.1007/s11627-018-9910-5
Shekari F, Asadi Danalo A, Mustafavi SH (2015) Exogenous polyamines improve seed germination of borage under salt stress via involvement in antioxidant defenses. WALIA 31:57–63
Sheteiwy M, Shen H, Xu J, Guan Y, Song W, Hu J (2017) Seed polyamines metabolism induced by seed priming with spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environ Exp Bot 137:58–72. https://doi.org/10.1016/j.envexpbot.2017.02.007
Shi J, Fu XZ, Peng T, Huang XS, Fan QJ, Liu JH (2010) Spermine pretreatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol 30:914–922. https://doi.org/10.1093/treephys/tpq030
Shoji T, Hashimoto T (2015) Polyamine-derived alkaloids in plants: molecular elucidation of biosynthesis. In Polyamines. Springer, Tokyo, pp 189-200
Sivakumar S, Siva G, Sathish S, Kumar GP, Vigneswaran M, Vinoth S, Kumar TS, Sathishkumar R, Jayabalan N (2019) Influence of exogenous polyamines and plant growth regulators on high frequency in vitro mass propagation of Gloriosa superba L. and its colchicine content. Biocat Agri Biotechnol 18:101030. https://doi.org/10.1016/j.bcab.2019.101030
Sivanandhan G, Mariashibu TS, Arun M, Rajesh M, Kasthurirengan S, Selvaraj N, Ganapathi A (2011) The effect of polyamines on the efficiency of multiplication and rooting of Withania somnifera (L.) Dunal and content of some withanolides in obtained plants. Actaphysiolplantarum 33:2279. https://doi.org/10.1007/s11738-011-0768-y
Tabart J, Franck T, Kevers C, Dommes J (2015) Effect of polyamines and polyamine precursors on hyperhydricity in micropropagated apple shoots. Plant Cell Tissue Organ Cult 120:11–18. https://doi.org/10.1007/s11240-014-0568-3
Takahashi T, Kakehi J (2010) Polyamines: ubiquitous polycations with unique roles in growth and stress responses. Ann Bot 105:1–6. https://doi.org/10.1093/aob/mcp259
Takeda T, Hayakawa F, Oe K, Matsuoka H (2002) Effects of exogenous polyamines on embryogenic carrot cells. Biochem Eng J 12:21–28. https://doi.org/10.1016/S1369-703X(02)00037-2
Thiruvengadam M, Chung IM, Chun SC (2012) Influence of polyamines on in vitro organogenesis in bitter melon (Momordica charantia L.). J Med Plants Res 6:3579–3585. https://doi.org/10.5897/JMPR12.246
Tiburcio AF, Altabella T, Bitrián M, Alcázar R (2014) The roles of polyamines during the lifespan of plants: from development to stress. Planta 240(1):1–18. https://doi.org/10.1007/s00425-014-2055-9
Wang X, Zeng J, Li Y, Rong X, Sun J, Sun T, Li M, Wang L, Feng Y, Chai R, Chen M (2015) Expression of TaWRKY44, a wheat WRKY gene, in transgenic tobacco confers multiple abiotic stress tolerances. Front Plant Sci 6:615. https://doi.org/10.3389/fpls.2015.00615
Vasudevan V, Subramanyam K, Elayaraja D, Karthik S, Vasudevan A, Manickavasagam M (2017) Assessment of the efficacy of amino acids and polyamines on regeneration of watermelon (Citrullus lanatus Thunb.) and analysis of genetic fidelity of regenerated plants by SCoT and RAPD markers. Plant Cell Tissue Organ Cult (PCTOC) 130:681–687. https://doi.org/10.1007/s11240-017-1243-2
Yamaguchi K, Takahashi Y, Berberich T, Imai A, Miyazaki A, Takahashi T, Michael A, Kusano T (2006) The polyamine spermine protects against high salt stress in Arabidopsis thaliana. FEBS Lett 580:6783–6788. https://doi.org/10.1016/j.febslet.2006.10.078
Yamaguchi K, Takahashi Y, Berberich T, Imai A, Takahashi T, Michael AJ, Kusano T (2007) A protective role for the polyamine spermine against drought stress in Arabidopsis. Biochem Bioph Res Co 352:486–490. https://doi.org/10.1016/j.bbrc.2006.11.041
Yamasaki H, Cohen MF (2006) NO signal at the crossroads: polyamine-induced nitric oxide synthesis in plants? Trends Plant Sci 11:522–524. https://doi.org/10.1016/j.tplants.2006.09.009
Yang YK, Lee SY, Park WT, Park NI, Park SU (2010) Exogenous auxins and polyamines enhance growth and rosmarinic acid production in hairy root cultures of ’Nepeta cataria’ L. Plant Omics 3:190
Yang C, Chen M, Zeng L, Zhang L, Liu X, Lan X, Tang K, Liao Z (2011) Improvement of tropane alkaloids production in hairy root cultures of Atropa belladonna by overexpressing pmt and h6h genes. Plant Omics 4(1):29–33
Yang L, Hong X, Xiao-xia W, Yun-cheng L (2016) Effect of polyamine on seed germination of wheat under drought stress is related to changes in hormones and carbohydrates. J Integr Agric 15(12):2759–2774. https://doi.org/10.1016/S2095-3119(16)61366-7
Yan-hua Z, Yu-ping Z, Jing X, Hui W, Hui-zhe C, Yi-kai Z, De-feng Z (2016) Effects of chilling tolerance induced by spermidine pretreatment on antioxidative activity, endogenous hormones and ultrastructure of Indica Japonica hybrid rice seedlings. J Integr Agric 15(2):295–308. https://doi.org/10.1016/S2095-3119(15)61051-6
Yi J, Lee G, Chung J, Lee Y, Gwag J, Lee S (2015) Efficient micropropagation of pear germplasm using soot tips and nodal explants. Korean J Plant Res 28:690–696. https://doi.org/10.7732/kjpr.2015.28.6.690
Yu Y, Zhang WB, Li XY, Piao XC, Jiang J, Lian ML (2016) Pathogenic fungal elicitors enhance ginsenoside biosynthesis of adventitious roots in Panax quinquefolius during bioreactor culture. Ind Crops Prod 94:729–735. https://doi.org/10.1016/j.indcrop.2016.09.058
Yu Y, Qin W, Li Y, Zhang C, Wang Y, Yang Z, Ge X, Li F (2019) Red light promotes cotton embryogenic callus formation by influencing endogenous hormones, polyamines and antioxidative enzyme activities. Plant Growth Reg 87:187–199. https://doi.org/10.1007/s10725-018-0461-x
Zeid FA, Omer EA, Amin AY, Hanafy SAH (2014) Effect of putrescine and salicylic acid on ajwain plant (Trachyspermum ammi) at vegetative stage grown under drought stress. Int J Agric Sci Res 4(6):61–80
Zhang L, Ding R, Chai Y, Bonfill M, Moyano E, Oksman-Caldentey KM, Xu T, Pi Y, Wang Z, Zhang H, Kai G (2004) Engineering tropane biosynthetic pathway in Hyoscyamus niger hairy root cultures. PNAS 101:6786–6791. https://doi.org/10.1073/pnas.0401391101
Zhang L, Yang B, Lu B, Kai G, Wang Z, Xia Y, Ding R, Zhang H, Sun X, Chen W, Tang K (2007) Tropane alkaloids production in transgenic Hyoscyamus niger hairy root cultures over-expressing putrescine N-methyltransferase is methyl jasmonate-dependent. Planta 225:887–896. https://doi.org/10.1007/s00425-006-0402-1
Zhao J, Shi G, Yuan Q (2007) Polyamines content and physiological and biochemical responses to ladder concentration of nickel stress in Hydrocharis dubia (Bl.) Backer leaves. Biometals 21:665. https://doi.org/10.1007/s10534-008-9151-x
Acknowledgements
The first author thankfully acknowledges the research grant provided by DST-INSPIRE (Fellowship sanction letter no. and date: C/4588/IFD/2014-15 and 25.11.2014). The corresponding author thankfully acknowledges the “Faculty Research and Professional Development Fund” (FRPDF) for financial assistance from Presidency University.
Author information
Authors and Affiliations
Contributions
SN, DKP, and MSS, prepared the manuscript and did the literature survey. TD, CKT, TM, and MG revised the manuscript and designed the figures. VSG, CKT, UA, and MK prepared the tables. TB, NKS, NKJ, DKP, and PD edited the primary draft; AD designed, edited, and supervised the entire work. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nandy, S., Das, T., Tudu, C.K. et al. Unravelling the multi-faceted regulatory role of polyamines in plant biotechnology, transgenics and secondary metabolomics. Appl Microbiol Biotechnol 106, 905–929 (2022). https://doi.org/10.1007/s00253-021-11748-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11748-3