Abstract
Polyamines are amine-containing, low molecular weight, and ubiquitous polycationic molecules present in almost all cells and free-living microbes, which are formed by aliphatic hydrocarbons replaced with multiple amino groups. They have been considered as a new kind of plant biostimulant, which play vital roles in diverse plant growth and developmental processes, and environmental stress responses. However, little is known regarding the effects of polyamines specifically on the elicitation of bioactive compounds in medicinal plant production. Therefore, in this review, we attempt to cover these gaps of information. Supply of polyamines, whether by exogenous application or through genetic engineering, could positively affect medicinal plant growth, productivity, and stress tolerance; however, these effects depend on type and dose of polyamine application and plant species. Furthermore, polyamines play as precursor for the several groups of alkaloids (pyrrolizidine, tropane, and quinolizidine alkaloids) and phenolamides, so these bioactive compounds could significantly increase the concentration of the above-mentioned natural products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A plant biostimulant (phytostimulator) is defined as any nontoxic substance (mostly natural origin) or microorganism applied to plant systems that aim to improve nutrition-use efficiency, stimulate plant life processes, and abiotic stress tolerance (Jardin 2015). Plant biostimulants impact some of the metabolic pathways of plants such as photosynthesis, respiration, and nucleic acid synthesis, and when applied in proper quantities, improve the plant growth and development without changing their natural pathway (Rafiee et al. 2016). Some major biostimulants are humic and fulvic acids, N-containing compounds (e.g., polyamines and betaines), seaweed extracts and botanicals, biopolymers (e.g., chitosan), inorganic compounds (e.g., Al, Co, Na, Se, and Si), and beneficial fungi and bacteria (Jardin 2015).
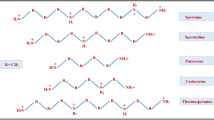
Polyamines (PAs) are organic polycations present in almost all cells and free-living microbes, and due to their stimulatory roles in plant metabolic processes, they are regarded as plant biostimulants. In higher plants, the most common PAs are spermidine (Spd), spermine (Spm), and their diamine precursor putrescine (Put). They are formed by aliphatic hydrocarbons substituted with two or more amino groups (Fig. 1). In plant cells, PAs are found in the vacuole, cytoplasm, plastids, mitochondria, and in the cell wall, but Spm is also present in the nucleus (Alcázar et al. 2010a). Polyamines are present in the cells not only in free form but also due to their polycationic nature at physiological pH they are able to form linkages to phenolic acids, especially hydroxycinnamic acid and other compounds with low molecular weight or to nucleic acids and proteins. The free forms of PAs are easily translocated within cells due to their water-soluble properties; this property promotes the direct action of free PAs. Apart from ionic linkages, PAs are able to form electrostatic linkages with negatively charged molecules, causing conformational stabilization/destabilization of DNA, RNA, chromatin, and proteins (Alcázar et al. 2010a; Wimalasekera et al. 2011). It is suggested that the formation of conjugated PAs plays a key role in the regulation of the free PA level (control intracellular concentration) and may indirectly contribute to development and resistance regulation.
Polyamines as a new type of plant growth biostimulant play vital roles in a range of developmental and physiological processes such as gene expression, protein and DNA synthesis, and cell division and differentiation; in growth and developmental processes such as somatic embryogenesis, organogenesis, and dormancy breaking of tubers; and in seed germination, development of flowers and fruits, and senescence (Shi and Chan 2014; Tavladoraki et al. 2012). Numerous studies have shown that PAs are able to enhance the tolerance to environmental stresses (Minocha et al. 2014; Hussain et al. 2011). Interactions of PAs with signal molecules such as nitric oxide (NO), γ-aminobutyric acid (GABA), proline (Pro), and phytohormones underlie some of their functions in plant growth and development, and environmental stress responses (Martin-Tanguy 2001; Wimalasekera et al. 2011; Subramanyam et al. 2015). Intensive research on PA function in plants has illustrated that they are important regulators of growth and development. Apart from their stimulatory roles in medicinal plant growth and tolerance to environmental stresses, PAs have been reported to facilitate the accumulation of several economically important secondary metabolites. The well-known association between PAs and secondary metabolism is the creation of various alkaloids (mainly derived from Put) (Shoji and Hashimoto 2015) and other secondary products such as phenolic compounds (Bassard et al. 2010). In spite of significant knowledge on PAs and their roles in plants, any researcher did not mention practical ways for consumers, so farmers are still bewildered when they want to use these materials. In addition, there is no specific report on the effects of these biostimulants on medicinal herb growth and productivity. Therefore, the present review not only highlights the prospects of PA application for stimulating growth and development and enhanced the quality of medicinal plants but also prepares forward-looking information on polyamines.
PA biosynthesis and catabolism
The PA homeostasis in the plant cells is mainly attained via the regulation of its interconversion, biosynthesis, and catabolism. In addition, conjugation, transport, and compartmentalization affect PA homeostasis in a cellular/extracellular point. The PA biosynthetic and catabolism pathways have been comprehensively studied in many organisms such as plants and reviewed in detail (Hussain et al. 2011; Tiburcio et al. 2014). In plants, Put is a major diamine and a direct substrate for triamine Spd and tetra-amine Spm. Two alternative synthesis pathways have been demonstrated for the Put biosynthesis from various plant species, directly from either ornithine (Orn) by Orn decarboxylase (ODC) or arginine (Arg) by Arg decarboxylase (ADC) with two intermediates, agmatine and N-carbamoylputrescine (Fig. 2). Spermidine synthase catalyzes Spd synthesis from Put by adding an aminopropyl moiety provided by decarboxylated S-adenosylmethionine (dcSAM). The conversion of SAM to dcSAM by SAM decarboxylase is a rate-limiting step in PA pathway. Similarly, Spm is synthesized from Spd and dcSAM, which is catalyzed by Spm synthase. Polyamine biosynthetic genes and enzymes are listed in Table 1. Among the PA biosynthesis enzymes, ADC, ODC, and SAM decarboxylase are the most important for the proper regulation of cellular PAs. Hence, the control of biosynthesis of PAs in plants might be attained by the genetic manipulation of mentioned enzyme activity. The diamine cadaverine (Cad) is derived from lysine either via the action of lysine decarboxylase (LDC) activity or as a result of ODC (Slocum and Flores 1991).
Schematic representation of PA metabolism and connections with other metabolic pathways. Biosynthesis is indicated by continuous lines and degradation pathway by dashed lines. ADC arginine decarboxylase, AIH agmatine iminohydrolase, CPA N-carbamoylputrescine aminohydrolase, ODC ornithine decarboxylase, SPDS spermidine synthase, SPMS spermine synthase, SAMS S-adenosylmethionine synthase, SAMDC S-adenosylmethionine decarboxylase, ACCS 1-amino-cyclopropane-1-carboxylic-acid synthase, ACCO 1-amino-cyclopropane-1-carboxylic-acid oxidase, LDC lysine decarboxylase, Spd spermidine, Spm spermine, Put putrescine, DAO diamine oxidases, NO nitric oxide, NOS nitric oxide synthase, PAO polyamine oxidases, PDH pyrroline dehydrogenase, Cad cadaverine, GABA gamma aminobutyric acid, Δ1 2-acetyl-1-pyrroline
Apart from biosynthesis, PA catabolism plays an important role in the regulation of cellular PA titers, which is primarily ascribed to two amine oxidases, diamine oxidase (DAO) and PA oxidase (PAO) (Fig. 2), which is completely reviewed by Moschou et al. (2008) and Kusano et al. (2015). Diamine oxidase [copper amine oxidases (CuAOs)] catalyzes the oxidation of Put to give Δ1-pyrroline, hydrogen peroxide (H2O2), and ammonia (NH4), and PAO catalyzes the conversion of Spd and Spm to pyrroline and 1,5-diabicyclononane and 1,3-diaminopropane (DAP) and H2O2, respectively. Then, Δ1-pyrroline can be converted to GABA by pyrroline dehydrogenase (PDH). The γ-aminobutyric acid can then be oxidized and converted to succinic acid, a central intermediate in Krebs cycle. This ensures that carbon and nitrogen resulting from Put breakdown is recycled in the PA cycle (Pal et al. 2015).
As shown in Fig. 2, PA metabolism is unique, which has crosstalk with several important metabolites. Put, Pro, and GABA share glutamate as a common substrate, and these compounds are synthesized by alternate pathways from glutamate (Glu) (Hyun et al. 2013). Furthermore, Arg is the substrate for ADC, NO synthases (NOS), and urea cycle, which produce Put, NO, and Orn, respectively (Bitrián et al. 2012).
Mode of application
There are two main types of application ways that supply more PAs for plant to grow up and stress tolerance. These include (1) exogenously application (seed priming, foliar spray, or adding to media) and (2) genetic engineering through up-regulation of PA biosynthesis in plants.
Seed priming is defined as a presowing technique by which seeds are partially hydrated to the induction and activation of pregerminative metabolism, but radicle emergence inhibited (Bradford 1986). Primed seeds mainly displayed improved germination rate and uniformity and sometimes higher germination percentage (Farooq et al. 2011; Savvides et al. 2016). Recently, several researches demonstrated that seed priming with PAs positively affects the germination performance of medicinal plants and was effective in producing the vigorous seedlings (Xu et al. 2011; Mustafavi et al. 2015a). In the next stages of plant growth, foliar application technique by which PAs are directly contacted with leaf surface is more effective than seed priming (Farooq et al. 2009b). Recently, genetic engineering was used to produce and extend novel plant/green sources with enhanced therapeutic properties. In this way, up-regulation of the rate-limiting enzyme gene has been mostly used for increasing the synthesis and accumulation of biologically active ingredients (Gill and Tuteja 2010).
Stimulatory roles of PAs in medicinal plants
Polyamine in seed germination, plant growth, and development
Seed germination and seedling growth are the two most vital and sensitive stages in the life cycle of plants (Ahmad et al. 2009). Polyamine contents alter during the seed germination process, with the increase during imbibition and decrease from radicle protrusion onward (Dias et al. 2009; Pieruzzi et al. 2011), so this may be an important evidence that they contributed to the seed germination process. For instance, it has been showed that Put has a fundamental function at the beginning of the embryogenesis, when cell division rate is high, while at the end of embryo growth and development in which growth depends on cellular elongation, supply of Spm and Spd would be necessary (Astarita et al. 2003). These findings highlight the importance of increment in PA levels during germination process. Pieruzzi et al. (2011) reported that Spd and Spm could play more important role in the seed germination process than Put. After imbibition, Spd and Spm transport from the megagametophyte and accumulate in corrosion cavity, the space where the embryo develops and serves as a nutritional and hormonal interphase between the developing embryo and megagametophyte (Carmanet al. 2005). Therefore, (Spm + Spd):Put ratio, which mainly increases prior to radicle emergence, could be a crucial index for germination. Recently, several reports showed that exogenously applied PAs affect germination and dormancy breaking of seeds although it depends on the type of PA and its concentration (Farooq et al. 2011; Savvides et al. 2016; Huang et al. 2017). Role of PAs and their alteration during the seed germination process had been previously reviewed by Matilla (1996). Positive effects of seed priming with PAs on seed germination, seedling vigor, and growth and development of different medicinal plants are well documented (Table 2). Moreover, hot pepper (Capsicum annuum L.) seed primed with PAs (75 mM for 48 h) led to rapid and uniform germination of the seeds compared with control (Khan et al. 2012). Polyamines positively affect seed germination not only under favorable conditions but also under environmental stresses. Tobacco (Nicotiana tabacum L.) seeds pretreated with Put showed high germination percentage and seedling dry weight under chilling condition (Xu et al. 2011).
In addition to the germination stage, PAs are involved in different types of fundamental events such as growth and development, replication, cell proliferation, cell differentiation, stimulation of embryogenesis, and senescence (Tiburcio et al. 2014; Hussain et al. 2011). In higher plants, PAs appear in large quantities in meristem tissues and in highly active growing tissues, and decline towards the cell-elongation zone (Flores and Galston 1982). Takahashi and Kakehi (2010) studied the individual functions of each PA in plant cells and showed that among the PAs, Spd is the most critical PA for plant growth. Spermidine is a substrate for hypusine synthesis (a polyamine-derived amino acid), which is required for cell division. Spermidine conjugates contribute in pollen development (Tiburcio et al. 2014). Thermospermine, which is synthesized from Spd in a similar reaction to Spm synthesis, plays a critical role in stem elongation (Takahashi and Kakehi 2010). Due to stimulatory role of PAs on plant growth and productivity, many researchers attempt to exogenously apply them in medicinal plant production. For instance, foliar application of Put on Pelargonium graveolens L. plants significantly increased plant height, fresh, and dry weight of leaves/plant (Ayad et al. 2010). Talaat et al. (2005) found that the application of Put at 1 mM significantly improved the growth and productivity of periwinkle plants. Polyamines also are important in primary root growth, and lateral and adventitious root formation, and the depletion of their pool has dramatic effects on root development (Tisi et al. 2011). Polyamines can be the precursors of several alkaloids that are synthesized in roots and seem to be correlated with root growth (Couee et al. 2004). Translocation of PAs produced in the turmeric leaves to rhizomes affected the growth and thereby increased biomass (Ferreira et al. 2016). Chriqui et al. (1986) demonstrated that there is a synergistic effect between auxins and PAs in rhizogenesis in Datura innoxia (Mill.) leaf explants. These findings are in line with the results of Bais et al. (1999) on hairy root culture of Cichorium intybus (L.).
Plant roots associated with microorganisms play a key role in plant nutrition, health, and productivity (Xie et al. 2014). Research indicates that the establishment of mutualism and symbiosis relationship between plants and beneficial microorganisms such as plant growth-promoting rhizobacteria (PGPR) and mycorrhizae depends on PA metabolism (Jiménez-Bremont et al. 2014). In addition, it has been shown that exogenous PAs have the potential to promote mycorrhizal colonization and development in the in vitro and greenhouse cultivation systems (Rezvanypour et al. 2015). Niemi et al. (2006) revealed that PA content and the activity of PA metabolic enzymes were increased in infected tissues after microbial inoculation. There is some evidence for a link between PAs and biofilm formation by PGPR, which plays an important role in protecting plants. Biofilms are multicellular communities of bacteria enclosed in an extracellular matrix of protein, exopolysaccharide, and sometimes DNA, in which their formation is necessary for the plant root colonization (Ramey et al. 2004; Karatan and Michael 2013). Burrell et al. (2010) reported that in Bacillus subtilis, ADC enzyme and PAs are essential for biofilm formation, and this seems to be the most important role of polyamines in bacteria. Xie et al. (2014) showed that stimulatory role of B. subtilis is attributed to PAs (especially Spd) produced by themselves.
Polyamines play a crucial role in improving the photosynthesis performance, stomatal conductance, and quantum yield (as reviewed by Shu et al. 2012). Exogenous Spd increased photosynthetic rate and plant growth by promoting the Rubisco activity and decreasing the carbohydrate accumulation in leaves, which reduced feedback inhibition of photosynthesis (Chen et al. 2011). It was also reported that among the PAs, Spd could directly interact with thylakoid membranes, so that they become more resistant to degradation during leaf senescence (Legocka and Zajchert 1999). Hajiboland and Ebrahimi (2011) showed that exogenous 0.5 mM PAs (Spd, Spm, and Put) added to the nutrient solution increased net assimilation rate of tobacco (Nicotiana rustica L. cv. Basmas) plants. In Catharanthus roseus (L.) plants, Put treatments increased Chl a, b and carotenoids content (Talaat et al. 2005). Foliar spray of Lactuca sativa (L.) with 0.2 mM Spm could ameliorate the adverse effect of senescence on degradation of Chl a and Chl b by increasing TGase activity (Serafini-Fracasini et al. 2010). Exogenously applied PAs were able to stimulate photophosphorylation in tobacco leaves, which led to an increase in ATP synthesis up to 70% (Ioannidis and Kotzabasis 2007).
Polyamines (especially conjugated PAs) are involved in the regulation of the reproductive process of plants as reported in several studies. Plant PAs occur not only as free forms but also as a conjugated form with hydroxycinnamic acids such as caffeic, p-coumaric, and ferulic acids (Tiburcio et al. 2014). Conjugated PAs including hydroxycinnamoyl Put, Spd, and Spm conjugates are found in many plant species and they are essential for certain developmental processes (Bassard et al. 2010). Biondi et al. (2001) found that MeJA treatment induced PA conjugate accumulation in tobacco root culture. PA conjugates mainly are found in roots, then transmitted and accumulated in shoot apices upon floral initiation (Martin-Tanguy 1997). They are closely related to bud dormancy and development, flower development, floral initiation and development, pollen development, fruit set, and flower fertility (El-Yazal and Rady 2012; Aloisi et al. 2016). Promotion of flowering by exogenous PAs has been demonstrated in some medicinal plants. For instance, exogenous Spd promoted floral bud development by 20% in tobacco tissue cultures, while all the buds were vegetative when cultures were lacking in Spd (Kaur-Sawhney et al. 1988). Mahgoub et al. (2006) stated that exogenous application of Put (200 mg l−1) increased number of flowers/plant, fresh, and dry weight of Dianthus caryophyllus (L.) flowers. Similar results were also reported by Youssef et al. (2004) on Datura innoxia (Mill.). In addition, a few examples of the beneficial effects of exogenous PAs on the growth and development of medicinal plants are presented in Table 2.
Polyamine in secondary metabolite production
In addition to the effect of PAs on germination, growth, and development of medicinal plants, these biostimulants affect plants’ secondary metabolite production. Polyamines affect the production of secondary metabolites in two ways. One of them is the direct impact of PAs on the production of these metabolites and the other by indirectly on plant growth. For instance, Bais et al. (2000) found that the incorporation of Put and Spd enhanced betalain and thiophene contents through increased biomass accumulation. Elicitation of ginsenoside synthesis and accumulation by Alternaria panax and Cylindrocarpon destructans in Panax quinquefolius (L.) were attributed to Put signaling (Yu et al. 2016). A wide range of plant secondary compounds can be metabolized from PAs through greatly unelucidated pathways. Secondary metabolites that are derived from PAs are as follows.
Polyamine-related alkaloids
Alkaloids are nitrogenous substances synthesized from primary metabolites such as PAs in plant cells, offering huge adaptive benefits to plants eliciting them. Polyamines are metabolized into nicotine, pyrrolizidine, and tropane alkaloids. According to Fig. 3, Put acts as precursor for a wide variety of alkaloids. The pyrrolidine rings of tobacco alkaloids (nornicotine and nicotine), pyrrolizidine (retronecine), and tropane alkaloids (hyoscyamine, hyoscine, and meteloidine) are Put derivatives (Shoji and Hashimoto 2015). In addition, Put is incorporated into the tropane ring in tropane alkaloids and has also been involved as precursor in biosynthesis of calystegines (Drager 2004). According to Graser and Hartmann (2000), spermidine is a vital and direct precursor for pyrrolizidine alkaloid biosynthesis. It provides aminobutyl group that is subsequently combined with Put and produces the first specific precursor of pyrrolizidine alkaloids, namely homospermidine. Homospermidine synthase (HSS, EC 2.5.1.44) catalyzes the formation of homospermidine from Put and Spd as substrates. Cadaverine is an essential free intermediate in the production of quinolizidine and piperidine alkaloids derived from lysine through a Cad precursor (Fig. 3). Biosynthesis pathway of these alkaloids is completely reported by Bhattacharya and Rajam (2007).
Nicotine is the predominant alkaloid in the family of Solanaceae and species of Nicotiana especially tobacco (Nicotiana tabacum L.) and highly toxic, which serves as an efficient and safe fumigant and insecticide. Tropane alkaloids that comprise the pharmaceutically important anticholinergic compounds, hyoscyamine and scopolamine, cocaine, and calystegines have been traditionally used for their medicinal, hallucinogenic, and poisonous characteristics. Besides the Solanaceae family, tropane alkaloids are also found in the Convolvulaceae, Cruciferae, Rhizophoraceae, and Brassicaceae. Cocaine is mainly produced in Erythroxylum coca (Erythroxylaceae). Pyrrolizidine alkaloids, however, are constitutively formed in some genera of Asteraceae, Boraginaceae, Fabaceae, and Orchidaceae as defense compounds against herbivores (Hartmann and Witte 1995).
Phenolamides (hydroxycinnamic acid amides)
A large proportion of the PAs found in plants are predominantly as conjugated form with phenolic acids including caffeic, coumaric, and ferulic acids. Phenylpropanoid pathway contributed to phenolamide production by providing conjugation partners for PAs. Therefore, the activity of this pathway significantly regulates plant growth (Mader and Hanke 1997). Conjugation of PA with phenolics changes some of their chemical characteristics such as polarity and hydrophilicity, and thus promotes their translocation, compartmentation, and stability. Furthermore, conjugates were considered as stored and final products, and they could regulate the phenolics and bioactive PA pool in the cells through decomposition and conversion (Bassard et al. 2010). Phenylamides are quantitatively major phytochemicals, which are firmly regulated during plant ontogeny and pollen development processes as well as adaptative responses (Edreva et al. 2007). Biological roles of phenolamides in plant growth, development, and stress tolerance have been previously discussed in two reviews (Edreva et al. 2007; Bassard et al. 2010), which are mainly associated with their phenolic and PA constituents. Kakkar and Rai (1993) reported that plant floral induction and development are most often related to high concentrations of phenolamides. In addition, phenolamides are often considered as bioactive substances with various therapeutic effects. For example, N-feruloyltyramine has been reported as the most active ingredient in garlic (Allium sativum L.), which plays a major positive role in cardiovascular system (Park 2009). Coumarins, a group of phenylpropanoids, have also been reported to accumulate in different diseased plants (Murray et al. 1982).
Polyamines and environmental stress tolerance of medicinal plants
Plants are mainly exposed to various environmental fluctuations, such as drought, cold, salinity, and heavy metal, which affect their growth, development, and productivity. To cope with these stresses and their negative effects, plants possess efficient mechanisms of defense (Bartels and Sunkar 2005). One of the important defensive systems is the synthesis and accumulation of certain functional substances such as protective proteins and compatible solute (Farooq et al. 2009a). These solutes maintain cellular turgor and water potential, and play as chaperones to stabilize protein and membrane structures or scavenge of reactive oxygen species. Like their stimulatory role in plant growth and development processes, PAs also have an important function in modulating the defense response against stressors. Contribution of PAs in the induction of abiotic stress tolerance in plants has been reviewed in several reports (Alcázar et al. 2010a, b; Hussain et al. 2011; Shi and Chan 2014; Pal et al. 2015; Liu et al. 2015).
Literature has demonstrated that environmental stresses caused the accumulation of PAs in many plant species, and these findings illustrated the possible roles of PAs in plant stress tolerance. In most cases, Spd and Put contents were decreased under stresses as reported by Do et al. (2013) under drought and by Sheteiwy et al. (2017) under chilling stress; in contrast, Spm contents were either unchanged or somewhat increased. Takahashi and Kakehi (2010) revealed that among the PAs, Spm plays versatile role in stress responses. Although there is much evidence demonstrating the regulating role of Spm in the control of ion channel, one of the most important roles of Spm has been thought to be in protecting DNA from reactive oxygen species and subsequent mutation (Takahashi and Kakehi 2010). Recently, many researches proved that the supply of PAs for plants whether by exogenous application or genetic transformation with PA biosynthetic genes could enhance the tolerance of medicinal plants to environmental stresses (Tables 2, 3). For instance, Mustafavi et al. (2016) found that the foliar application of PAs ameliorated the adverse effect of water stress on valerian (Valeriana officinalis L.) plants, and Spm was effective than others. Under salinity stress, exogenous Spd reduced H2O2 and superoxide molecules in ginseng (Panax ginseng CA Meyer) seedlings by activating antioxidant-based defense system (Parvin et al. 2014). Seed priming with Spd improved the germination percentage and seedling growth as compared with those of unprimed seeds (Sheteiwy et al. 2017). Under chilling stress, α-amylase activity decreased and starch degradation limited subsequently (Hussain et al. 2016), which priming with Spd significantly improved it (Sheteiwy et al. 2017). Apart from exogenous PA application, overexpression of PA biosynthesis-related genes is an alternative tool to enhance the status of endogenous PA and to alter environmental stress tolerance. For instance, overexpression of ADC genes from Datura stramonium (L.) increased Put content in the transgenic plants and resulted in enhanced drought tolerance (Roy and Wu 2001; Capell et al. 2004). In addition, overexpression of other genes involved in the biosynthesis of PA such as ODC, SPDS, and SAMDC in transgenic plants has been shown to improve tolerance to specific stresses, such as drought and salt (listed in Table 3). In general, overexpression of a PA-related biosynthetic gene has been verified to increase tolerance to different environmental stresses in plants (Wang et al. 2011; Kasukabe et al. 2006; Liu et al. 2017), showing that modification in the endogenous PA level has an extreme influence on stress tolerance.
Polyamines: mechanism of action
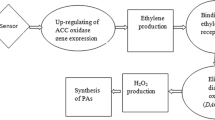
As PAs interact with primary and secondary metabolism of plants, it is impossible to suggest one common mode of action. They stimulate the synthesis and/or activation of natural hormones, root growth, and protein synthesis. At the cellular level, PAs affect cell division and differentiation, membrane integrity, DNA replication, and ion channel regulation (Handa and Mattoo 2010). In addition, PAs are implicated in physiological processes including seed germination, floral initiation and development, modulation of carbon and nitrogen (C/N) balance, organogenesis, and fruit development and ripening. Protective role of PAs during plant response to environmental stresses is also demonstrated in several researches. Possible mechanism of action of PAs is summarized in Fig. 4.
Nitrogen metabolism and signal molecules
Carbon and nitrogen are crucial for plant growth, development, and stress responses; therefore, their sufficient application is critical, and the N:C ratio also participates in the sensing of environmental changes. Nitrogen metabolism is a critical network related to photosynthetic C assimilation, and is necessary for preparing energy and C skeletons during the assimilation of N, particularly during conditions with restricted photosynthesis which take place under environmental perturbations.
Polyamines and several other N-rich metabolites play important physiological roles in C:N assimilation and balancing, and finally plant development and stress response (Moschou et al. 2012; Minocha et al. 2014; Majumdar et al. 2016). For instance, excess NH4+ originated from acquisition, nitrate reduction, photorespiration processes, and also stress conditions is chiefly converted to Glu through the action of glutamine synthetase and glutamate synthase. Glutamate is a precursor of two major N-rich metabolites, namely Put and Pro, which also produces intermediates such as NO and the non-protein amino acid GABA that has critical function in plant growth, development, and abiotic stress tolerance (Mattoo et al. 2010, and reviewed by Shelp et al. 2012). Therefore, the N movement towards PAs makes these cells accumulate N nutrient and may act as potential source for excess NH4+, thereby decreasing its toxic effects (Serapiglia et al. 2008).
Not only PA biosynthesis, but also their catabolism, which produces H2O2 and Δ1-pyrroline, is also involved in maintaining the balance of C:N in plants (Fait et al. 2011). Polyamine-derived H2O2 could act as a signal and play key roles in cell wall maturation events and stress-induced stiffening, signaling of stomata opening, and in programmed cell death (PCD) (Angelini et al. 2010). Furthermore, Δ1-pyrroline can be catabolized to GABA (a key metabolite in signal transduction during the oxidative stress), and subsequently transaminated inside the Krebs (TCA) cycle for C recycling (Moschou et al. 2008). In addition, PA conjugates, which are regarded as final products and accumulated in different organs such as seed, serve as N sources for seed germination, growth, and developmental processes (Bassard et al. 2010). In the alternative way, PAs affect N metabolism by promoting the nitrate reductase (NR), a rate-limiting enzyme, activity (Rosales et al. 2012; Miura 2013). Moreover, enhanced photosynthetic capacity by Spd may increase NR activity through improvement in the reductant pool.
Polyamine–phytohormone cross-talk
As PAs are considered as growth biostimulant, their physiological processes are often similar to phytohormones. It is found that plant hormone roles in plant growth and development are correlated with PA metabolism. The changes in the PA levels and their biosynthetic enzymes accompany many hormonal responses, and in a few cases, PAs appear to mimic the effects of plant hormones (Rastogi and Davies 1991; Yan-hua et al. 2016).
Abscisic acid (ABA) is an essential phytohormone that plays an important role in response to different abiotic stresses and stress signaling. Several reports indicated a positive interaction between Put and ABA, which promotes each other’s biosynthesis in regulating abiotic stress responses (Alcázar et al. 2010a). Priming with Put increased leaf ABA (Farooq et al. 2011) by which can adjust the photoassimilate utilization pathways in leaves through allocation of carbon flows either to the synthesis of macromolecules (e.g., proteins, cellulose, and hemicellulose), or to the synthesis of transportable types of carbohydrates (Kiseleva and Kaminskaya 2002). Polyamines have also a positive feedback with gibberellin (GA), and these two hormones synergistically induced cell division (Anwar et al. 2015). Kaur-Sawhney et al. (1986) suggested that GA-induced growth stimulation might be due to increase in PA levels. Another hormone that has crosstalk with PA is auxin. Tissues treated with auxins display increases in PA level prior to the occurrence of cell division and other morphological changes (Kanchanapoom et al. 1991). Several reports demonstrate that endogenous indole-3-acetic acid (IAA) levels interfere with PA application, therefore, causing a synergistic effect in plant growth (Pieruzzi et al. 2011). The treatment with Put at 1.5 mM concentration increased endogenous IAA contents as compared with the control plants; it supported faster growth and total coumarin production (Bais and Ravishankar 2003). Liu et al. (2013) and Yang et al. (2016) demonstrated that the promotion of seed germination by PAs was closely associated with rapid seed starch degradation induced by GAs.
Polyamine has a strong relation with ethylene (Eth). During PA biosynthesis, SAM is used as an Eth precursor; therefore, these two compounds compete with each other for SAM (Tiburcio et al. 2014). Inhibition of Eth synthesis by PA application enhanced membrane stability (Apelbaum et al. 1985). A few reports indicated that the PAs and Eth play an important role in the regulation of somatic embryogenesis of cultured cell/tissues, and Eth alone does not aid in somatic embryogenesis (Anwar et al. 2015). The jasmonate (JA) family of signaling molecules plays a key role in regulating the PA biosynthesis. Caffeoylputrescine (CP) in tomato is a conjugate between caffeic acid and Put and, therefore, belongs to the hydroxycinnamic acid amide compound group. Chen et al. (2006) revealed that the biosynthesis of CP in cells of tomato plants is regulated by the JA signaling pathway.
Recently, Huang et al. (2017) reported that improving the germination behavior of sweet corn seeds by Spd application might be due to the metabolism of hormones such as GA, ABA, and Eth, and with the increase of H2O2 in the radical produced partly from Spd oxidation. Yang et al. (2016) reported that high contents of IAA, GA, and ABA induced by exogenous Spd and Spm hastened the seed starch breakdown and improved the concentration of soluble sugars in seeds during seed germination. Signaling roles of phytohormones and ROS in seed germination process have been previously reviewed by Oracz and Karpinski (2016). Furthermore, the improvement of biotic and abiotic stress tolerance after the application of PAs could be due to the changes in endogenous phytohormones by which antioxidant system increased (Li et al. 2016).
Polyamine and cell growth response: mechanism of action
Polyamines use several mechanisms to regulate cell growth. They are important factors regulating gene expression, transcription and translation, membrane stabilization, cell cycle, post-translational modification, and conformational transition of DNA (Dey et al. 2014). The most important mode of action involves the property of the cationic amine groups of PAs that can bind to anionic phosphate groups (present in DNA, RNA, and phosphoproteins) and carboxyl groups (present in acidic proteins) in the cell, which can cause conformational changes, altering the activity or function of the bound molecule or membrane (Mattoo et al. 2015). For instance, such a binding may stabilize the double helix structure in DNA (Lindemose et al. 2005) and affect post-translational alteration of proteins (Serafini-Fracasini et al. 2009). In the alternative way, the PA Spd is required in the post-translational formation of hypusine in eukaryotic, which converts a eukaryotic translation initiation factor 5A (eIF5A) precursor from inactive form to a high active protein. This is an essential factor for protein biosynthesis and cell growth (Wolff and Park 2015). Therefore, the hypusine modification determines a link between PAs and cell growth/proliferation via promotion of translation.
Polyamine and stress response in plants: potential mechanism of action
In general, different roles of PAs in ameliorating the adverse effects of stress on plants may include: (1) they serve as compatible solute (Lepri et al. 2001), and their catabolism is also closely related to Pro, GABA, soluble sugars, and β-alanine betaine production (Moschou et al. 2008); (2) interact with plant cell macromolecules such as proteins, DNA, RNA, transcriptional and translational systems, and cellular and organellar membrane structures for their stabilization (Mattoo et al. 2015); (3) play directly in scavenging oxygen and free radicals and enhance the generation of their conjugates, which are known as the most efficient antioxidant (Li et al. 2014; Sheteiwy et al. 2017); (4) act as cellular signaling molecules in the ABA and NO regulatory stress response networks (e.g., regulating stomatal responses) and through the production of H2O2 (reviewed by Wimalasekera et al. 2011); (5) regulation of several ion channels and Ca2+ homeostasis via direct binding to the channel proteins and/or their associated membrane components (Pottosin and Shabala 2014), and (6) participation in PCD by regulating the activity of transglutaminase (TGase) enzymes (Del Duca et al. 2014).
Polyamines and medicinal plant in vitro culture
Medicinal plants in the most developing countries are the major source of medicines. Biologically active metabolites derived from plants are not only being used as influential healthcare and remedy products, but also constitute the primary material of food additives, fragrance and flavors, insecticides, pigments, cosmetics, and perfumes. Medicinal plant species are largely gathered from the wild, and relatively few genera are cultivated on a commercial scale. This exploitation has resulted in habitats disappearing. Therefore, in vitro regeneration or micropropagation provides novel tools for the economical processing of rare plants and the metabolites they produce. Many plant growth regulators (e.g., auxins and cytokinins) have been used in vitro. Recently, beneficial effects of PAs on in vitro regeneration and somatic embryogenesis in tissue culture and micropropagation technique have been evidenced in several medicinal plant species (listed in Table 4). These investigations have been mainly implemented by two strategies: by following the endogenous levels of these compounds during the development of somatic embryos, and by exogenously applying PAs.
Literature mostly demonstrated that PA applications could stimulate callus organogenesis, somatic embryogenesis, and also root induction. For instance, in Curcuma longa L., the exogenous application of PAs led to the production of developed callus with numerous roots that showed good regeneration and produced vigorous plants (Viu et al. 2009). A positive involvement of Put in the inductive phases of cell proliferation was also suggested by Scaramagli et al. (1995) in tissue culture of tobacco (Nicotiana tabacum L.).
Somatic embryogenesis whether directly from the explants without any callus phase or indirectly after a callus phase is a tool for clonal propagation, and these somatic embryos may develop into intact plants generating flowers and seeds (Neumann et al. 2009). Stimulatory effects of PAs in the conversion of somatic embryos or shoot regeneration have been proved in several medicinal plant species. Santa-Catarina et al. (2007) suggested that Put induced the generation of new somatic embryos, whereas Spd and Spm resulted in the development and maturation of somatic embryo. Not only Put but also the incorporation of its precursors, Arg and Orn, into the induction or regeneration media may lead to an increase in the number of embryos produced in Panax ginseng CA Meyer (Kevers et al. 2000). In liquid cultures of Panax ginseng CA Meyer, the supplementation of Spd to the basal medium remarkably enhanced the production of somatic embryos (Monteiro et al. 2002).
Rooting is probably one of the most challenging aspects of the regenerating process of a plant in vitro. Auxin is one of the essential hormones in the rooting process. Most results demonstrated that high levels of PAs, particularly Put, play a regulatory role in consonance with auxin. Rooting and growth of artichoke (Cynara scolymus L.) explants were promoted by adding the Put to rooting medium (Le Guen-Le Saos and Hourmant 2001). In addition to Put, other PAs could positively affect rooting. In Olea europaea L., the PAs’ Put, Spd, and Spm when added at 1 mM concentration into the in vitro rooting medium accelerated the rooting and improved the final rooting percentage and the number or roots per explants (Rugini 1992). In general, regeneration and micropropagation of medicinal plants could be considerably improved by the exogenous application of PAs.
Stimulatory roles of PAs in secondary metabolite production
To supply the world demand, secondary metabolites are mostly obtained from cultivated plants. Their chemical synthesis is usually difficult and not economically feasible. One of the most common challenges in the current plant production system is poor yielding and the high cost of bioactive compound separation/and or purification. Accordingly, the application of agronomical and biotechnological approaches to enhance their production and accumulation has been widely used in the past decades. Several researches demonstrate that PAs can elicit the production of bioactive compounds in plants.
Exogenous application
Although much investigation has been done on the growth stimulation and stress tolerance roles of exogenous PAs, there has been much less work about the increased production of bioactive substances through elicitation of whole medicinal plants. Foliar application of a PA catabolite enhanced the quercetin content in black chokeberry (Aronia melanocarpa Michx. Elliott) up to 60% (Hudec et al. 2006). Abd El Wahed and Gamal El Din (2004) indicated that Spd treatment enhanced the major terpenic components (bisabolol oxide B, chamazulene and bisabolol A). Recently, Orabi et al. (2015) exogenously applied Spm on growth and essential oil accumulation in lemongrass (Cymbopogon citratus DC. Stapf) plants. They found that the 100 mg L−l Spm significantly promoted plant height, fresh and dry mass, total phenols, and essential oil content (especially relative percentage of citronellol). In addition, it is reported that the foliar application of Put on drought-stressed ajwain plants (Trachyspermum ammi L.) not only ameliorated the adverse effect of stress, but also increased essential oil accumulation (Zeid et al. 2014). Mustafavi (2016) showed that foliar PA application on water-stressed valerian plants not only counteracted the adverse effects of the stress, but also increased essential oil content.
In vitro application
Alteration of the nutritional regime in growth media is one of the effective strategies used to improve the production of natural products from in vitro cultures (Isah et al. 2017). Several reports have been shown that the incorporation of PAs in plant growth media or in vitro culture could influence the content and composition of natural products. For instance, Yang et al. (2010) found that the addition of 50 mg L−1 Put increased the growth and rosmarinic acid content in hairy culture of Nepeta cataria L. Moreover, the incorporation of Put to hairy root cultures of witloof chicory enhanced the production of esculetin and esculin (Bais and Ravishankar 2003). Similar results were also reported by Bais et al. (1999) and Suresh et al. (2004), who showed that Put improved hairy root growth and coumarin and betalaine contents on Cichorium intybus L. and Beta vulgaris L. Hao et al. (2012) reported that amounts of salvianic acid A and salvianolic acid B in hairy root cultures of Salvia miltiorrhiza Bge.f.alba. treated with PAs, especially Put (50 mg L−1), were higher than those in the control. It has also been reported that Put treatment (0.1 mmol L−1) facilitated the increment of capsaicin biosynthesis in cell suspension cultures of Capsicum frutescens L. (Sudha and Ravishankar 2003). In addition, Kumar et al. (2008) showed that the application of PAs and/or their synthesis inhibitors improved the embryogenesis and caffeine content in in vitro cultures of Coffea canephora P. ex Fr.
Metabolic engineering
Biotechnological approaches provide alternative strategies to enhance the production of important bioactive compounds in plant cells or organ cultures. Molecular engineering for secondary metabolite biosynthesis has great potential to enhance the product quantity and quality. This technique applied to plants also resulted in the creation of transgenic plants (GMO) in which the value, or composition of natural products is modified to provide therapeutically and commercially important characteristics. Over the last decades, genetic engineering approach has been applied to improve the biosynthesis of these metabolites by manipulating the PA biosynthetic pathway genes (Table 5). Putrescine N-methyltransferase (PMT) is an important enzyme in flowing the nitrogen away from PA biosynthesis to nicotine and tropane alkaloid biosynthesis. Several reports showed that PMT gene overexpressing in plant cell cultures improved nicotine and scopolamine production (Sato et al. 2001; Moyano et al. 2003). Ornithine decarboxylase and arginine decarboxylase are other important enzymes that the manipulation of their related genes could have significant effect on PA-based bioactive compounds. Hamill et al. (1990) reported that overexpression of yeast ODC gene in Nicotiana rustica L. enhanced accumulation of both Put and the alkaloid nicotine. Narula et al. (2004) introduced ODC and ADC separately into Datura innoxia (Mill.) calli culture using Agrobacterium tumefaciens. The transgenic lines exhibited greater PA levels, and also resulted in a maximum content of hyoscyamine compared with control plants. Overexpression of a bacterial gene encoding LDC, an important rate-limiting step in anabasine synthesis in Nicotiana tabacum L., increased the production of Cad and anabasine (Fecker et al. 1993). Hao et al. (2012) indicated that exogenous PAs enhanced the amounts of two salvianolic acids in hairy root culture of Salvia miltiorrhiza Bge.f.alba.
Conclusions
In this review, an attempt has been made to discuss on the stimulatory roles of PA on growth, productivity, and abiotic stress tolerance of medicinal plants. Several agronomical and biotechnological utilities of the role of PA have been dealt in detail. To sum up, it is inferred that PAs, which are economical and environment-friendly alternatives not only positively affect medicinal plant productivity, depending on type and dose of PA application, but also could significantly increase the concentration of PA-derived natural products. It should also be noted that individual PAs have defined action in plant biology, so for the proper utilization of these materials, attention to their roles is essential. In this regard, seed germination is mainly affected by (Spm + Spd):Put ratio, and increase in Spm and Spd contents during germination process is vital. Therefore, to select an appropriate type of PA by which germination of our target medicinal plants improve, the application of Spd and Spm is better than Put. Plant stress tolerance and cell growth are mainly affected by Spm and Spd, respectively.
Author contribution statement
Collected available literature and prepared first draft of the manuscript: SHM and HR. Wrote the manuscript: HNB and AM. Edited the manuscript: AS, TJ, and MG.
Abbreviations
- ADC:
-
Arginine decarboxylase
- AIH:
-
Agmatine iminohydrolase
- Cad:
-
Cadaverine
- SAM:
-
S-Adenosylmethionine
- SAMDC:
-
SAM decarboxylase
- CPA:
-
N-Carbamoylputrescine amidohydrolase
- DAO:
-
Diamine oxidases
- DAP:
-
1,3-Diaminopropane
- eIF5A:
-
Eukaryotic translation initiation factor 5A
- GABA:
-
γ-Aminobutyric acid
- H6H:
-
Hyoscyamine 6β-hydroxylase
- JA:
-
Jasmonate
- LDC:
-
Lysine decarboxylase
- NO:
-
Nitric oxide
- ODC:
-
Ornithine decarboxylase
- PA:
-
Polyamine
- PAO:
-
Polyamine oxidases
- PDH:
-
Pyrroline dehydrogenase
- PMT:
-
Putrescine N-methyltransferase
- Pro:
-
Proline
- Put:
-
Putrescine
- Spd:
-
Spermidine
- SPDS:
-
Spermidine synthase
- SPMS:
-
Spermine synthase
- Spm:
-
Spermine
- TGase:
-
Transglutaminase
References
Abd El Wahed MA, Gamal El Din KM (2004) Stimulation effect of spermidine and stigmasterol on growth, flowering, biochemical constituents and essential oil of chamomile plant (Chamomilla recutita L., Rausch). Bulg J Plant Physiol 30:48–60
Ahmad S, Ahmad R, Ashraf MY, Waraich EA (2009) Sunflower (Helianthus annuus L.) response to drought stress at germination and growth stages. Pak J Bot 41:647–654
Alcázar R, Marco F, Cuevas JC, Patrón M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T (2006) Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett 28:1867–1876
Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Knocz C, Carrasco P, Tiburcio AF (2010a) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Alcázar R, Planas J, Saxena T, Zarza X, Bortolotti C, Cuevas J (2010b) Putrescine accumulation confers drought tolerance in transgenic Arabidopsis plants over-expressing the homologous arginine decarboxylase2 gene. Plant Physiol Biochem 48:547–552
Ali RM, Abbas HM, Kamal RK (2007) The effects of treatment with polyamines on dry matter and oil and flavonoid contents in salinity stressed chamomile and sweet marjoram. Plant Soil Environ 55(11):477–483
Ali RM, Abbas HM, Kamal RK (2009) The effects of treatment with polyamines on dry matter and some metabolites in salinity–stressed chamomile and sweet marjoram seedlings. Plant Soil Environ 55(11):477–483
Aloisi I, Cai G, Serafini-Fracassini D, Del Duca S (2016) Polyamines in pollen: from microsporogenesis to fertilization. Front Plant Sci 7:155. https://doi.org/10.3389/fpls.2016.00155
Alsokari SS (2011) Synergistic effect of kinetin and spermine on some physiological aspects of seawater stressed Vigna sinensis plants. Saudi J Biol Sci 18:37–44
Amin AA, Fatma AE, Ghrib M, El-awadi M, Rashed ESM (2011) Physiological response of onion plants to foliar application of putrescine and glutamine. Sci Hort 129(3):353–360
Angelini R, Cona A, Federico R, Fincato P, Tavladoraki P, Tisi A (2010) Plant amine oxidases ‘on the move’: an update. Plant Physiol Biochem 48:560–564.
Anwar R, Mattoo AK, Handa AK (2015) Polyamine interactions with plant hormones: crosstalk at several levels. In: Kusano T, Suzuki H (eds) Polyamines. Springer, Japan, pp 267–304
Apelbaum A, Burgoon AC, Anderson JD, Lieberman M, Ben-Arie R, Mattoo AK (1985) Polyamines inhibit biosynthesis of ethylene in higher plant tissue and fruit protoplasts. Plant Physiol 68:453–456
Arena ME, Pastur GM, Benavides MP, Curvetto N (2005) Polyamines and inhibitors used in successive culture media for in vitro rooting in Berberis buxifolia. N Z J Bot 43(2):373–380
Astarita LV, Handro W, Floh EIS (2003) Changes in polyamines content associated with zygotic embryogenesis in the Brazilian pine, Araucaria angustifolia (Bert.) O. Ktze. Rev Braz J Bot 26:163–168
Ayad HS, Reda F, Abdalla MSA (2010) Effect of putrescine and zinc on vegetative growth, photosynthetic pigments, lipid peroxidation and essential oil content of geranium (Pelargonium graveolens L.). World J Agri Sci 6:601–608
Bais HP, Ravishankar GA (2003) Synergistic effect of auxins and polyamines in hairy roots of Cichorium intybus L. during growth, coumarin production and morphogenesis. Acta Physiol Plant 25:193–220
Bais HP, George J, Ravishankar GA (1999) Influence of polyamines on growth of hairy root cultures of witloof chicory (Cichorium intybus L. cv. Lucknow Local) and formation of coumarins. J Plant Growth Regul 18:33–37
Bais HP, Madhusudhan R, Bhagyalakshmi N, Rajasekaran T, Ramesh BS, Ravishankar GA (2000) Influence of polyamines on growth and formation of secondary metabolites in hairy root cultures of Beta vulgaris and Tagetes patula. Acta Physiol Plant 22(2):151–158
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24:23–58
Bassard J-E, Ullmann P, Bernier F, Werck-Reichhart D (2010) Phenolamides: Bridging polyamines to the phenolic metabolism. Phytochem 71:1808–1824
Bhattacharya E, Rajam MV (2007) Polyamine biosynthetic pathway: a potential target for enhancing alkaloid production. In: Verpoorte R, Alfermann AW, Johnson TS (eds) Applications of plant metabolic engineering. Springer, Netherlands, pp 129–144
Biondi S, Scaramagli S, Capitani F, Altamura MM, Torrigiani P (2001) Methyl jasmonate upregulates biosynthetic gene expression, oxidation and conjugation of polyamines, and inhibits shoot formation in tobacco thin layers. J Exp Bot 52:231–242
Bitrián M, Zarza X, Altabella T, Tiburcio AF, Alcázar R (2012) Polyamines under abiotic stress: metabolic crossroads and hormonal crosstalks in plants. Metabolites 2:516–528
Bradford KJ (1986) Manipulation of seed water relations via osmotic priming to improve germination under stress conditions. Hortic Sci 21:1105–1112
Burrell M, Hanfrey CC, Murray EJ, Stanley-Wall NR, Michael AJ (2010) Evolution and multiplicity of arginine decarboxylases in polyamine biosynthesis and essential role in Bacillus subtilis biofilm formation. J Biol Chem 285(50):39224–39238
Capell T, Bassie L, Christou P (2004) Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci USA 101:990–991
Carman JG, Rodney GR, Fuller J, Ghermay T, Timmis R (2005) Nutrient and hormone levels in Douglas-fir corrosion cavities, megagametophytes, and embryos during embryony. Can J For Res 35:2447–2456
Chae SC (2016) Shoot organogenesis of Echinacea angustifolia DC as influenced by polyamines. LifeSci 13(1):16–19
Chen H, Jones AD, Howe GA (2006) Constitutive activation of the jasmonate signaling pathway enhances the production of secondary metabolites in tomato. FEBS Lett 580:2540–2546
Chen LF, Lu W, Sun J, Guo SR, Zhang ZX, Yang YJ (2011) Effects of exogenous spermidine on photosynthesis and carbohydrate accumulation in roots and leaves of cucumber (Cucumis sativus L.) seedlings under salt stress. J Nanjing Agri Univ 34(3):31–36
Chriqui D, D’Orazi D, Bagni N (1986) Ornithine and arginine decarboxylases and polyamine involvement during in vivo differentiation of Datura innoxia leaf explant. Physiol Planta 68:589–596
Couee I, Hummel I, Sulmon C, Gouesbet G, El Amrani A (2004) Involvement of polyamines in root development. Plant Cell Tissue Organ Cult 76:1–10
Del Duca S, Serafini-Fracassini D, Cai G (2014) Senescence and programmed cell death in plants: polyamine action mediated by trans-glutaminase. Front Plant Sci 5:1–17
Dey A, Gupta K, Gupta B (2014) Role of polyamines in plant–pathogen interactions. In: Anjum NA, Gill SS, Gil R (eds) Plant adaptation to environmental change. CAB International, USA, pp 222–244
Dias LLC, Santa-Catarina C, Silveira V, Pieruzzi FP, Floh EIS (2009) Polyamines, amino acids, IAA and ABA contents during Ocotea catharinensis seed germination. Seed Sci Technol 37:42–51
Do PT, Degenkolbe T, Erban A, Heyer AG, Kopka J, Karin IK, Hincha DK, Ellen Z (2013) Dissecting rice polyamine metabolism under controlled long-term drought stress. PLOS One 8(4):44–54
Drager B (2004) Chemistry and biology of calystegines. Nat Prod Rep 21:211–223
Edreva AM, Velikova VB, Tsonev TD (2007) Phenylamides in plants.Russ. J Plant Physiol 54:287–301
El-Yazal MAS, Rady MM (2012) Changes in nitrogen and polyamines during breaking bud dormancy in “Anna” apple trees with foliar application of some compounds. Sci Hort 136:75–80
Fait A, Nesi AN, Angelovici R, Lehmann M, Pham PA, Song L, Haslam RP, Napier JA, Galili G, Fernie AR (2011) Targeted enhancement of glutamate-to-γ-aminobutyrate conversion in Arabidopsis seeds affects carbon–nitrogen balance and storage reserves in a development-dependent manner. Plant Physiol 157:1026–1042
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009a) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212
Farooq M, Wahid A, Lee DJ (2009b) Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol Plant 31:937–945
Farooq M, Aziz T, Rehman A, Cheema SA, Rehman H (2011) Evaluating surface drying and re-drying for wheat seed priming with polyamines: effects on emergence, early seedling growth and starch metabolism. Acta Physiol Plant 33:1707–1713
Fecker LF, Rugenhagen C, Berlin J (1993) Increased production of cadaverine and anabasine in hairy root cultures of Nicotiana tabacum expressing a bacterial lysine decarboxylase gene. Plant Mol Biol 23:11–21
Ferreira MI, Uliana MR, Costa SM, Magro M, Vianello F, Ming LC, Lima GPP (2016) Exclusion of solar UV radiation increases the yield of curcuminoid in Curcuma longa L. Ind Crops Prod 89:188–194
Flores HE, Galston AW (1982) Polyamines and plant stress: activation of putrescine biosynthesis by osmotic shock. Science 217:1259–1261
Gill SS, Tuteja N (2010) Polyamines and abiotic stress tolerance in plants. Plant Signal Behav 5(1):26–33
Graser G, Hartmann T (2000) Biosynthesis of spermidine, a direct precursor of pyrrolizidine alkaloids in root cultures of Senecio vulgaris L. Planta 211:239–245
Hajiboland R, Ebrahimi N (2011) Effect of exposure to UV radiation on growth, photosynthesis and antioxidant defense system in tobacco (Nicotiana rustica L. cv. Basmas) plants treated with exogenous polyamines. Genet Plant Physiol 1:76–90
Hamill JD, Robins RJ, Parr AJ (1990) Over-expression of a yeast ornithine decarboxylase gene in transgenic roots of Nicotiana rustica can lead to enhanced nicotine accumulation. Plant Mol Biol 15:27–38
Handa AK, Mattoo AK (2010) Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol Biochem 48:540–546
Hao G, Ji H, Li Y, Shi R, Wang J, Feng L, Huang L (2012) Exogenous ABA and polyamines enhanced salvianolic acids contents in hairy root cultures of Salvia miltiorrhiza Bge.f.alba. Plant Omics 5(5):446–452
Hartmann T, Witte L (1995) Pyrrolizidine alkaloids: chemical, biological and chemoecological aspects. In: Pelletier SW (ed) Alkaloids: chemical and biological perspectives. Oxford, Pergamon, pp 155–233
Hashimoto T, Matsuda J, Yamada Y (1993) Two-step epoxidation of hyoscyamine to scopolamine is catalyzed by bifunctional hyoscyamine 6-hydroxylase. FEBS Lett 329:35–39
Herminghaus S, Tholl D, Rugenhagen C (1996) Improved metabolic action of a bacterial lysine decarboxylase gene in tobacco hairy root cultures by its fusion to an rbcS transit peptide coding sequence. Transgenic Res 5:193–201
Huang Y, Lin C, He F, Li Z, Guan Y, Hu Q, Hu J (2017) Exogenous spermidine improves seed germination of sweet corn via involvement in phytohormone interactions, H2O2 and relevant gene expression. BMC Plant Biol 17(1):1–16
Hudec J, Bakos D, Mravec D, Kobida L, Burdova M, Turianica I, Hlusek J (2006) Content of phenolic compounds and free polyamines in black chokeberry (Aronia melanocarpa) after application of polyamine biosynthesis regulators. J Agric Food Chem 54:3625–3628
Hussain SS, Ali M, Ahmad M, Siddique KHM (2011) Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol Adv 29:300–311
Hussain S, Khan F, Hussain HA, Nie L (2016) Physiological and biochemical mechanisms of seed priming-induced chilling tolerance in rice cultivars. Front Plant Sci 7:1–14
Hyun TK, Eom SH, Jeun YC, Han SH, Kim J-S (2013) Identification of glutamate decarboxylases as a γ-aminobutyric acid (GABA) biosynthetic enzyme in soybean. Ind Crops Prod 49:864–870
Ioannidis NE, Kotzabasis K (2007) Effects of polyamines on the functionality of photosynthetic membrane in vivo and in vitro. Biochim Biophys Acta 1767:1372–1382
Isah T, Umar S, Mujib A, Sharma MP, Rajasekharan PE. Zafar N, Fruk A (2017) Secondary metabolism of pharmaceuticals in the plant in vitro cultures: strategies, approaches, and limitations to achieving higher yield. Plant Cell Tiss Organ Cult. https://doi.org/10.1007/s11240-017-1332-2
Jardin PD (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hort 196:3–14
Jiménez-Bremont JF, Marina M, Guerrero-González ML, Rossi FR, Sánchez-Rangel D, Rodríguez-Kessler M, Ruiz OA, Gárriz A (2014) Physiological and molecular implications of plant polyamine metabolism during biotic interactions. Front Plant Sci 5:95. https://doi.org/10.3389/fpls.2014.00095
Jouhikainen K, Lindgren L, Jokelainen T (1999) Enhancement of scopolamine production in Hyoscyamus muticus L. hairy root cultures by genetic engineering. Planta 208:545–551
Kakkar RK, Rai VK (1993) Plant polyamines in flowering and fruit ripening. Phytochem 33:1281–1288
Kanchanapoom M, Antognoni F, Pistocchi R, Bagni N (1991) Effect of auxins on spermidine uptake into carrot protoplasts. Physiol Plant 82:19–23
Kang YM, Lee OS, Jung HY (2005) Overexpression of hyoscyamine 6-hydroxylase (h6h) gene and enhanced production of tropane alkaloids in Scopolia parviflora hairy root lines. J Microbiol Biotechnol 15:91–98
Karatan E, Michael AJ (2013) A wider role for polyamines in biofilm formation. Biotechnol Lett. https://doi.org/10.1007/s10529-013-1286-3
Kasukabe Y, He L, Watakabe Y, Otani M, Shimada T, Tachibana S (2006) Improvement of environmental stress tolerance of sweet potato by introduction of genes for spermidine synthase. Plant Biotech 23:75–83
Kaur-Sawhney R, Dai Y, Galston AW (1986) Effect of inhibitors of polyamine biosynthesis on gibberellin-induced inter-node growth in light-grown dwarf peas. Plant Cell Physiol 27:253–260
Kaur-Sawhney R, Tiburcio AF, Galston AW (1988) Spermidine and flower bud differentiation in thin-layer explants of tobacco. Planta 173:282–284
Kevers C, Le Gal N, Monteiro M, Dommes J, Gaspar T (2000) Somatic embryogenesis of Panax ginseng in liquid cultures: a role for polyamines and their metabolic pathways. Plant Growth Regul 31:209–214
Khan HA, Ziaf K, Amjad M, Iqbal Q (2012) Exogenous application of polyamines improves germination and early seedling growth of hot pepper. Chil J Agric Res 72:429–433
Kiseleva IS, Kaminskaya OA (2002) Hormonal regulation of assimilate utilization in barley leaves in relation to the development of their source function. Russ J Plant Physiol 49:534–540
Kumar V, Giridhar P, Chandrashekar A, Ravishankar GA (2008) Polyamines influence morphogenesis and caffeine biosynthesis in in vitro cultures of Coffea canephora P. ex Fr. Acta Physiol Plant 30:217–223
Kumría R, Rajam MV (2002) Ornithine decarboxylase transgene in tobacco affects polyamines, in vitro morphogenesis and response to salt stress. J Plant Physiol 159:983–990
Kusano T, Kim DW, Liu T, Berberich T (2015) Polyamine catabolism in plants. In: Kusano T, Suzuki H (eds) Polyamines. Springer., Japan, pp 47–60
Le G-L Saos, Hourmant F A (2001) Stimulation of putrescine biosynthesis via the ornithine decarboxylase pathway by gibberellic acid in the in vitro rooting of globe artichoke (Cynara scolymus). Plant Growth Regul 35:277–284
Lee OS, Kang YM, Jung HY (2005) Enhanced production of tropane alkaloids in Scopolia parviflora by introducing the PMT (putrescine N-methyltransferase) gene. In Vitro Cell Dev Biol 41:167–172
Legocka J, Zajchert I (1999) Role of spermidine in the stabilization of the apoprotein of the light-harvesting chlorophyll a/b-protein complex of photo-system II during leaf senescence process. Acta Physiol Plant 21:127–132
Lepri O, Bassie L, Safwat G, Thu-Hang P, Trung-Nghia P, Hölttä E, Chris-tou P, Capell T (2001) Over-expression of human ornithine decarboxylase cDNA in transgenic rice plants alters the polyamine pool in a tissue-specific manner. Mol Gen Genet 266:303–312
Li Z, Peng Y, Zhang XQ, Ma X, Huang LK, Yan YH (2014) Exogenous spermidine improves seed germination of white clover under water stress via involvement in starch metabolism, antioxidant defenses and relevant gene expression. Molécules 19:18003–18024
Li Z, Zhang Y, Zhang X, Peng Y, Merewitz E, Ma X, Huang L (2016) The alterations of endogenous polyamines and phytohormones induced by exogenous application of spermidine regulate antioxidant metabolism, metallothionein and relevant genes conferring drought tolerance in white clover. Environ Exp Bot 124:22–38
Lindemose S, Nielsen PE, Mollegaard NE (2005) Polyamines preferentially interact with bent adenine tracts in double-stranded DNA. Nucleic Acids Res 33:1790–1803
Liu Y, Gu DD, Wu W, Wen XX, Liao YC (2013) The Relationship between polyamines and hormones in the regulation of wheat grain filling. PloS One 8:e78196
Liu JH, Wang W, Wu H, Gong X, Moriguchi T (2015) Polyamines function in stress tolerance: from synthesis to regulation. Front Plant Sci 6:827. https://doi.org/10.3389/fpls.2015.00827
Liu Z, Liu P, Qi D, Peng X, Liu G (2017) Enhancement of cold and salt tolerance of Arabidopsis by transgenic expression of the S-adenosylmethionine decarboxylase gene from Leymus chinensis. J Plant Physiol 211:90–99
Mader JC, Hanke DE (1997) Polyamine sparing may be involved in the prolongation of cell division due to inhibition of phenylpropanoid synthesis in cytokinin-starved soybean cells. J Plant Growth Regul 16:89–93
Mahgoub MH, El-Ghorab AH, Bekheta HM (2006) Effect of some bioregulators on the endogenous phytohormones, chemical composition, essential oil and its antioxidant activity of carnation (Dianthus caryophyllus L.). Mansoura Univ J Agri Sci 31:4229–4245
Mahgoub MH, Abdel ANG, Mazhar AMA (2011) Response of Dahlia pinnata L. plant to foliar spray with putrescine and thiamine on growth, flowering and photosynthetic pigments. Am Eurasian J Agric Environ Sci 10(5):769–775
Mahros KM, Badawy EM, Mahgoub MH, Habib AM, Sayed IME (2011) Effect of putrescine and uniconazole treatments on flower characters and photosynthetic pigments of Chrysanthemum indicum L. plant J Am Sci 7(3):399–408
Majumdar R, Barchi B, Turlapati SA, Gagne M, Minocha R, Long S, Minocha SC (2016) Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: the pathway is regulated at the post-transcriptional level. Front Plant Sci 7:78. https://doi.org/10.3389/fpls.2016.00078
Martin-Tanguy J (1997) Conjugated polyamines and reproductive development: biochemical, molecular and physiological approaches. Physiol Plant 100:675–688
Martin-Tanguy J (2001) Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul 34:135–148
Matilla AJ (1996) Polyamines and seed germination. Seed Sci Res 6:81–93
Mattoo AK, Minoscha SC, Minocha R, Handa A (2010) Polyamines and cellular metabolism in plants: transgenic approaches reveal different responses to diamine putrescine versus higher polyamines spermidine and spermine. Amino Acids 38:405–413
Mattoo AK, Fatima T, Upadhyay RK, Handa AK (2015) Polyamines in plants: biosynthesis from arginine, and metabolic, physiological and stress-response roles. In: D’Mello JPF (ed) Amino acids in higher plants. CAB International, USA, pp 177–194
Minocha R, Majumdar R, Minocha SC (2014) Polyamines and abiotic stress in plants: a complex relationship. Front Plant Sci 5:175. https://doi.org/10.3389/fpls.2014.00175
Miura K (2013) Nitrogen and phosphorus nutrition under salinity stress. In: Ahmad P, Prasad MNV, Azooz MM (eds) Ecophysiology and responses of plants under salt stress. Springer, New York, pp 425–441
Monteiro M, Kevers C, Dommes J, Gaspar T (2002) A specific role for spermidine in the initiation phase of somatic embryogenesis in Panax ginseng CA Meyer. Plant Cell Tissue Organ Cult 68:225–232
Moschou PN, Delis ID, Paschalidis KA, Roubelakis-Angelakis KA (2008) Transgenic tobacco plants overexpressing polyamine oxidase are not able to cope with oxidative burst generated by abiotic factors. Physiol Plant 133:140–156
Moschou PN, Wu J, Cona A, Tavladoraki P, Angelini R, Roubelakis-Angelakis KA (2012) The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J Exp Bot 63:5003–5015
Moyano E, Jouhikainen K, Tammela P (2003) Effect of pmt gene overexpression on tropane alkaloid production in transformed root cultures of Datura metel and Hyoscyamus muticus. J Exp Bot 54:203–221
Murray RDH, Mendez J, Brown SA (1982) The natural coumarin: Occurrence, chemistry and biochemistry. John Wiley, New York
Mustafavi SH (2016) Effect of plant growth regulators on growth and productive characteristics of valerian at different environmental conditions. Dissertation. University of Maragheh
Mustafavi SH, Shekari F, Abbasi A (2015a) Putrescine improve low temperature tolerance of fennel (Foeniculum vulgare Mill.) seeds. Cercet Agron Mold 48(1):69–76
Mustafavi SH, Shekari F, Nasiri Y, Hatami-Maleki H (2015b) Nutritional and biochemical response of water-stressed valerian plants to foliar application of spermidine. Biol Forum 7(1):1811–1815
Mustafavi SH, Shekari F, Hatami Maleki H (2016) Influence of exogenous polyamines on antioxidant defence and essential oil production in valerian (Valeriana officinalis L.) plants under drought stress. Acta Agri Slov 107(1):81–91
Nahed GAA, Lobna ST, Soad MMI (2009) Some studies on the effect of putrescine, ascorbic acid and thiamine on growth, flowering and some chemical constituents of gladiolus plants at Nubaria. Ozean J App Sci 2:169–179
Narula A, Kumar SV, Pande D (2004) Agrobacterium mediated transfer of arginie decarboxylase and ornithine decarboxylase genes to Datura innoxiaenhances shoot regeneration and hyoscyamine biosynthesis. J Plant Biochem Biotech 13:127–130
Neumann KH, Kumar A, Imani J (2009) Plant propagation—meristem cultures, somatic embryogenesis. In: Neumann KH, Kumar A, Imani J (eds) Plant cell and tissue culture—a tool in biotechnology, principles and practice. Springer, Berlin, pp 75–137
Niakan M, Rezapour Mahjoob S, Ghorbanli M (2015) Effect of exogenous putrescine on growth, photosynthesis and alkaloid compounds of Datura (Datura stramonium L.) in response to salinity stress under hydroponic conditions. J Sci Tech Greenhouse Cult 6(21):15–26
Niemi K, Sutela S, Haggman H, Scagel C, Vuosku J, Jokela A, Sarjala T (2006) Changes in polyamine content and localization of Pinus sylvestris ADC and Suillus variegatus ODC mRNA transcripts during the formation of mycorrhizal interaction in an in vitro cultivation system. J Exp Bot 57(11):2795–2804
Orabi SA, Talaat IM, Balbaa LK, Abdalla AE (2015) Influence of pyridoxine and spermine on lemongrass (Cymbopogon citratus) plants. Nusantara bioscie 7:139–143
Oracz K, Karpinski S (2016) Phytohormones signaling pathways and ROS involvement in seed germination. Front Plant Sci 7:1–6
Oshmarina VI, Shevyakova NI, Shamina ZB (1982) Dynamics of free amino acids and amides in Nicotiana sylvestris cell cultures at different concentrations of putrescine in the medium. Soviet Plant Physiol 29:477–281
Pal M, Szalai G, Janda T (2015) Speculation: polyamines are important in abiotic stress signaling. Plant Sci. https://doi.org/10.1016/j.plantsci..05.003
Palazón J, Moyano E, Rcusidó RM (2003) Alkaloid production in Duboisia hybrid hairy roots and plants overexpressing the h6h gene. Plant Sci 165:1289–1295
Park JB (2009) Isolation and characterization of N-feruloyltyramine as the P-selectin expression suppressor from garlic (Allium sativum). J Agric Food Chem 57:8868–8872
Parvin S, Lee OR, Sathiyaraj G, Khorolragchaa A, Kim YJ, Yang DC (2014) Spermidine alleviates the growth of saline-stressed ginseng seedlings through antioxidative defense system. Genetics 53:70–78
Pieruzzi FP, Dias LLC, Balbuena TS, Santa-Catarina C, Dos Santos ALW, Floh EIS (2011) Polyamines, IAA and ABA during germination in two recalcitrant seeds: Araucaria angustifolia (Gymnosperm) and Ocotea odorifera (Angiosperm). Ann Bot 108:337–345
Pottosin I, Shabala S (2014) Polyamines control of cation transport across plant membranes: implications for ion homeostasis and abiotic stress signaling. Front Plant Sci 5:1–16
Rafiee H, Naghdi Badi H, Mehrafarin A, Qaderi A, Zarinpanjeh N, Sekara A, Zand E (2016) Application of plant biostimulants as new approach to improve the biological responses of medicinal plants- a critical review. J Med Plants 15(59):6–39
Ramey BE, Koutsoudis M, Bodman SB, Fuqua C (2004) Biofilm formation in plant–microbe associations. Curr Opin Micro Biol 7:602–609
Rastogi R, Davies PJ (1991) Polyamine metabolism in ripening tomato fruit. Plant Physiol 95:41–45
Rezvanypour S, Hatamzadeh A, Elahinia SA, Asghari HR (2015) Exogenous polyamines improve mycorrhizal development and growth and flowering of Freesia hybrida. J Hort Res 23(2):17–25
Rocha P, Stenzel O, Parr A (2002) Functional expression of tropinone reductase I (trI) and hyoscyamine-6-hydroxylase (h6h) from Hyoscyamus niger in Nicotiana tabacum. Plant Sci 162:905–913
Rosales EP, Iannone MF, Groppa MD, Benavides MP (2012) Polyamines modulate nitrate reductase activity in wheat leaves: involvement of nitric oxide. Amino Acids 42:857–865
Roy M, Wu R (2001) Arginine decarboxylase transgene expression and analysis of environmental stress tolerance in transgenic rice. Plant Sci 160:869–875
Rugini E (1992) Involvement of polyamines in auxin and Agrobacterium rhizogenes-induced rooting of fruit trees in vitro. J Am Soc Horti Sci 117:532–536
Santa-Catarina C, Silveira V, Scherer GFE, Floh EIS (2007) Polyamine and nitric oxide levels relate with morphogenetic evolution in somatic embryogenesis of Ocotea catharinensis. Plant Cell Tissue Organ Cult 90:93–101
Sato F, Hashimoto T, Hachiya A (2001) Metabolic engineering of plant alkaloid biosynthesis. Proc Natl Acad Sci 98:367–372
Savvides A, Ali S, Tester M, Fotopoulos V (2016) Chemical priming of plants against multiple abiotic stresses: mission possible? Trends Plant Sci 21(4):329–340
Scaramagli S, Bueno M, Torrigiani P, Altamura MM, Capitani F, Bagni N (1995) Morphogenesis in cultured thin layers and pith explants of tobacco. II. Early hormone-modulated polyamine biosynthesis. J Plant Physiol 147:113–117
Serafini-Fracasini D, Della-Mea M, Tasco G, Casadio R, Del-Duca S (2009) Plant and animal transglutaminases: do similar functions imply similar structures? Amino Acids 36:643–657
Serafini-Fracasini D, Di Sandro A, Del Duca S (2010) Spermine delays leaf senescence in Lactuca sativa and prevents the decay of chloroplast photosystems. Plant Physiol Biochem 48:602–611
Serapiglia MJ, Minocha R, Minocha CS (2008) Changes in polyamines, inorganic ions and glutamine synthetase activity in response to nitrogen availability and form in red spruce (Picea rubens). Tree Physiol 28:1793–1803
Shekari F, Asadi Danalo A, Mustafavi SH (2015) Exogenous polyamines improve seed germination of borage under salt stress via involvement in antioxidant defenses. WALIA 31(S6):57–63
Shelp BJ, Bozzo GG, Trobacher CP, Zarei A, Deyman KL, Brikis CJ (2012) Hypothesis/review: contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci 194:130–135
Sheteiwy M, Shen H, Xu J, Guan Y, Song W, Hu J (2017) Seed polyamines metabolism induced by seed priming with spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environ Exp Bot 137:58–72
Shi H, Chan Z (2014) Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J Integr Plant Biol 40:20–30
Shoji T, Hashimoto T (2015) Polyamine-derived alkaloids in plants: molecular elucidation of biosynthesis. In: Kusano T, Suzuki H (eds) Polyamines. Springer, Japan, pp 189–200
Shu S, Guo SR, Yuan LY (2012) A review: polyamines and photosynthesis. In: Advances in photosynthesis—fundamental aspects. InTech, Rijeka, pp 439-464
Sivanandhan G, Mariashibu TS, Arun M, Rajesh M, Kasthurirengan S, Selvaraj N, Ganapathi A (2011) The effect of polyamines on the efficiency of multiplication and rooting of Withania somnifera (L.) Dunal and content of some withanolides in obtained plants. Acta Physiol Plant 33:2279–2288
Slocum RD, Flores HE (1991) Biochemistry and physiology of polyamines in plants. CRC Press, Boca Raton
Subramanyam S, Sardesai N, Minocha SC, Zheng C, Shukle RH, Williams CE (2015) Hessian fly larval feeding triggers enhanced polyamine levels in susceptible but not resistant wheat. BMC Plant Biol. https://doi.org/10.1186/s12870-014-0396y
Sudha G, Ravishankar GA (2003) Putrescine facilitated enhancement of capsaicin production in cell suspension cultures of Capsicum frutescens. J Plant Physiol 160:339–346
Suresh B, Thimmaraju R, Bhagyalakshmi N, Ravishankar GA (2004) Polyamine and methyl jasmonate-influenced enhancement of betalaine production in hairy root cultures of Beta vulgaris grown in a bubble column reactor and studies on efflux of pigments. Process Biochem 39:2091–2096
Takahashi T, Kakehi J (2010) Polyamines: ubiquitous polycations with unique roles in growth and stress responses. Ann Bot 105:1–6
Talaat IM, Balbaa LK (2010) Physiological response of sweet basil plants (Ocimum basilicum L.) to putrescine and trans-cinnamic acid. Am Eurasian J Agric Environ Sci 8(4):438–445
Talaat IM, Bekhea MA, Mahgoub MH (2005) Physiological response of periwinkle plants (Catharanthus roseus L.) to tryptophan and putrescine. Int J Agric Biol 2:210–213
Tavladoraki P, Cona A, Federico R, Tempera G, Viceconte N, Saccoccio S, Battaglia V, Toninello A, Agostinelli E (2012) Polyamine catabolism: target for antiproliferative therapies in animals and stress tolerance strategies in plants. Amino Acids 42:411–426
Tiburcio AF, Altabella T, Bitrián M, Alcázar R (2014) The roles of polyamines during the lifespan of plants: from development to stress. Planta 240(1):1–18
Tisi A, Angelini R, Cona A (2011) Does polyamine catabolism influence root development and xylem differentiation under stress conditions? Plant Signal Behav 6(11):1844–1847
Veerasamy G, Chinnagounder S (2013) Effect of polyamines on in vitro organogenesis using shoot tip explants of Stevia rebaudiana Bert. Int J Rec Biotechnol 21(2):5–10
Viu AFM, Viu MAO, Tavares AR, Vianello F, Lima GPP (2009) Endogenous and exogenous polyamines in the organogenesis in Curcuma longa L. Sci Hort 121:501–504
Waie B, Rajam MV (2003) Effect of increased polyamine biosynthesis on stress responses in trans-genic tobacco by introduction of human S-adenosylmethionine gene. Plant Sci 164:727–734
Wang BQ, Zhang QF, Liu JH, Li GH (2011) Overexpression of PtADC confers enhanced dehydration and drought tolerance in transgenic tobacco and tomato: effect on ROS elimination. Biochem Biophys Res Commun 413:10–16
Wi SJ, Kim WT, Park KY (2006) Over expression of carnation S-adenosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep 25:1111–1121
Wimalasekera R, Tebartz F, Scherer GF (2011) Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci 181:593–603
Wolff EC, Park MH (2015) Role of the polyamine spermidine as a precursor for hypusine modification in eIF5A. In: Kusano T, Suzuki H (eds) Polyamines. Springer, Japan, pp 121–130
Xie SS, Wu HJ, Zang HY, Wu LM, Zhu QQ, Gao XW (2014) Plant growth promotion by spermidine-producing Bacillus subtilis OKB105. Mol. Plant Microbe Interact 27(7):655–663
Xu S, Hu JL, Ma W, Zheng Y, Zhu S (2011) Chilling tolerance in Nicotiana tabacum induced by seed priming with putrescine. Plant Growth Regul 63:279–290
Yang YK, Lee SY, Park WT, Park NI, Park SU (2010) Exogenous auxins and polyamines enhance growthand rosmarinic acid production in hairy root cultures of Nepeta cataria L. Plant omics 3(6):190–193
Yang C, Chen M, Zeng L, Zhang L, Liu X, Lan X, Tang K, Liao Z (2011) Improvement of tropane alkaloids production in hairy root cultures of Atropa belladonna by overexpressing pmt and h6h genes. Plant Omics 4(1):29–33
Yang L, Hong X, Xiao-xia W, Yun-cheng L (2016) Effect of polyamine on seed germination of wheat under drought stress is related to changes in hormones and carbohydrates. J Integr Agric 15(12):2759–2774
Yan-hua Z, Yu-ping Z, Jing X, Hui W, Hui-zhe C, Yi-kai Z, De-feng Z (2016) Effects of chilling tolerance induced by spermidine pretreatment on antioxidative activity, endogenous hormones and ultrastructure of indica japonica hybrid rice seedlings. J Integr Agric 15(2):295–308
Youssef AA, Mahgoub MH, Talaat IM (2004) Physiological and biochemical aspects of Matthiola incana L. plants under the effect of putrescine and Kinetin treatments. Egypt J App Sci 19:492–510
Yu Y, Zhang WB, Li XY, Piao XC, Jiang J, Lian ML (2016) Pathogenic fungal elicitors enhance ginsenoside biosynthesis ofadventitious roots in Panax quinquefolius during bioreactor culture. Ind Crops Prod 94:729–735
Zeid FA, Omer EA, Amin AY, Hanafy SAH (2014) Effect of putrescine and salicylic acid on ajwain plant (Trachyspermum ammi) at vegetative stage grown under drought stress. Int J Agric Sci Res 4(6):61–80
Zhang L, Ding R, Chai Y (2004) Engineering tropane biosynthetic pathway in Hyoscyamus niger hairy root cultures. Proc Natl Acad Sci 101:6786–67910
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by L. A. Kleczkowski.
Rights and permissions
About this article
Cite this article
Mustafavi, S.H., Naghdi Badi, H., Sękara, A. et al. Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol Plant 40, 102 (2018). https://doi.org/10.1007/s11738-018-2671-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2671-2