Abstract
Mannitol is a naturally occurring six-carbon sugar alcohol that has wide applications in the food and pharmaceutical industry because of its many properties, namely being a natural sweetener with a low metabolism and no glycemic index. The increasing demand for mannitol has spurred many studies of its production. Compared with its chemical synthesis and extraction from plants, both of which are difficult to satisfy for industrial requirements, biotechnological production of mannitol has received considerably more attention and interest from scientists because of its known advantages over those two methods. Accordingly, in this review, we summarize recent advances made in the production of mannitol through various biotechnological methods. The physicochemical properties, sources, and physiological functionalities and applications of mannitol are systematically covered and presented. Then, different determination methods for mannitol are also described and compared. Furthermore, different biotechnological strategies for the production of mannitol via fermentation engineering, protein engineering, and metabolic engineering receive a detailed overview in terms of mannitol-producing strains, enzymes, and their key reaction parameters and conditions.
Key points
• Physiological functionalities and applications of mannitol are presented in detail.
• Different determination methods for mannitol are also described and compared.
• Various biotechnological strategies for the production of mannitol are reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugar alcohols, a class of polyols, are formed by the reduction of an aldehyde or ketone group on the monosaccharide, disaccharide, oligosaccharide, and polysaccharide molecule to a hydroxyl group, thus differing markedly from other polyols, such as ethylene glycol, propylene glycol, and pentaerythritol that are synthesized by the petrochemical industry (Song and Vieille 2009). In the past few decades, many kinds of sugar alcohols have emerged due to their properties and broad applications, especially in the food industry as sweeteners, cooling agents, humectants, and thickeners in baking goods, hard candies, and spreads (Rice et al. 2019). Currently, these common sugar alcohols used in food include mannitol, sorbitol, erythritol, maltitol, lactitol, xylitol, and isomalt (Fig. 1). For example, by using an unsupported mesoporous Ni/NiO catalyst in the temperature range of 100–150 °C under a 50-bar pressure, sorbitol was hydrogenated from glucose with a yield of 84 wt% (Singh et al. 2018). Using an Ni/SiO2 catalyst derived from nickel phyllosilicate, xylitol was obtained from xylose via hydrogenation and distinguished by high xylitol selectivity of approximately 99% (Du et al. 2020). When prepared a ternary Co-P-B amorphous alloy catalyst with KBH4 and NaH2PO2 in an aqueous solution, these catalysts exhibited 100% selectivity for maltitol during liquid-phase hydrogenation of maltose (Li et al. 2007). Erythritol is mainly produced through biological processes involved in fungi fermentation. It can be formed from an erythrose-4-phosphate, a metabolic product of the pentose phosphate pathway in erythritol-producing yeasts, by dephosphorylation and reduction with the concomitant oxidation of NAD(P)H to NAD(P)+ (Rzechonek et al. 2018). Furthermore, lactitol and isomalt may also be chemically hydrogenated from lactose (Mishra et al. 2018) and sucrose (Queneau et al. 2008), respectively. Using glucose and fructose (50:50, w/w) as substrates, 25% of mannitol was obtained from this mixture solution via chemical hydrogenation (Dai et al. 2017; Lu et al. 2020).
Recently, demand for these sugar alcohols has gradually increased in the food and medical fields due to their low-metabolism, no glycemic index, and natural sweetening traits (Mitchell 2006). These properties make them a better food substitute for conventional sugar as they reduce the risk of diseases, such as diabetes and obesity, closely associated with the excess intake of high-sugar foods. However, the chemical production of these sugar alcohols has many disadvantages that are not conducive to their commercial industrialization, such as complex reaction steps, weakly selective chemical inorganic catalysts, and many accompanied by-products. Accordingly, the biotechnological production of these sugar alcohols has garnered considerable attention and interest.
Mannitol is a naturally occurring sugar alcohol widely used in the food, pharmaceutical, and chemical industry. The biological production of mannitol has been extensively studied in many reports through various biotechnological strategies. A great variety of microorganisms, including lactic acid bacteria (LAB), yeast, and fungi, have been found to harbor the potential ability for mannitol production through fermentation techniques (Saha and Racine 2011). To reduce the cost of producing mannitol, much research is focusing on how to utilize low-cost substrates in this process. In addition, various enzymes from different species, including mannitol-1-phosphate dehydrogenase (Mt1D, EC 1.1.1.17), mannitol-1-phosphatase (M1Pase, EC 3.1.3.22), and mannitol-2-dehydrogenase (MtDH, EC 1.1.1.67 or EC 1.1.1.138), have also been discovered and reported on for their roles in mannitol’s production. Furthermore, with advances in micro-biotechnology, metabolic engineering may also be applied to the production of mannitol. Yet few systematic summaries or reviews of these reports for the biotechnological production of mannitol are currently available (Dai et al. 2017; Zhang et al. 2018).
In this review article, a kind of sugar alcohol, mannitol, is discussed in terms of its physicochemical properties, sources, and physiological functionalities and applications. Then, a suite of determination methods for mannitol are also summarized and described. Next, recent advances made in how to produce mannitol using different biotechnological strategies, namely fermentation engineering, protein engineering, and metabolic engineering approaches, are discussed in detail.
Physicochemical properties of mannitol
Along with sorbitol, xylitol, and erythritol, mannitol is a key compound in the sugar alcohol field of research. Also called mannite or mannitolum, its chemical structure is similar to sorbitol except for the orientation of hydroxyl groups at the C-2 position that varies (Fig. 1), having a molecular formula and molecular weight of C6H14O6 and 182.17 g/mol, respectively. With a sweetness level that is 50–70% that of sucrose, mannitol will melt and boil at 165–168 °C and 290–295 °C, respectively, having a caloric value of 1.99 kcal/g and a relative density of 1.49 (25 °C). Mannitol is noted for its good stability properties in dilute acid/alkali solution and for not being easily oxidized when exposed to air. Furthermore, mannitol can be dissolved in glycerin, pyridine, and aniline and is slightly soluble in ethanol and methanol, but almost insoluble in ethers, ketones, and hydrocarbons, to name a few (Xiang and Wang 2009; Zhou and Fan 2005).
Existing sources of mannitol
Mannitol is a six-carbon sugar alcohol naturally found in many consumed vegetables and fruits (Saha and Racine 2011). In particular, mannitol is widely distributed in pumpkins, celery, carrot, pineapple, and marine algae, albeit in small quantities (Stoop et al. 1996). Mannitol was first isolated and characterized from the honeydew honey tree in 1806 by Proust, who is the source of its name (Wei 2010). The content of mannitol will vary depending on the species identity in which it occurs. For example, through the photosynthesis, marine algae such as brown algae could generate 10–20% mannitol (Ikawa et al. 1972; Dai et al. 2017). Another abundant source of mannitol is derived from manna; after heating the bark of the Fraxinus ornus tree, approximately 30–50% mannitol was produced, and this method was used up till the 1920s for the preparation of commercial mannitol (Soetaert 1990). Additionally, in some cases, the content of mannitol is closely related to the role it plays in higher plants. Guicherd et al. (1997) reported the mannitol content of leaves of F. excelsior L. under drought conditions was twice that under well-watered conditions, as this accumulated mannitol was involved in this tree’s osmotic and tolerance adjustment in response to water stress. Moreover, there are bacteria, yeast, fungi, seaweed, lichen, and other microorganisms that can also produce mannitol.
Physiological functionalities and applications of mannitol
As noted above, mannitol is widespread in nature. It is the only non-hygroscopic crystal among the functional sugar alcohols. Since its discovery, many of its important physiological functions have been revealed, and this compound is now used extensively in the food and pharmaceutical industry. Due to its certain nutritional value, mannitol also has utility as a functional food. After its absorption into the human body, 75% of it is fermented and absorbed by the intestinal microbiota; the remaining 25% is absorbed before being discharged into urine without having been metabolized for energy (Livesey 2003; Song and Vieille 2009). In addition, since the glycemic and insulinemic indexes of mannitol are zero, it is highly suitable for dieters and diabetics as a food constituent (Meng et al. 2017). Mannitol has been recognized as GRAS (Generally Recognized As Safe) by the FDA, which facilitates its application in prepared foods. For example, mannitol can serve as a sweetener or anti-sticking agent in sugar-free chewing gum. When making flavored ice cream and chocolate, the addition of mannitol could increase the hard shape of the shuck (Saha and Racine 2011). In the baking industry, because of its non-reducing properties which would not cause the Maillard reaction, mannitol can maintain a product’s color appearance by diminishing the likelihood of cooking. Furthermore, mannitol has promising use for removing free radicals, by activating superoxide dismutase and eliminating the accumulation of lipid peroxides in the body (Dai et al. 2017).
Mannitol also has broad applications in the clinical and pharmaceutical industry. Being a highly effective dehydrating agent and osmotic diuretic, mannitol can help to minimize the risk of acute renal failure in patients after they undergo renal transplantation. It can reduce the elevated intracranial pressure and protect perioperative renal functioning in those patients undergoing major vascular and cardiac surgery, and in those with jaundice (Rockwell 2015; Shawkat et al. 2012). Additionally, mannitol has found use as a chemically synthesized pharmaceutical raw material. As a hexahydric alcohol, the hydroxyl groups within mannitol are prone to substitution, esterification, and condensation reactions, thereby generating a series of biologically active derivatives or their intermediates. For example, the preparation of dibromomannitol, by substitution reaction of mannitol as the raw material with bromine water, has demonstrated clinical benefits in treating chronic myelogenous leukemia and particular positive effects for Hodgkin’s disease and polycythemia (Zhan and Song 2003). In the plastics industry, using mannitol and propylene oxide as starting (raw) materials with KOH as the catalyst under pressure at 100–150 °C, produced polymannitol-propylene oxide ether, which is used to manufacture rigid polyurethane foam, possessing improved oil resistance, oxidation resistance, and dimensional stability, whose heat resistance reaches 180 °C (Qianqian 2010).
Determination methods for mannitol
As an important sugar alcohol, mannitol has received considerable attention due to its broad applications in food, chemical, and pharmaceuticals industry. Accordingly, the detection and analysis of mannitol have become critical for its large-scale production and sound application. Generally, detecting mannitol has two parts: qualitative and quantitative analyses (Xiang and Wang 2009). The principle of qualitative analysis is that, under alkaline conditions, crystalline mannitol can react with ferric chloride to form a brownish-yellow precipitate that is not dissolvable unless excess alkaline solution is added (Zhou and Fan 2005). Although this method is relatively simple and lets one quickly judge whether the mannitol is present, it is limited to mannitol of higher crystalline and purity. If the ingredients in solution are complex, the accuracy is greatly diminished, thereby posing major limitations in practical and industrial applications. Quantitative analysis of mannitol mainly includes thin-layer chromatography (TLC), colorimetric method (Li et al. 1999), high-performance liquid chromatography (HPLC) (Liu et al. 2004), gas chromatography–mass spectrometry (GC-MS) (Kiyoshima et al. 2001), and capillary electrophoresis–electrochemical detection (CE-ED) (Cheng et al. 2008).
Spectroscopic methods
Spectrophotometry is a method of qualitatively and quantitatively analyzing a substance by measuring its absorbance of light, at a specific wavelength or a certain wavelength range. It has the advantages of high sensitivity, easy operation, and rapid monitoring and now is the most commonly used experimental method for the analysis and determination of many chemical compounds. In a previous article, Li et al. (1999) employed the spectrophotometric method to determine the mannitol content of Cordyceps sinensis. Actually, mannitol itself does not display any characteristic absorption peak. But if sodium periodate is added to the mannitol solution, a yellow compound named 3,5-diacetyl-1,4-dehydrodimethylpyridine is generated after the oxidation reaction, and this compound exhibits a characteristic maximum absorption value at 412 nm. Furthermore, some monosaccharides, such as d-galactose, d-glucose, and d-mannose, have a negligible effect on this reaction but the presence of d-fructose will interfere with mannitol’s determination by the reaction system (Jiang et al. 2005). To reduce this unwanted interference, Jiang et al. (2005) developed a method whereby the sample is co-heated with hydrochloric acid in advance which ensures the d-fructose become furfural after dehydration, leaving it inert and unable react with sodium periodate.
Chromatographic methods
High-performance liquid chromatography (HPLC) has a wide range of applications in many basic and applied fields due to its accuracy and practicality. With the popularization of HPLC, this approach has been used more often recently to detect mannitol, especially when it occurs in a solution with complex components. Fleming et al. (1990) reported on a rapid and simultaneous determination method for detecting lactulose and mannitol in urine samples, by using HPLC coupled with pulsed amperometric detection, in which the lactulose and mannitol separated well from each other and also from other carbohydrates in the urine. Likewise, work by Liu et al. (2004) showed to determine mannitol by using HPLC with a refractive index detector. A GC-MS method was also devised to measure the level of mannitol in various human brain tissues obtained at autopsy (Kiyoshima et al. 2001): after derivatization with 1-butaneboronic acid, mannitol was subjected to GC-MS which revealed its detection limit in distilled water could reach as low as 1 ng/0.1 g.
Other methods
Polyhydroxy saccharide easily incurs redox reactions on a copper electrode under alkaline conditions. When using an electrochemical detector, the saccharide could be directly detected and so it does not require a derivatization reaction. Cheng et al. (2008) applied the CE-ED method to detect carbohydrates in Chrysanthemum plant tissues. After optimizing the electrode potential, concentration of operating solution, electrophoresis voltage, and injection time, the content of mannitol could thus be measured. Using this method, mannitol in the woody plant Ligustrum lucidum Ait at different growth stages was also determined (Chen et al. 2005).
As highlighted above, different determination methods of mannitol are characterized by their own advantages that depend on the properties of various samples. To assay the free mannitol present in a mixed solution, HPLC may well be the preferable choice. Yet, if the component of the sample is clear and no interference compound(s) is present, adopting a spectroscopic method is apt because of its higher efficiency. But when the test sample contains many saccharides, CE-ED may be a better choice for determining the mannitol content.
Mannitol production
Extraction methods for mannitol production
In recent decades, mannitol has been found widely in seaweed, fruits, plant leaves, and almost all vegetables, but in small amounts. Therefore, extensive studies of mannitol’s extraction from these sources have been made to obtain this valuable sugar alcohol. In China, the extraction of mannitol from natural sea products, such as kelp and seaweed, is a major mode by which to obtain mannitol (Wei 2010). To do this, in the main extraction process used, the kelp and seaweed are first prepared with sodium alginate and then soaked in alkali water, precipitated, and filtered, after which the filtrate is further acidified, oxidized, and treated by an ion exchange resin to adsorb the iodine. The remaining acidic aqueous solution containing mannitol is further processed by electrodialysis or a recrystallization method, thereby yielding pure mannitol (Wei 2010; Xie et al. 1998).
Chemical synthesis for mannitol production
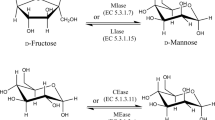
Currently, the commercial production of mannitol is done primarily by applying the catalytic hydrogenation method at high temperature and pressure with the use of chemical catalysts (Fig. 1) (Tang et al. 1998; Zhang et al. 2018). To do this, chemists rely mostly on d-fructose or d-glucose, or both, to synthesize mannitol; in this approach, β-fructose is converted to mannitol, whereas α-fructose and glucose are converted to sorbitol as a by-product, when using Raney-nickel as a catalyst and hydrogen gas as the reducing reagent (Dai et al. 2017). In their patented technique, Takemura et al. (1978) proposed that glucose be first converted to mannose (yielding 30–36%) via chemical isomerization, after which the produced mannose is further hydrogenated into mannitol. Later, Wang et al. (2005) used dual isomerization coupled with a chemical hydrogenation method to produce crystalline mannitol. For this, the starting material glucose was first isomerized to mannose by 0.3% ammonium molybdate at 100 °C, pH 3–4 for 3 h and then further isomerized by adding glucose isomerase. Next, the glucose-mannose-fructose mixed solution was chemically hydrogenated, using Raney-nickel as the catalyst, and the formed sorbitol and mannitol products then separated by recrystallization to obtain a mannitol purity of 97.5% and a mannitol yield of 59% (Wang et al. 2005). Because mannitol is much less soluble than sorbitol (von Weymarn et al. 2002), if a starting material consisting of a glucose-fructose mixture of 1:1 is used, its hydrogenation would produce mixed mannitol-sorbitol (1:3) products due to the poor selectivity of the nickel catalyst (Soetaert 1990). Nonetheless, some shortcomings and disadvantages inevitably emerge in the chemical synthesis process as a whole. The accompanied byproduct sorbitol and added metal catalysts should be removed during downstream processing, which increases the total separation costs. In addition, this reaction often requires a high temperature, high pressure, and low pH environment, in addition to high purity substrates, which together drive up overall production costs while also having low yield deficiencies.
Biological strategies for mannitol production
Fermentation engineering for mannitol production
There are many extensive studies investigating the production of mannitol through microbial fermentation. Based on the differences in the mannitol synthesis pathway during fermentation, these mannitol-producing bacteria are generally divided into homofermentative LAB (HO-LAB) and heterofermentative LAB (HE-LAB) (Fig. 2). Glucose and fructose are the most effective substances for mannitol production. For HO-LAB, when the glucose and/or fructose substrate is absorbed and metabolized to fructose-6-phosphate, mannitol could be produced from fructose-6-phosphate by the action of M1Pase and Mt1D. For HE-LAB, when the fructose substrate enters into the cell via permease, mannitol is obtainable from fructose under the action of MtDH. Recently, producing mannitol from agricultural waste and forestry resources is receiving much attention and interest by scientists trying to reduce overall production costs and prevent environmental pollution by changing these waste products into something harmless or useful (Table 1). For example, Saha (2006) developed a simultaneous enzymatic saccharification and fermentation (SSF) strategy to produce mannitol from inulin, a polyfructan occurring in Jerusalem artichoke, chicory, and dahlia. The HE-LAB L. intermedius NRRL B-3693 was capable of producing 207.4 g L−1 of mannitol in 72 h by SSF, whose corresponding productivity was 2.88 g L−1 h−1. If applying the fed-batch fermentation (FBF) strategy, this strain could generate 160.7 g L−1 of mannitol with a volumetric productivity of 4.0 g L−1 h−1 from the medium containing 50 g L−1 of corn steep liquor, 250 g L−1 of fructose, and 33 mg L−1 of MnSO4 (Saha and Racine 2010). When using cashew apple juice as the substrate, which contained 50 g L−1 of total reducing sugars (28 g L−1 of fructose), 18 g L−1 of mannitol was obtained with a 67% fructose conversion and productivity of 1.8 g L−1 h−1 by the action of Leuconostoc mesenteroides B-512F (Fontes et al. 2009). When grown in a medium supplemented with carob syrup—this containing sucrose, fructose, glucose, and pinitol—L. fructosum provided the maximum mannitol concentration, at 43.7 g L−1, and the highest volumetric productivity of 2.36 g L−1 h−1 among the eight tested LAB strains (Carvalheiro et al. 2011). Sugar molasses, a by-product in the sugar industry that is rich in sucrose, glucose, and fructose, has also been employed in the production of mannitol. After agitating the culture grown at 37 °C on DeMan–Rogosa–Sharpe (MRS) media supplemented with sugarcane molasses, 211.3 g L−1 of mannitol was produced from 7.5% of molasses with a productivity of 1.60 g L−1 h−1 by L. reuteri CRL 1101 (Ortiz et al. 2012). In another study, to effectively use raw glycerol, Yoshikawa et al. (2014) isolated and characterized 13 mannitol-producing yeast strains from environmental samples. Their results showed Candida azyma NBRC10406 attained a mannitol production level of 50.8 g L−1 over 7 days and a yield of 0.30 g g−1 glycerol, in jar fermenters under batch culture conditions.
Protein engineering for mannitol production
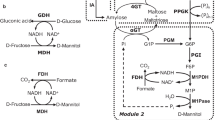
As Fig. 2 shows, MtDH is a crucial enzyme for the conversion of fructose to mannitol by HE-LAB. Not surprisingly then, many researchers have attempted to synthesize mannitol in vitro through protein engineering (Table 2). A thermostable MtDH from hyperthermophilic Thermotoga neapolitana DSM 4359 was cloned and expressed in E. coli. This recombinant TnMtDH featured an optimal pH and temperature of 6.5 and 90 °C, respectively. The recombinant purified TnMtDH could produce mannitol from 1% (w/v) fructose at a conversion rate of 40%, with TnMtDH showing no activity toward xylitol, inositol, sorbitol, rahmanose, mannose, and xylose under high temperatures (Koko et al. 2016). Earlier, Song et al. (2008) had cloned and expressed a gene encoding MtDH from T. maritime TM0298 in E. coli and investigated the biochemical characteristics of this recombinant enzyme, TmMtDH. Firstly, it was highly thermostable, with half-lives of 57 min at 80 °C and 6 min at 95 °C; secondly, using a two-step enzymatic transformation of glucose to mannitol with xylose isomerase from T. neapolitana and TmMtDH, 19 mM of mannitol was produced from 180 mM of glucose. The low former concentration may be attributed to the absence of a regenerating co-factor; hence, the MtDH from L. pseudomesenteroides ATCC 12291, coupled with the formate dehydrogenase (FDH, EC 1.17.1.9) from Candida boidinii, was used for co-factor NADH regeneration from NAD+ to enhance enzymatic production of mannitol (Fig. 3) (Parmentier et al. 2005). By combining 25 U L−1 of MtDH and commercial 25 U L−1 of FDH and reacting this at 25 °C and pH 6.5 with 100 mM of fructose and 100 mM of Na-formate, all the fructose was converted into mannitol in the reaction mixture. Similarly, to solve the problem of insufficient cofactor regeneration during the mannitol production process, an NAD(P)-dependent glucose dehydrogenase could be relied upon because it catalyzes glucose into gluconic acid, with the NAD+ reduced to NADH (Fig. 3) (Kulbe et al. 1987).
To reduce the multiple transformation steps and prevent the loss of intermediate compound via efficient substrate channeling, fusion enzymes consisting of M1Pase and Mt1D were heterologously expressed in E. coli, leading to the synthesis of mannitol (Madsen et al. 2018). Although 218 mg L−1 of mannitol, corresponding to 2% molar yield of glucose, was achieved in the experimental conditions, this concentration of mannitol was clearly lower when compared with an earlier report (Kaup et al. 2005). Hence, further research should focus on substrate transport and metabolic flux regulation of substrates to increase the yield of mannitol.
Metabolic engineering for mannitol production
MtDH can catalyze the direct reaction between fructose and mannitol, playing a pivotal role in the production of mannitol. However, given that MtDH is a NADH-dependent enzyme, using it to produce mannitol will undoubtedly increase the production costs. To overcome this limitation, Baumchen et al. (2007) created an oxidation/reduction cycle for the conversion of fructose to mannitol by using the resting cells of Corynebacterium glutamicum. The MtDH gene from Leuconostoc pseudomesenteroides and the FDH gene from Mycobacterium vaccae were overexpressed in the strain C. glutamicum. The recombinant C. glutamicum cells harboring pEKEx2–fdh–mdh could produce mannitol at a constant rate of 0.22 g (g dcw)−1 h−1 under the co-substrates of fructose and sodium formate (Table 2). If the resting whole cell of Bacillus megaterium co-expressing the above source MtDH and FDH was used for the biotransformation of fructose to mannitol, 22.0 g L−1 of mannitol was produced in a FBF strategy with a productivity of 0.32 g (g dcw)−1 h−1 (Baumchen et al. 2007). In the redox cycle, the uptake rate of fructose was a limiting factor for mannitol’s production rather than the FDH activity per se. Therefore, the glucose/fructose facilitator gene glf from Zymomonas mobilis was ligated to vector pVWEx2 and overexpressed in C. glutamicum (pEKEx2–fdh–mdh); this led to a 5.5-fold greater productivity, of 1.25 g (g dcw)−1 h−1, and 87 g L−1 of mannitol obtained from 93.7 g L−1 of fructose (Baumchen and Bringer-Meyer 2007). Previous work found that HO-LAB L. lactis MG1363 strains deficient in lactate dehydrogenase (LDH) could produce mannitol during glucose catabolism (Neves et al. 2002). However, as this strain was able to metabolize mannitol, the metabolite was consumed once glucose was depleted, which does not benefit the production of mannitol. The outcome’s corollary is that mannitol transport system of this LDH-deficient strain was disrupted. Accordingly, the genes mltA and mtlF responsible for the mannitol transport were deleted, by a double-crossover recombinant in strain L. lactis FI9630 derived from a food-grade LDH-deficient strain MG1363 (Gaspar et al. 2004). Results using this altered strain showed that 90% of mannitol accumulated in the extracellular medium when the glucose was completely exhausted, with one-third of the glucose converted into mannitol. Among the produced products, which included 2,3-butanediol, ethanol, lactate, acetoin, and acetate, mannitol was the major metabolite, present in a concentration of 13.1 mM, thus indicating that this engineering strategy has potential applications for the production of mannitol by resting bacterial cells. Coincidentally, to produce mannitol from glucose substrate, a systematic synthesis platform was constructed using a novel E. coli strain.
As shown in Fig. 2 outlining the synthetic route of mannitol for HO-LAB, M1Pase coupled with Mt1D enzymes plays an important catalytic role in producing mannitol from glucose. Evidently, co-factor NADH is needed during the reaction of fructose-6-phosphate to mannitol-1-phosphate when catalyzed by Mt1D. Reshamwala et al. (2014) constructed an efficient system that overexpressed M1Pase, Mt1D, and phosphite dehydrogenase (PtxD) in E. coli. The PtxD enzyme was used for the co-factor’s (NADH) regeneration with the concomitant oxidation of phosphite to phosphate (Fig. 3). Using whole-recombinant resting E. coli cells in the medium containing glucose, with sodium phosphite serving as the biotransformation catalyst, a molar yield of 87% mannitol was achieved from glucose, indicating the feasibility for bioproduction of this valued sugar alcohol. Recently, to achieve superior mannitol production, Xiao et al. (2020) provided new insight into the utility of adaptive evolution by blocking metabolic pathways that consume NADH and inactivating the mannitol uptake strategy in HO-LAB L. lactis under its native metabolism, rather than expressing the above foreign enzyme. In this way, they overcame bottlenecks in metabolism and increased the supply of NADH, a key factor influencing the yield of mannitol, such that 60% of glucose was ultimately converted into mannitol.
Conclusions
Owing to its properties, mannitol has vast, important applications in the food, chemical, medical, and pharmacy industry. Compared with its chemical synthesis and extraction from plants, the biotechnological strategy for mannitol production offers many notable advantages; hence, it is a promising method for obtaining mannitol in the future. In this respect, LAB is the mainstream strain for the production of mannitol among fungi and yeast. Finding ways to augment the yield of mannitol should be the focus of future research work. Developing a new strategy to regulate the metabolic flux of reducing co-factors to improve the yield of mannitol may represent a feasible way forward. Not to be overlooked are immobilized cells containing M1Pase and Mt1D or MtDH, or immobilizing these enzymes, which could also be harnessed to produce more mannitol.
References
Baumchen C, Bringer-Meyer S (2007) Expression of glfZ.m. increases D-mannitol formation in whole cell biotransformation with resting cells of Corynebacterium glutamicum. Appl Microbiol Biotechnol 76(3):545–552. https://doi.org/10.1007/s00253-007-0987-8
Baumchen C, Roth AH, Biedendieck R, Malten M, Follmann M, Sahm H, Bringer-Meyer S, Jahn D (2007) D-mannitol production by resting state whole cell biotransformation of D-fructose by heterologous mannitol and formate dehydrogenase gene expression in Bacillus megaterium. Biotechnol J 2(11):1408–1416. https://doi.org/10.1002/biot.200700055
Cao H, Yue M, Liu G, Du Y, Yin H (2018) Microbial production of mannitol by Lactobacillus brevis 3-A5 from concentrated extract of Jerusalem artichoke tubers. Biotechnol Appl Biochem 65(3):484–489. https://doi.org/10.1002/bab.1590
Carvalheiro F, Moniz P, Duarte LC, Esteves MP, Girio FM (2011) Mannitol production by lactic acid bacteria grown in supplemented carob syrup. J Ind Microbiol Biotechnol 38(1):221–227. https://doi.org/10.1007/s10295-010-0823-5
Chen G, Zhang L, Wu X, Ye J (2005) Determination of mannitol and three sugars in Ligustrum lucidum Ait. by capillary electrophoresis with electrochemical detection. Anal Chim Acta 530(1):15–21. https://doi.org/10.1016/j.aca.2004.08.053
Cheng H, Qian H, Cao Y (2008) Capillary electrophoresis - electrochemical detection of D-mannitol and sugars in Chrysanthemum. J Univ Sci Technol Suzhou (in Chinese) 25(3):46–50 http://www.cnki.com.cn/Article/CJFDTotal-TDSY200803014.htm
Dai Y, Meng Q, Mu W, Zhang T (2017) Recent advances in the applications and biotechnological production of mannitol. J Funct Foods 36:404–409. https://doi.org/10.1016/j.jff.2017.07.022
Du H, Ma X, Jiang M, Yan P, Zhao Y, Conrad Zhang Z (2020) Efficient Ni/SiO2 catalyst derived from nickel phyllosilicate for xylose hydrogenation to xylitol. Catal Today. https://doi.org/10.1016/j.cattod.2020.04.009
Fleming SC, Kapembwa MS, Laker MF, Levin GE, Griffin GE (1990) Rapid and simultaneous determination of lactulose and mannitol in urine, by HPLC with pulsed amperometric detection, for use in studies of intestinal permeability. Clin Chem 36(5):797–799. https://doi.org/10.1093/clinchem/36.5.797
Fontes CP, Honorato TL, Rabelo MC, Rodrigues S (2009) Kinetic study of mannitol production using cashew apple juice as substrate. Bioprocess Biosyst Eng 32(4):493–499. https://doi.org/10.1007/s00449-008-0269-6
Gaspar P, Neves AR, Ramos A, Gasson MJ, Shearman CA, Santos H (2004) Engineering Lactococcus lactis for production of mannitol: high yields from food-grade strains deficient in lactate dehydrogenase and the mannitol transport system. Appl Environ Microbiol 70(3):1466–1474. https://doi.org/10.1128/aem.70.3.1466-1474.2004
Guicherd P, Peltier JP, Gout E, Bligny R, Marigo G (1997) Osmotic adjustment in Fraxinus excelsior L . : malate and mannitol accumulation in leaves under drought conditions. Trees 11(3):155–161. https://doi.org/10.1007/PL00009664
Ikawa T, Watanabe T, Nisizawa K (1972) Enzymes involved in the last steps of the biosynthesis of mannitol in brown algae. Plant Cell Physiol 13(6):1017–1029. https://doi.org/10.1093/oxfordjournals.pcp.a074808
Jiang H, Chen W, Zhao J, Zhang H (2005) Determination of mannitol in lactic acid bacteria fermentation system by spectrophotometry. Food Ferment Ind (in Chinese) 31(4):105–107. https://doi.org/10.13995/j.cnki.11-1802/ts.2005.04.028
Kaup B, Bringer-Meyer S, Sahm H (2005) D-Mannitol formation from D-glucose in a whole-cell biotransformation with recombinant Escherichia coli. Appl Microbiol Biotechnol 69(4):397–403. https://doi.org/10.1007/s00253-005-1996-0
Kiyoshima A, Kudo K, Hino Y, Ikeda N (2001) Sensitive and simple determination of mannitol in human brain tissues by gas chromatography–mass spectrometry. J Chromatogr B 758(1):103–108. https://doi.org/10.1016/S0378-4347(01)00145-1
Koko MYF, Hassanin HAM, Letsididi R, Zhang T, Mu W (2016) Characterization of a thermostable mannitol dehydrogenase from hyperthermophilic Thermotoga neapolitana DSM 4359 with potential application in mannitol production. J Mol Catal B Enzym 134:122–128. https://doi.org/10.1016/j.molcatb.2016.10.010
Kulbe KD, Schwab U, Gudernatsch W (1987) Enzyme-catalyzed production of mannitol and gluconic acid. Ann N Y Acad Sci 506(1):552–568. https://doi.org/10.1111/j.1749-6632.1987.tb23850.x
Li X, Bao T, Wang Y (1999) Determination of mannitol in Cordyceps sinensis by colorimetric method. Chin Herb Med (in Chinese) 30(1):19–21 http://www.cnki.com.cn/Article/CJFDTotal-ZCYO199901008.htm
Li H, Yang P, Chu D, Li H (2007) Selective maltose hydrogenation to maltitol on a ternary Co–P–B amorphous catalyst and the synergistic effects of alloying B and P. Appl Catal A Gen 325(1):34–40. https://doi.org/10.1016/j.apcata.2007.02.007
Liu H, Zhang S, Yu A, Qu L, Zhao Y, Huang H, Li J (2004) Studies on intestinal permeability of cirrhotic patients by analysis lactulose and mannitol in urine with HPLC/RID/MS. Bioorg Med Chem Lett 14(9):2339–2344. https://doi.org/10.1016/j.bmcl.2004.01.107
Livesey G (2003) Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr Res Rev 16(2):163–191. https://doi.org/10.1079/NRR200371
Lu F, Xu W, Wu H, Guang C, Zhang W, Mu W (2020) Purification and characterization of recombinant mannitol dehydrogenase from P. bacterium1109. Sci Technol Food Ind (in Chinese) http://kns.cnki.net/kcms/detail/11.1759.TS.20200403.0920.004.html
Madsen MA, Semerdzhiev S, Amtmann A, Tonon T (2018) Engineering mannitol biosynthesis in Escherichia coli and Synechococcus sp. PCC 7002 using a green algal fusion protein. ACS Synth Biol 7(12):2833–2840. https://doi.org/10.1021/acssynbio.8b00238
Meng Q, Zhang T, Wei W, Mu W, Miao M (2017) Production of mannitol from a high concentration of glucose by Candida parapsilosis SK26.001. Appl Biochem Biotechnol 181(1):391–406. https://doi.org/10.1007/s12010-016-2219-0
Mishra DK, Dabbawala AA, Truong CC, Alhassan SM, Jegal J, Hwang JS (2018) Ru–NiOx nanohybrids on TiO2 support prepared by impregnation-reduction method for efficient hydrogenation of lactose to lactitol. J Ind Eng Chem 68:325–334. https://doi.org/10.1016/j.jiec.2018.08.003
Mitchell H (2006) Sweeteners and sugar alternatives in food technology. Blackwell Publishing, Oxford
Neves AR, Ramos A, Shearman C, Gasson MJ, Santos H (2002) Catabolism of mannitol in Lactococcus lactis MG1363 and a mutant defective in lactate dehydrogenase. Microbiology 148(11):3467–3476. https://doi.org/10.1099/00221287-148-11-3467
Ortiz ME, Fornaguera MJ, Raya RR, Mozzi F (2012) Lactobacillus reuteri CRL 1101 highly produces mannitol from sugarcane molasses as carbon source. Appl Microbiol Biotechnol 95(4):991–999. https://doi.org/10.1007/s00253-012-3945-z
Parmentier S, Arnaut F, Soetaert W, Vandamme EJ (2005) Enzymatic production of D-mannitol with the Leuconostoc pseudomesenteroides mannitol dehydrogenase coupled to a coenzyme regeneration system. Biocatal Biotransfor 23(1):1–7. https://doi.org/10.1080/10242420500071664
Qianqian W (2010) Research progress in preparation and application of mannitol. Sci Technol Food Ind (in Chinese) 31(12):401–404. https://doi.org/10.13386/j.issn1002-0306.2010.12.009
Queneau Y, Chambert S, Besset C, Cheaib R (2008) Recent progress in the synthesis of carbohydrate-based amphiphilic materials: the examples of sucrose and isomaltulose. Carbohydr Res 343(12):1999–2009. https://doi.org/10.1016/j.carres.2008.02.008
Reshamwala SMS, Pagar SK, Velhal VS, Maranholakar VM, Talangkar VG, Lali AM (2014) Construction of an efficient Escherichia coli whole-cell biocatalyst for D-mannitol production. J Biosci Bioeng 118(6):628–631. https://doi.org/10.1016/j.jbiosc.2014.05.004
Rice T, Zannini E, Arendt EK, Coffey A (2019) A review of polyols– biotechnological production, food applications, regulation, labeling and health effects. Crit Rev Food Sci Nutr:1–18. https://doi.org/10.1080/10408398.2019.1625859
Rockwell K (2015) Therapeutic review: Mannitol. J Exo Pet Med 24(1):120–122. https://doi.org/10.1053/j.jepm.2014.11.002
Rzechonek DA, Dobrowolski A, Rymowicz W, Mirończuk AM (2018) Recent advances in biological production of erythritol. Crit Rev Biotechnol 38(4):620–633. https://doi.org/10.1080/07388551.2017.1380598
Saha BC (2006) Production of mannitol from inulin by simultaneous enzymatic saccharification and fermentation with Lactobacillus intermedius NRRL B-3693. Enzym Microb Technol 39(5):991–995. https://doi.org/10.1016/j.enzmictec.2006.02.001
Saha BC, Racine FM (2010) Effects of pH and corn steep liquor variability on mannitol production by Lactobacillus intermedius NRRL B-3693. Appl Microbiol Biotechnol 87(2):553–560. https://doi.org/10.1007/s00253-010-2552-0
Saha BC, Racine FM (2011) Biotechnological production of mannitol and its applications. Appl Microbiol Biotechnol 89(4):879–891. https://doi.org/10.1007/s00253-010-2979-3
Shawkat H, Westwood MM, Mortimer A (2012) Mannitol: a review of its clinical uses. Contin Edu Anaesth Crit Care Pain 12(2):82–85. https://doi.org/10.1093/bjaceaccp/mkr063
Singh H, Rai A, Yadav R, Sinha AK (2018) Glucose hydrogenation to sorbitol over unsupported mesoporous Ni/NiO catalyst. Mol Catal 451:186–191. https://doi.org/10.1016/j.mcat.2018.01.010
Soetaert W (1990) Production of mannitol with Leuconostoc mesenteroides. Mededelingen van de Faculteit Landbouwwetenschappen, Rijksuniversiteit Gent (Netherlands journal) 55(4):1549–1552 https://www.cabdirect.org/cabdirect/abstract/19920313622
Song SH, Vieille C (2009) Recent advances in the biological production of mannitol. Appl Microbiol Biotechnol 84(1):55–62. https://doi.org/10.1007/s00253-009-2086-5
Song SH, Ahluwalia N, Leduc Y, Delbaere LT, Vieille C (2008) Thermotoga maritima TM0298 is a highly thermostable mannitol dehydrogenase. Appl Microbiol Biotechnol 81(3):485–495. https://doi.org/10.1007/s00253-008-1633-9
Stoop JMH, Williamson JD, Mason Pharr D (1996) Mannitol metabolism in plants: a method for coping with stress. Trends Plant Sci 1(5):139–144. https://doi.org/10.1016/S1360-1385(96)80048-3
Takemura M, Iijima M, Tateno Y, Osada Y, Maruyama H (1978) Process for preparing D-mannitol. Google Patents https://patentimages.storage.googleapis.com/be/56/9f/548421a0cafc5c/US4083881.pdf
Tang Y, Liu Y, Zhang Y, Zhang J, Liu Y (1998) Hydrolytic hydrogenation of cane juice to product of mannitol and sorbitol. Sugarcane Canesugar (in Chinese) 2:41–44 http://www.cnki.com.cn/Article/CJFDTotal-GZTY199802011.htm
von Weymarn N, Hujanen M, Leisola M (2002) Production of D-mannitol by heterofermentative lactic acid bacteria. Process Biochem 37(11):1207–1213. https://doi.org/10.1016/S0032-9592(01)00339-9
Wang G, Zhao G, He D, Li J (2005) Preparation of crystalline mannitol by double isomerization. Food Res Develop (in Chinese) 26(4):82–84. https://doi.org/10.3969/j.issn.1002-1124.2005.03.022
Wei Q (2010) Research progress in preparation and application of mannitol. Sci Technol Food Ind (in Chinese) 31(12):401–404. https://doi.org/10.13386/j.issn1002-0306.2010.12.009
Xiang D, Wang X (2009) Research progress in the analysis and determination methods of mannitol. Sci Technol Food Ind (in Chinese) 30(12):420–423. https://doi.org/10.13386/j.issn1002-0306.2009.12.012
Xiao H, Wang Q, Bang-Berthelsen CH, Jensen PR, Solem C (2020) Harnessing adaptive evolution to achieve superior mannitol production by Lactococcus lactis using its native metabolism. J Agric Food Chem 68(17):4912–4921. https://doi.org/10.1021/acs.jafc.0c00532
Xie X, Zhang C, Zeng B (1998) Study on extraction method of mannitol in kelp. Appl Technol Market (in Chinese) 11:8–9 http://www.cnki.com.cn/Article/CJFDTotal-KJCK199811004.htm
Yoshikawa J, Habe H, Morita T, Fukuoka T, Imura T, Iwabuchi H, Uemura S, Tamura T, Kitamoto D (2014) Production of mannitol from raw glycerol by Candida azyma. J Biosci Bioeng 117(6):725–729. https://doi.org/10.1016/j.jbiosc.2013.11.016
Zhan T, Song J (2003) Progress in studies of mannitol in medicinal application. Chin J Mar Drugs 3:57–61 http://www.cnki.com.cn/Article/CJFDTotal-HYYW200303016.htm
Zhang M, Gu L, Cheng C, Ma J, Xin F, Liu J, Wu H, Jiang M (2018) Recent advances in microbial production of mannitol: utilization of low-cost substrates, strain development and regulation strategies. World J Microbiol Biotechnol 34(3):41. https://doi.org/10.1007/s11274-018-2425-8
Zhou W, Fan J (2005) Review of manufacturing mannitol. Sugarcane Canesugar (in Chinese) 5:46–50 http://www.cnki.com.cn/Article/CJFDTotal-GZTY200505011.htm
Funding
This work was supported by the National Natural Science Foundation of China (No. 31801583 and No. 31922073), the Natural Science Foundation of Jiangsu Province (No. BK20180607), and the National First-Class Discipline Program of Food Science and Technology of China (JUFSTR20180203).
Author information
Authors and Affiliations
Contributions
MC and HW wrote the paper. HW, WZ, and WM collected references and drew pictures. HW and CG designed the structure and discussed the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain studies with human participants or animals carried out by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, M., Zhang, W., Wu, H. et al. Mannitol: physiological functionalities, determination methods, biotechnological production, and applications. Appl Microbiol Biotechnol 104, 6941–6951 (2020). https://doi.org/10.1007/s00253-020-10757-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10757-y