Abstract
D-Mannose is an epimer of glucose at the C-2 position and exists in nature as a component of mannan. It has 60 and 86% sweetness than that of sucrose and D-glucose, respectively. Because of its low-calorie and nontoxic features, D-mannose is used widely in food, medicine, cosmetic, and food-additive industries. Besides, it exhibits many physiologic benefits on health: immune system, diabetes mellitus, intestinal diseases, and urinary tract infections. It is used as a starting material to synthesize immunostimulatory agents, anti-tumor agents, vitamins, and D-mannitol. However, D-mannose production using chemical synthesis and plant extraction cannot meet the requirements of the industry. This article presents recent research on the biological production of D-mannose. The physiologic benefits and applications of D-mannose are summarized. Besides, different D-mannose-producing enzymes from various sources are discussed in detail with regard to their biochemical characteristics, catalytic efficiency, and reaction kinetics for D-mannose production. Furthermore, attempts to use enzymatic conversion to produce D-mannose are reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, the incidence of several chronic diseases, such as diabetes mellitus, hyperlipidemia, and hypertension, has increased rapidly worldwide. The occurrence of these diseases is closely related to the overconsumption of high-sugar and high-fat foods (Zhang et al. 2017b). Therefore, it is necessary to pay close attention to the effects of diet on human health.
Functional sugars have received considerable attention due to their excellent physiologic properties, such as low calories and low sweetness, and they have broad applications in the food, medicinal, and beverage industries (Huang et al. 2018a). For example, D-tagatose has numerous health benefits, including few calories, anti-biofilm effects, promotion of weight loss, and no glycemic effect (Oh 2007). D-Tagatose is widely used as a low-calorie functional sweetener. D-Allose possesses anti-tumor, anti-inflammatory, and anti-hypertensive properties (Chen et al. 2018b). d-Mannose shows several health benefits, too, such as being a prebiotic (Korneeva et al. 2012), promoting insulin secretion (Machicao et al. 1990), and aiding treatment for a deficiency in phosphomannose isomerase (Lonlay and Seta 2009). This monosaccharide can also be used as a starting material for the production of vitamins (Chen et al. 2007), anti-tumor agents (El-Nakkady et al. 2012), and immunostimulatory agents (Etchison and Freeze 1997). Because of its valuable properties and wide applications, D-mannose has gained much attention and interest.
D-Mannose is mostly found in nature as a component of mannan, hemicellulose, and cellulose in dietary fiber (Hu et al. 2016a). The structure of D-mannose is extremely similar to that of D-glucose and D-fructose. In detail, D-mannose is an epimer of D-glucose at the C-2 position and the aldose isomer of D-fructose. The content of D-mannose varies in different plants. It has been reported that the contents of D-mannose were 0.04 to 0.08% and 0 to 0.03% in the fresh apple flesh (Gheyas et al. 1997) and mango (Yashoda et al. 2007), respectively. The content of D-mannose reaches up to 21.2% in spent coffee grounds (Mussatto et al. 2011). Therefore, these D-mannose-containing plants are important raw materials for the preparation of D-mannose. The current top-selling brands of D-mannose including NOW Foods, Source Naturals, and Vibrant Health are derived from plant sources and most of them are made into capsules or powdered form (Hu et al. 2016a). The extraction of D-mannose from plants is mainly carried out by extraction. In addition, D-mannose could also be synthesized by chemical methods. Due to the poor specificity of the inorganic catalyst on substrate, the chemical reactions are often accompanied by the formation of many byproducts, which are not suitable for industrial production of D-mannose. With the development of microbiotechnology, the enzymatic production of D-mannose has gradually attracted considerable interest. At present, four types of microbial enzymes are reported to have the potential applications for the production of D-mannose, including D-lyxose isomerase (LIase, EC 5.3.1.15), D-mannose isomerase (MIase, EC 5.3.1.7), cellobiose 2-epimerase (CEase, EC 5.1.3.11), and D-mannose 2-epimerase (MEase, EC 5.1.3.-). The four enzymes could produce D-mannose directly based on D-glucose or D-fructose as substrate through the isomerization or epimerization reactions.
Here, we have summarized recent studies on the biological production of D-mannose. The physiologic benefits and applications of D-mannose have also been discussed in detail. Besides, different D-mannose-producing enzymes, such as MEase, LIase, CEase, and MEase, from various sources, are compared with regard to their biochemical characteristics, catalytic efficiency, and reaction kinetics for D-mannose production. Furthermore, some attempts to use enzymatic conversion to produce D-mannose have been reviewed.
Beneficial effects and applications of d-mannose
D-Mannose is a white crystal or crystalline powder. It has sweetness of 60 and 86% compared with that of sucrose and D-glucose, respectively (Hu et al. 2016a). It is easy to dissolve in water but slightly soluble in ethanol; at 17 °C, 248 g of D-mannose can be dissolved in 100 g water to give a 71-wt% solution (Hu et al. 2016a). Upon heating of D-mannose, the Maillard reaction occurs (Yaylayan and Forage 1992). The caloric value of D-mannose is 3.75 kcal/g, which is lower than that of many types of sugars (Pohl et al. 2012). D-Mannose is transported and absorbed in the human body through free diffusion, and its transportation rate is one tenth to that of glucose in the small intestine. D-Mannose in the human body is not converted readily into glycogen, and 95 to 98% of it is catalyzed to D-fructose-6-phosphate by phosphomannose isomerase, and only ~ 2% of D-mannose is used for N-glycosylation (Sharma et al. 2014).
As an important hexose, scientists are interested in the function of D-mannose. Several studies have found that D-mannose has many functions in the human body. Iwasaki and Medzhitov (2015) showed that D-mannose plays an important part in the human immune system. D-Mannose-binding lectin is an important component of the innate immune system. It can recognize D-mannose on the surface of pathogens and is the first line of defense in human immunity (Turner 2003). Deletion or genetic mutation of D-mannose-binding lectin leads to the susceptibility and severity of diseases (Müller et al. 2010; Sharma et al. 2012). In addition, D-mannose receptors can recognize the specific molecules or pathogens on the surface of hepatocytes and maintain the internal environment by participating in receptor-mediated endocytosis and phagocytosis (Ohnishi et al. 2012).
D-Mannose can be used as a raw material to synthesize high-value products, such as immunostimulants (Ranta et al. 2012), vitamins (Chen et al. 2007), anti-tumor-related drugs (Kamel et al. 2010), anti-human immunodeficiency-related drugs (Botos et al. 2002), and D-mannitol (Mishra and Hwang 2013). These products can be used in disease treatment and nutrient metabolism. For example, D-mannitol is widely used in food and pharmaceutical products because of its low-caloric and cariogenic properties (Dai et al. 2017). Besides, it is chemically inert and is used commonly in the pharmaceutical formulation of chewable tablets and granulated powders (Saha and Racine 2011).
Also, D-mannose has been used widely in food, medicine, cosmetic, and food-additive industries. As mentioned above, D-mannose is a low-calorie monosaccharide and has 60% of the sweetness of sucrose, making it a potential alternative sweetener for use in food processing. The general public is paying increasing attention to health issues, and the demand for low-calorie and low-sweet sugars is increasing (Zhang et al. 2017b). The biochemical property of D-mannose as a food component is very stable (Montero et al. 2004). D-Mannose is added to ice cream as a stabilizer (Sutton and Wilcox 2010). Besides, the combination of a certain ratio of D-galactose and D-mannose exhibits recrystallization behavior to locust bean gum. As a reducing monosaccharide, D-mannose can take part in the Maillard reaction, which can increase the melting point and improve the color, flavor, and taste of food (Yaylayan and Forage 1992). Elghaouth et al. (1995) declared that D-mannose can slow down the decay rate of apples and peaches.
Many antibiotics are added to the fodder used for the growth of livestock and poultry, but excessive use of antibiotics can cause environmental pollution and/or drug resistance (van Immerseel et al. 2002). Therefore, finding an alternative method to replace antibiotics has become very important. Researchers have demonstrated that D-mannose can inhibit the infection by Salmonella typhi in chickens and that D-mannose has no side effects, indicating the potential of replacing the antibiotics used against S. typhi (van Immerseel et al. 2002). In addition, D-mannose has been found to inhibit the colonization and abscission of pathogens in the intestinal tract (Berge and Wierup 2012).
Aloe vera plays an important part in the chemical industry as a moisturizer, whitening agent, and skin sunscreen (Chen and Dong 2008). Its physiologic functions are closely related to the presence of Aloe polysaccharides, which are rich in mannan and glucomannan (Eshun and He 2004). D-Mannose can make the skin more moisturized, softer, and smoother after washing (Schmidt et al. 1991). Wivell and Deckner (1995) invented a skincare product containing D-glucose, D-mannose, and D-glucuronic acid, which can cleanse and moisturize the skin. In addition to the aforementioned physiologic benefits, D-mannose can be used as an antibiotic screening agent in research on transgenic plants (Joersbo 2001). D-Mannose can also provide energy for transgenic maize plants from protoplasts (Wang et al. 2000).
D-Mannose production
Plant extraction
D-Mannose is extracted mainly from fruits, herbs, and palm (Hu et al. 2016a). Currently, the main extraction methods included acid hydrolysis, thermal hydrolysis, and enzymatic hydrolysis. Zhang et al. (2009) presented a route for the purification of D-mannose from palm kernel. First, the palm kernel was hydrolyzed by sulfuric acid at the temperature of 100 °C and then the hydrolysis solution was further treated by endo-β-mannanase. Subsequently, the solution was filtered through a silica gel column and subjected to desalting treatment with an ion exchange resin. Finally, the D-mannose crystals were obtained after purified by ethanol with a yield of 48.4% (Zhang et al. 2009). Fan et al. (2014) used microwave-assisted coupled with sulfuric acid treatment method to extract D-mannose from deproteinized palm kernels. After optimizing the extraction condition by response surface methodology, D-mannose yield of 92.11% was achieved at 148 °C for 10 min 31 s at a substrate-to-solvent ratio (w/v) of 1:49.69. However, this method requires the consumption of a large amount of organic reagents, e.g., sulfuric acid, and is not friendly to the environment.
Chemical synthesis
D-Mannose can also be produced from D-glucose by chemical methods, which are catalyzed mostly through molybdate catalysts (Hu et al. 2016a). Using ammonium molybdate as a catalyst, 32.6% yield of D-mannose was obtained after reaction about 150 min at 98 °C, pH 2.0, and 55% glucose concentration (Zhang et al. 2017a). Using a mixed catalyst of ammonium molybdate and calcium oxide, 44.8% yield of D-mannose was achieved after reaction for 80 min at 150 ° C, pH 3.0 (Xu et al. 2014). However, this method also has disadvantages. For example, the chemical reaction must be carried out at a high-temperature and low-acid environment, which increases the production costs. Furthermore, many byproducts produced in the reaction system are difficult to isolate in subsequent downstream processes.
Biological production using microbial enzymes
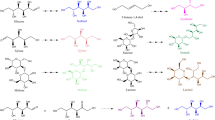
As a dietary supplement, D-mannose has many physiologic functions and is used widely in food, pharmaceutical, and cosmetic industries. However, the content of free D-mannose in nature is low and far below the demands of industrial applications. The synthesis of D-mannose by a chemical method requires strict control of temperature, pH, time, and pressure in the reaction process. Moreover, due to the weak specificity of the inorganic catalyst used in the reaction, many byproducts or toxic products are usually produced, which is unacceptable for consumers. An extraction method using acid hydrolysis from D-mannose-containing plants also has disadvantages. Due to the high crystallinity of plant cell walls, the hydrolysis of D-mannan requires a high temperature and high concentrations of acid/alkali/organic reagents, which are also not acceptable. Therefore, the biological production of D-mannose using microbial enzymes has attracted attention considerably because of the mild reaction conditions and few byproducts. As mentioned above, LIase, MIase, CEase, and MEase demonstrate the potential for the production of D-mannose on a large scale. According to the Izumori strategy, D-mannose can be converted from other hexoses by biological enzymes (Izumori 2002; Mu et al. 2015). The enzyme production of D-mannose is based mainly on D-fructose or D-glucose as the raw material, which is realized by isomerization or epimerization of monosaccharide (Fig. 1).
MIase
MIase (EC 5.3.1.7) is another important isomerase for D-mannose production. MIase reversibly catalyzes the isomerization of D-fructose and D-mannose. This enzyme has been isolated and characterized from Pseudomonas saccharophila (Palleroni and Doudoroff 1956), Xanthomonas rubrilineans S-48 (Takasaki and Takano 1964), Streptomyces aerocolorigenes (Takasaki 1967), Mycobacterium smegmatis (Hey-Ferguson and Elbein 1970), Pseudomonas cepacia (Allenza et al. 1990), Agrobacterium radiobacter M-1 (Hirose et al. 2001), Thermobifida fusca MBL10003 (Kasumi et al. 2014), and Marinomonas mediterranea (Saburi et al. 2018) and is also found in Escherichia coli and Salmonella enterica (Itoh et al. 2008).
The biochemical characteristics of these enzymes have also been investigated. Temperature and pH affect enzyme activity greatly. The optimal temperature and pH for the isomerase activity of these MIases ranged from 30 to 60 °C and 6.4 to 8.0, respectively (Table 1). Interestingly, maintenance of isomerization activity does not require the participation of metal ions in MIases. On the contrary, the addition of divalent metal ions, such as Cu2+, Cd2+, or Ca2+, can inhibit their activity significantly (Kasumi et al. 2014). If the isomerization is started by MIase using D-mannose as a substrate, the concentration ratio of D-fructose:D-mannose is 75:25 to 65:35 at reaction equilibrium (Hey-Ferguson and Elbein 1970; Hirose et al. 2001; Kasumi et al. 2014; Takasaki 1967). These results suggest a higher conversion rate from D-mannose to D-fructose. However, the low conversion ratio of D-mannose based on D-fructose leads to a shortage for D-mannose production by MIase on a large scale.
Hu et al. (2016b) reported that a recombinant MIase can also achieve the same conversion rate and productivity under the same concentration of substrate at pH 7.0 and 45 °C, in contrast to LIase from P. stuartii (Table 2). Hirose and colleagues tried to immobilize A. radiobacter cells containing MIase to produce D-mannose from D-fructose (Hirose et al. 2003). They prepared the cells by adsorption on chitosan or glutaraldehyde crosslinking in the presence of albumin. Finally, 9 g D-mannose accumulated in the effluent (180 mL) at pH 8.0 and 55 °C for 14 days.
Reports focusing on the crystal structure of MIases have been limited to MIase from M. mediterranea (PDB: 5X32) and a hypothetical protein YihS (later characterized as MIase) from S. enterica (PDB: 2AFA) (Itoh et al. 2008). MIase (PDB: 5X32) from M. mediterranea is formed by an (α/α)6-barrel, which is a typical characteristic structure in enzymes from the N-acylglucosamine 2-epimerase (AGE) superfamily (Saburi et al. 2018). Research has revealed that the residue amino acids responsible for catalysis and substrate binding are similar to those enzymes from the AGE superfamily, such as CEase and aldose–ketose isomerase. In the catalytic domain of MIase (PDB: 5X32), α7/α8 and α11/α12 loops play an important part in the opening of the substrate-binding site (Saburi et al. 2018). Elucidation of the crystal structure of MIase provides molecular insights into understanding the reaction mechanism of enzymes and substrates. However, insufficient information of the crystal structure of MIase has seriously restricted development studies on molecular modification of MIase and hampered attempts to increase its catalytic efficiency on D-fructose through site-directed mutagenesis.
LIase
LIase (EC 5.3.1.15) is an aldose–ketose isomerase that could be used to produce D-mannose (Cho et al. 2007). It can catalyze isomerization with various substrates. For example, it can catalyze isomerization at the C-2 position of D-lyxose, D-fructose, and L-ribose, thereby producing D-xylulose, D-mannose, and L-ribulose as products, respectively (Huang et al. 2018a). Isomerization of LIase has a critical role in microbial metabolism because these ketopentoses (D-xylulose, L-ribulose) can be phosphorylated further to form intermediates (D-xylulose-5-phosphate, L-ribulose-5-phosphate) through the action of kinases (Cho et al. 2007). These compounds are the common intermediates of the pentose phosphate pathway. Because of its broad spectrum of substrate specificity, LIase has been applied for the production of many functional sugars (Huang et al. 2018a). Several LIases have been produced by different microorganisms, including Cohnella laevoribosii RI-39 (Cho et al. 2007), Providencia stuartii (Kwon et al. 2010), Serratia proteamaculans (Park et al. 2010b), E. coli O157:H7 (van Staalduinen et al. 2010), Bacillus licheniformis (Patel et al. 2011), Dictyoglomus turgidum (Choi et al. 2012), and Thermosediminibacter oceani (Yu et al. 2016).
Recently, Yu et al. (2016) characterized a LIase from a hyperthermophile strain of T. oceani. The LIase exhibited potential for D-mannose production. The authors showed that 101.6 g L−1 of D-mannose could be obtained from 400 g L−1 of D-fructose in 9 h, with a conversion rate of 25.4% and productivity of 11.28 g L−1 h−1 at pH 6.5 and 60 °C. By using P. stuartii free LIase, 150 g L−1 of D-mannose could be produced from 600 g L−1 of D-fructose in 2 h, at pH 7.5 and 35 °C, with a conversion rate of 25% and productivity of 75 g L−1 h−1 (Park et al. 2010a). By immobilization of LIase from P. stuartii using Duolite resin A568, 75 g L−1 of D-mannose is obtained from 300 g L−1 of D-fructose with a conversion rate of 25% and a productivity of 75 g L−1 h−1 after 23 cycles (Park et al. 2010a). Additionally, to produce D-mannose from D-glucose directly, a one-pot enzyme process of D-mannose production from D-glucose has been constructed by co-expression of the D-glucose isomerase (GIase, EC 5.3.1.5) from Acidothermus cellulolyticus and LIase from T. oceani in E. coli BL21(DE3) cells (Huang et al. 2018b). Using this co-expression system, 60 g L−1 of D-mannose is obtained from 400 g L−1 of D-glucose in 8 h at pH 6.5 and 65 °C, suggesting the potential for industrial production of D-mannose.
In general, a high temperature is beneficial for the industrial production of functional sugars. A high temperature can accelerate the conversion rate of enzymatic reactions, reduce the risk of microbial contamination, and increase the solubility of products and substrates (Huang et al. 2018a). However, if the reaction temperature is high, the Maillard reaction occurs and some unexpected byproducts are produced because of nonenzymatic browning effect (Chen et al. 2018b). Hence, temperature control is very important in sugar processing. Besides, if the effect of nonenzymatic browning is to be reduced, control of the system pH in weak-acidic conditions is an effective strategy because the byproducts produced in the carbonyl ammonia reaction are hydrolyzed readily under acidic conditions (Shen and Wu 2004). These LIases show different biochemical properties with regard to temperature, pH, and kinetic parameters (Km, Kcat, Kcat/Km). Table 1 shows that the optimal temperature and pH range are from 40 to 75 °C and 6.5 to 8.5, respectively. Except for the LIase from C. laevoribosii (Cho et al. 2007) and T. oceani (Yu et al. 2016), the optimal pH of the other LIases have weak-alkaline characteristics. Therefore, for the industrial production of D-mannose, LIases with weak-acidic properties are needed, and the realization of this goal is dependent upon the molecular modification of LIase based on crystal structure or screening novel LIase-producing strains from nature. Different from MIase, LIase is a metal ion-dependent isomerase. For most LIases, Mn2+ is the optimal metal ion for the isomerase activity of LIase from C. laevoribosii (Cho et al. 2007), P. stuartii (Kwon et al. 2010), and S. proteamaculans (Park et al. 2010b), whereas Co2+ is the optimal metal ion for LIase from D. turgidum (Choi et al. 2012) (Table 3). With D-mannose as the substrate, D. turgidum shows the highest specific activity of 1668 ± 63 U/mg, whereas E. coli exhibits the lowest specific activity of 4.73 ± 0.05 U/mg.
CEase
CEase (EC 5.1.3.11) was isolated and identified first from the culture broth of Ruminococcus albus, in which CEase was found to catalyze cellobiose into glucosyl mannose through epimerization (Fig. 2) (Tyler and Leatherwood 1967). Subsequently, some studies revealed that CEase can convert other β-1,4-linked disaccharides, such as mannobiose and lactose, through epimerization at the C-2 position (Fig. 2). Disaccharides, such as mannobiose, cellobiose, and lactose, can be converted into mannosyl glucose, glucosyl mannose, and galactosyl mannose using CEase, respectively. Besides, CEase can catalyze the isomerization between keto-disaccharides and aldo-disaccharides (Chen et al. 2018a). To investigate the possibility of catalyzing monosaccharide substrates through epimerization, Park et al. (2011) studied the epimerization of CEase on different monosaccharides: D-glucose, D-mannose, D-xylose, D-lyxose, L-allose, L-gulose, L-arabinose, D-fructose, D-xylulose, L-psicose, L-sorbose, and L-ribulose. Their experimental results provided the first evidence that CEase from C. saccharolyticus can catalyze D-glucose, D-mannose, D-xylose, D-lyxose, and D-fructose through epimerization at the C-2 position. In particular, with D-glucose as a substrate, the value of Kcat/Km is ~ 25% that of D-mannose (Park et al. 2011). At 75 °C and pH 7.5, 75 g L−1 of D-mannose and 47.5 g L−1 of D-fructose are produced from 500 g L−1 of D-glucose after reaction for > 3 h by CEase. However, the reaction formed D-fructose as a byproduct at equilibrium because CEase can carry out isomerization and epimerization simultaneously.
MEase
Recently, Saburi et al. (2019) reported two novel MEases, named Runse_4512 and Dfer_5652, from Runella slithyformis and Dyadobacter fermentans, respectively, and showed that they are new members of the AGE superfamily. Similar to CEase, MEase also can convert D-glucose to D-mannose directly (Fig. 1). Upon reaction for 48 h at 50 °C and pH 8.0 with 500 g L−1 of D-glucose as substrate, 122 and 114 g L−1 of D-mannose are produced in the system using Runse_4512 and Dfer_5652 with a conversion rate of 24.4 and 22.8%, respectively (Saburi et al. 2019).
Future perspectives
D-Mannose exhibits many physiologic benefits for human health and is used widely in food, medicine, cosmetic, and food-additive industries. However, the detailed research about the action mechanism on human health is relatively scarce. Many studies should be performed to increase people’s understanding for this functional sugar. In addition, among the aforementioned four enzymes that were used for the production of D-mannose, most of them display properties with weakly alkaline optimal pH, and this property is not preferable for industrial production. To meet the requirements of the industry, required research is needed to improve the properties of such enzymes through protein-engineering techniques, such as site-directed mutagenesis or directed evolution. Besides, more MEases and CEases should be screened from nature because the two enzymes are capable of converting D-glucose to D-mannose directly. Furthermore, considering safety issues, a food-grade host, such as Bacillus subtilis, should be constructed for the expression of these enzymes in the future.
References
Allenza P, Morrell M, Detroy R (1990) Conversion of mannose to fructose by immobilized mannose isomerase from Pseudomonas cepacia. Appl Biochem Biotechnol 24(1):171–182. https://doi.org/10.1007/BF02920243
Berge AC, Wierup M (2012) Nutritional strategies to combat Salmonella in mono-gastric food animal production. Animal 6(4):557–564. https://doi.org/10.1017/S1751731111002217
Botos I, O’Keefe BR, Shenoy SR, Cartner LK, Ratner DM, Seeberger PH, Boyd MR, Wlodawer A (2002) Structures of the complexes of a potent anti-HIV protein cyanovirin-N and high mannose oligosaccharides. J Biol Chem 277(37):34336–34342. https://doi.org/10.1074/jbc.M205909200
Chen DY, Dong PC (2008) Aloe’s humectant in cosmetic. J Shantou Univ (Nat Sci) 23(4):42–46 (In Chinese) http://en.cnki.com.cn/Article_en/CJFDTOTAL-STDX200804010.htm
Chen FE, Zhao J-F, Xiong F-J, Xie B, Zhang P (2007) An improved synthesis of a key intermediate for (+)-biotin from D-mannose. Carbohydr Res 342(16):2461–2464. https://doi.org/10.1016/j.carres.2007.06.029
Chen Q, Xiao Y, Zhang W, Zhang T, Jiang B, Stressler T, Fischer L, Mu W (2018a) Current research on cellobiose 2-epimerase: enzymatic properties, mechanistic insights, and potential applications in the dairy industry. Trends Food Sci Technol 82:167–176. https://doi.org/10.1016/j.tifs.2018.09.009
Chen Z, Chen J, Zhang W, Zhang T, Guang C, Mu W (2018b) Recent research on the physiological functions, applications, and biotechnological production of D-allose. Appl Microbiol Biotechnol 102(10):4269–4278. https://doi.org/10.1007/s00253-018-8916-6
Cho EA, Lee DW, Cha YH, Lee SJ, Jung HC, Pan JG, Pyun YR (2007) Characterization of a novel D-lyxose isomerase from Cohnella laevoribosii RI-39 sp. nov. J Bacteriol 189(5):1655–1663. https://doi.org/10.1128/JB.01568-06
Choi JG, Hong SH, Kim YS, Kim KR, Oh DK (2012) Characterization of a recombinant thermostable D-lyxose isomerase from Dictyoglomus turgidum that produces D-lyxose from D-xylulose. Biotechnol Lett 34:1079–1085. https://doi.org/10.1007/s10529-012-0874-y
Dai Y, Meng Q, Mu W, Zhang T (2017) Recent advances in the applications and biotechnological production of mannitol. J Funct Foods 36:404–409. https://doi.org/10.1016/j.jff.2017.07.022
Elghaouth A, Wilson CL, Wisniewski ME (1995) Sugar analogs as potential fungicides for postharvest pathogens of apple and peach. Plant Dis 79(3):254–258. https://doi.org/10.1094/PD-79-0254
El-Nakkady SS, Hanna MM, Roaiah HM, Ghannam IAY (2012) Synthesis, molecular docking study and antitumor activity of novel 2-phenylindole derivatives. Eur J Med Chem 47(1):387–398. https://doi.org/10.1016/j.ejmech.2011.11.007
Eshun K, He Q (2004) Aloe vera: a valuable ingredient for the food, pharmaceutical and cosmetic industries—a review. Crit Rev Food Sci Nutr 44(2):91–96. https://doi.org/10.1080/10408690490424694
Etchison JR, Freeze HH (1997) Enzymatic assay of D-mannose in serum. Clin Chem 43(3):533–538 http://clinchem.aaccjnls.org/content/clinchem/43/3/533.full.pdf
Fan S, Jiang L, Chia C, Fang Z, Zakaria S, Chee K (2014) High yield production of sugars from deproteinated palm kernel cake under microwave irradiation via dilute sulfuric acid hydrolysis. Bioresour Technol 153:69–78. https://doi.org/10.1016/j.biortech.2013.11.055
Gheyas F, Blankenship SM, Young E, Mcfeeters R (1997) Dietary fibre content of thirteen apple cultivars. J Sci Food Agric 75(3):333–340. https://doi.org/10.1002/(sici)1097-0010(199711)75:3<333::aid-jsfa883>3.0.co;2-r
Hey-Ferguson A, Elbein AD (1970) Purification of a D-mannose isomerase from Mycobacterium smegmatis. J Bacteriol 101(3):777–780 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC250390/pdf/jbacter00384-0151.pdf
Hirose J, Maeda K, Yokoi H, Takasaki Y (2001) Purification and characterization of mannose isomerase from Agrobacterium radiobacter M-1. Biosci Biotechnol Biochem 65(3):658–661. https://doi.org/10.1271/bbb.65.658
Hirose J, Kinoshita Y, Fukuyama S, Hayashi S, Yokoi H, Takasaki Y (2003) Continuous isomerization of D-fructose to D-mannose by immobilized Agrobacterium radiobacter cells. Biotechnol Lett 25(4):349–352. https://doi.org/10.1023/A:1022301725817
Hu X, Shi Y, Zhang P, Miao M, Zhang T, Jiang B (2016a) D-Mannose: properties, production, and applications: an overview. Comp Rev Food Sci Food Safety 15(4):773–785. https://doi.org/10.1111/1541-4337.12211
Hu X, Zhang P, Miao M, Zhang T, Jiang B (2016b) Development of a recombinant D-mannose isomerase and its characterizations for D-mannose synthesis. Int J Biol Macromol 89:328–335. https://doi.org/10.1016/j.ijbiomac.2016.04.083
Huang J, Chen Z, Zhang W, Zhang T, Mu W (2018a) D-lyxose isomerase and its application for functional sugar production. Appl Microbiol Biotechnol 102(5):2051–2062. https://doi.org/10.1007/s00253-018-8746-6
Huang J, Yu L, Zhang W, Zhang T, Guang C, Mu W (2018b) Production of D-mannose from D-glucose by co-expression of D-glucose isomerase and D-lyxose isomerase in Escherichia coli. J Sci Food Agric 98(13):4895–4902. https://doi.org/10.1002/jsfa.9021
Itoh T, Mikami B, Hashimoto W, Murata K (2008) Crystal structure of YihS in complex with D-mannose: structural annotation of Escherichia coli and Salmonella enterica YihS-encoded proteins to an aldose–ketose isomerase. J Mol Biol 377(5):1443–1459. https://doi.org/10.1016/j.jmb.2008.01.090
Iwasaki A, Medzhitov R (2015) Control of adaptive immunity by the innate immune system. Nat Immunol 16(4):343–353. https://doi.org/10.1038/ni.3123
Izumori K (2002) Bioproduction strategies for rare hexose sugars. Naturwissenschaften 89(3):120–124. https://doi.org/10.1007/s00114-002-0297-z
Joersbo M (2001) Advances in the selection of transgenic plants using non-antibiotic marker genes. Physiol Plant 111(3):269–272. https://doi.org/10.1034/j.1399-3054.2001.1110301.x
Kamel MM, Ali HI, Anwar MM, Mohamed NA, Soliman AM (2010) Synthesis, antitumor activity and molecular docking study of novel sulfonamide-Schiff’s bases, thiazolidinones, benzothiazinones and their C-nucleoside derivatives. Eur J Med Chem 45(2):572–580. https://doi.org/10.1016/j.ejmech.2009.10.044
Kasumi T, Mori S, Kaneko S, Matsumoto H, Kobayashi Y, Koyama Y (2014) Characterization of mannose isomerase from a cellulolytic actinobacteria Thermobifida fusca MBL10003. J Appl Glycosci 61:21–25. https://doi.org/10.5458/jag.jag.JAG-2013_008
Korneeva OS, Cheeremushkina IV, Glushchenko AS, Miikhailova NA, Baturo AP, Romanenko EE, Zlygostev SA (2012) Prebiotic properties of mannose and its effect on specific resistance. Zh Mikrobiol Epidemiol Immunobiol 5:67–70 (in Russian) https://www.ncbi.nlm.nih.gov/pubmed/23163040
Kwon HJ, Yeom SJ, Park CS, Oh DK (2010) Substrate specificity of a recombinant D-lyxose isomerase from Providencia stuartii for monosaccharides. J Biosci Bioeng 110(1):26–31. https://doi.org/10.1016/j.jbiosc.2009.12.011
Lonlay PD, Seta N (2009) The clinical spectrum of phosphomannose isomerase deficiency, with an evaluation of mannose treatment for CDG-Ib. Biochim Biophys Acta 1792(9):841–843. https://doi.org/10.1016/j.bbadis.2008.11.012
Machicao F, Mushack J, Seffer E, Ermel B, Haring HU, Haring HU (1990) Mannose, glucosamine and inositol monophosphate inhibit the effects of insulin on lipogenesis. Further evidence for a role for inositol phosphate-oligosaccharides in insulin action. Biochem J 266(3):909–916 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1131225/pdf/biochemj00187-0275.pdf
Mishra DK, Hwang J-S (2013) Selective hydrogenation of D-mannose to D-mannitol using NiO-modified TiO2 (NiO-TiO2) supported ruthenium catalyst. Appl Catal A Gen 453(6):13–19. https://doi.org/10.1016/j.apcata.2012.11.042
Montero CM, Dodero MCR, Sánchez DAG, Barroso CG (2004) Analysis of low molecular weight carbohydrates in food and beverages: a review. Chromatographia 59(1-2):15–30. https://doi.org/10.1365/s10337-003-0134-3
Mu W, Yu L, Zhang W, Zhang T, Jiang B (2015) Isomerases for biotransformation of D-hexoses. Appl Microbiol Biotechnol 99(16):6571–6584. https://doi.org/10.1007/s00253-015-6788-6
Müller S, Schaffer T, Flogerzi B, Seibold-Schmid B, Schnider J, Takahashi K, Darfeuille-Michaud A, Vazeille E, Schoepfer AM, Seibold F (2010) Mannan-binding lectin deficiency results in unusual antibody production and excessive experimental colitis in response to mannose-expressing mild gut pathogens. Gut 59(11):1493–1500. https://doi.org/10.1136/gut.2010.208348
Mussatto SI, Carneiro LM, Silva JPA, Roberto IC, Teixeira JA (2011) A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr Polym 83(2):368–374. https://doi.org/10.1016/j.carbpol.2010.07.063
Oh DK (2007) Tagatose: properties, applications, and biotechnological processes. Appl Microbiol Biotechnol 76(1):1–8. https://doi.org/10.1007/s00253-007-0981-1
Ohnishi H, Oka K, Mizuno S, Nakamura T (2012) Identification of mannose receptor as receptor for hepatocyte growth factor β-chain novel ligand-receptor pathway for enhancing macrophage phagocytosis. J Biol Chem 287(16):13371–13381. https://doi.org/10.1074/jbc.M111.318568
Palleroni NJ, Doudoroff M (1956) Mannose isomerase of Pseudomonas saccharophila. J Biol Chem 218(1):535–548 http://www.jbc.org/content/218/1/535.full.pdf
Park CS, Kwon HJ, Yeom SJ, Oh DK (2010a) Mannose production from fructose by free and immobilized D-lyxose isomerases from Providencia stuartii. Biotechnol Lett 32(9):1305–1309. https://doi.org/10.1007/s10529-010-0300-2
Park CS, Yeom SJ, Lim YR, Kim YS, Oh DK (2010b) Substrate specificity of a recombinant D-lyxose isomerase from Serratia proteamaculans that produces D-lyxose and D-mannose. Lett Appl Microbiol 51(3):343–350. https://doi.org/10.1111/j.1472-765X.2010.02903.x
Park CS, Kim JE, Choi JG, Oh DK (2011) Characterization of a recombinant cellobiose 2-epimerase from Caldicellulosiruptor saccharolyticus and its application in the production of mannose from glucose. Appl Microbiol Biotechnol 92(6):1187–1196. https://doi.org/10.1007/s00253-011-3403-3
Patel DH, Wi SG, Lee SG, Lee DS, Song YH, Bae HJ (2011) Substrate specificity of the Bacillus licheniformis lyxose isomerase YdaE and its application in in vitro catalysis for bioproduction of lyxose and glucose by two-step isomerization. Appl Environ Microbiol 77(10):3343–3350. https://doi.org/10.1128/AEM.02693-10
Pohl JB, Baldwin BA, Dinh BL, Rahman P, Smerek D, III FJP, Sherazee N, Atkinson NS (2012) Ethanol preference in Drosophila melanogaster is driven by its caloric value. Alcohol Clin Exp Res 36(11):1903–1912. https://doi.org/10.1111/j.1530-0277.2012.01817.x
Ranta K, Nieminen K, Ekholm FS, Poláková M, Roslund MU, Saloranta T, Leino R, Savolainen J (2012) Evaluation of immunostimulatory activities of synthetic mannose-containing structures mimicking the β-(1→2)-linked cell wall mannans of Candida albicans. Clin Vaccine Immunol 19(11):1889–1893. https://doi.org/10.1128/CVI.00298-12
Saburi W, Jaito N, Kato K, Tanaka Y, Yao M, Mori H (2018) Biochemical and structural characterization of Marinomonas mediterranea D-mannose isomerase Marme_2490 phylogenetically distant from known enzymes. Biochimie 144:63–73. https://doi.org/10.1016/j.biochi.2017.10.016
Saburi W, Sato S, Hashiguchi S, Muto H, Iizuka T, Mori H (2019) Enzymatic characteristics of D-mannose 2-epimerase, a new member of the acylglucosamine 2-epimerase superfamily. Appl Microbiol Biotechnol 103(16):6559–6570. https://doi.org/10.1007/s00253-019-09944-3
Saha BC, Racine FM (2011) Biotechnological production of mannitol and its applications. Appl Microbiol Biotechnol 89(4):879–891. https://doi.org/10.1007/s00253-010-2979-3
Schmidt RR, Fortna RH, Beyer HH (1991) Mild skin cleansing aerosol mousse with skin feel and moisturization benefits. United States Patents 5002680. http://www.freepatentsonline.com/5002680.pdf
Sharma AA, Jen R, Butler A, Lavoie PM (2012) The developing human preterm neonatal immune system: a case for more research in this area. Clin Immunol 145(1):61–68. https://doi.org/10.1016/j.clim.2012.08.006
Sharma V, Ichikawa M, Freeze HH (2014) Mannose metabolism: more than meets the eye. Biochem Biophys Res Commun 453(2):220–228. https://doi.org/10.1016/j.bbrc.2014.06.021
Shen SC, Wu J (2004) Maillard browning in ethanolic solution. J Food Sci 69(4):273–279. https://doi.org/10.1111/j.1365-2621.2004.tb06328.x
Stevens F, Stevens P, Hovis J, Wu T (1981) Some properties of D-mannose isomerase from Escherichia coli K12. Microbiology 124(1):219–223. https://doi.org/10.1099/00221287-124-1-219
Sutton RL, Wilcox J (2010) Recrystallization in model ice cream solutions as affected by stabilizer concentration. J Food Sci 63(1):9–11. https://doi.org/10.1111/j.1365-2621.1998.tb15663.x
Takasaki Y (1967) Kinetic and equilibrium studies on D-mannose-D-fructose isomerization catalyzed by mannose isomerase from Streptomyces aerocolorigenes. Agric Biol Chem 31(4):435–440. https://doi.org/10.1080/00021369.1967.10858827
Takasaki Y, Takano S (1964) Studies on the isomerization of sugars by bacteria: part VIII. Purification and some properties of mannose isomerase from Xanthomonas rubrilineans S-48. Agric Biol Chem 28(9):605–609. https://doi.org/10.1080/00021369.1964.10858283
Turner MW (2003) The role of mannose-binding lectin in health and disease. Mol Immunol 40(7):423–429. https://doi.org/10.1016/S0161-5890(03)00155-X
Tyler TR, Leatherwood JM (1967) Epimerization of disaccharides by enzyme preparations from Ruminococcus albus. Arch Biochem Biophys 119(1):363–367. https://doi.org/10.1016/0003-9861(67)90466-3
van Immerseel F, Cauwerts K, Devriese LA, Haesebrouck F, Ducatelle R (2002) Feed additives to control Salmonella in poultry. World Poultry Sci J 58(4):501–513. https://doi.org/10.1079/WPS20020036
van Staalduinen LM, Park CS, Yeom SJ, Adams-Cioaba MA, Oh DK, Jia Z (2010) Structure-based annotation of a novel sugar isomerase from the pathogenic E. coli O157:H7. J Mol Biol 401(5):866–881. https://doi.org/10.1016/j.jmb.2010.06.063
Wang A, Evans R, Altendorf P, Hanten J, Doyle M, Rosichan J (2000) A mannose selection system for production of fertile transgenic maize plants from protoplasts. Plant Cell Rep 19(7):654–660. https://doi.org/10.1007/s002999900181
Wivell SC, Deckner GE (1995) Combined personal cleansing and moisturizing compositions. United States Patents 5439682. https://patentimages.storage.googleapis.com/3e/dc/b4/ed811bae2ea2db/US5439682.pdf
Xu L, Guo D, Liu J, Zhao P, Wang Y (2014) A mixed catalyst for improving conversion rate of D-glucose to D-mannose. Chinese Patents. CN103831122A http://pss-system.cnipa.gov.cn/sipopublicsearch/patentsearch/showViewList-jumpToView.shtml
Yashoda HM, Prabha TN, Tharanathan RN (2007) Mango ripening—role of carbohydrases in tissue softening. Food Chem 102(3):691–698. https://doi.org/10.1016/j.foodchem.2006.06.001
Yaylayan VA, Forage NG (1992) A kinetic model for the reaction of tryptophan with glucose and mannose—the role of diglycation in the Maillard reaction. Food Chem 44(3):201–208. https://doi.org/10.1016/0308-8146(92)90188-8
Yu L, Zhang W, Zhang T, Jiang B, Mu W (2016) Efficient biotransformation of D-fructose to D-mannose by a thermostable D-lyxose isomerase from Thermosediminibacter oceani. Process Biochem 51(12):2026–2033. https://doi.org/10.1016/j.procbio.2016.08.023
Zhang T, Pan Z, Qian C, Chen X (2009) Isolation and purification of D-mannose from palm kernel. Carbohydr Res 344(13):1687–1689. https://doi.org/10.1016/j.carres.2009.06.018
Zhang W, Zhang C, Liang G (2017a) Study on improving the yield of D-mannose by differential isomerization of glucose (in Chinese). Technol Dev Chem Ind 46(3):18–21 http://www.cnki.com.cn/Article/CJFDTOTAL-GXHG201703005.htm
Zhang W, Zhang T, Jiang B, Mu W (2017b) Enzymatic approaches to rare sugar production. Biotechnol Adv 35(2):267–274. https://doi.org/10.1016/j.biotechadv.2017.01.004
Zhang W, Huang J, Jia M, Guang C, Zhang T, Mu W (2019) Characterization of a novel D-lyxose isomerase from Thermoflavimicrobium dichotomicum and its application for D-mannose production. Process Biochem 83:131–136. https://doi.org/10.1016/j.procbio.2019.05.007
Funding
This work was supported by the National Natural Science Foundation of China (No. 31801583); the Natural Science Foundation of Jiangsu Province (No. BK20180607); the Research Program of State Key Laboratory of Food Science and Technology, Jiangnan University (SKLF-ZZA-201901); and the Postgraduate Research and Practice Innovation Program of Jiangsu Province (No. KYCX19_1817).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain studies with human participants or animals carried out by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, H., Zhang, W. & Mu, W. Recent studies on the biological production of D-mannose. Appl Microbiol Biotechnol 103, 8753–8761 (2019). https://doi.org/10.1007/s00253-019-10151-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10151-3