Abstract
Cheese whey (CW), the liquid resulting from the precipitation and removal of milk casein during cheese-making, and the second cheese whey (SCW) derived from the production of cottage and ricotta cheeses are the main byproducts of dairy industry. The major constituent of CW and SCW is lactose, contributing to the high BOD and COD content. Because of this, CW and SCW are high-polluting agents and their disposal is still a problem for the dairy sector. CW and SCW, however, also consist of lipids, proteins, and minerals, making them useful for production of various compounds. In this paper, microbial processes useful to promote the bioremediation of CW and SCW are discussed, and an overview on the main whey-derived products is provided. Special focus was paid to the production of health-promoting whey drinks, vinegar, and biopolymers, which may be exploited as value-added products in different segments of food and pharmaceutical industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cheese whey (CW), the liquid resulting from the precipitation and removal of milk casein during cheese-making, and the second cheese whey (SCW) derived from the production of cottage and ricotta cheeses are the main byproducts of dairy industry. CW is the most abundant pollutant in dairy wastewaters, representing 85–95% of the milk volume. The global production of CW is estimated to be over 108 tons per year (Carvalho et al. 2013). In the European Union (EU), the total CW production is estimated around 40 × 106 tons/year with the annual surplus of CW is 13 × 106 tons. Approx. 10 L CW is produced from 1 kg cheese (Mollea et al. 2013).
Properties of CW are affected by the type of milk used for cheese production. Therefore, casein precipitation leads to the formation of two CW types: acidic whey (pH 5) obtained by fermentation or addition of organic or mineral acids, and sweet whey (pH 6.0–7.0) obtained by addition of proteolytic enzymes (Panesar et al. 2006).
Generally, CW exhibits high chemical oxygen demand (COD; 50–70 g/L) and biological oxygen demand (BOD; 27–60 g/L) because it retains about 55% of its total milk nutrients. The most abundant components are lactose (45–50 g/L), soluble proteins (6–8 g/L), lipids (4–5 g/L), and mineral salts (8–10% of dried extract). The latter include NaCl and KCl (more than 50%), calcium salts (primarily phosphate), and others. CW also contains lactic (0.5 g/L) and citric acids, non-protein nitrogen compounds (urea and uric acid), and group B vitamins (Carvalho et al. 2013).
CW is usually used as feedstock for animal feeding or to produce ricotta cheese, generating another byproduct that is SCW. Similar to CW, SCW is also a highly polluting effluent and maintains significant BOD and COD values (up to 50 and 80 g/L of O2, respectively), high lactose content (around 50 g/L), and high salinity (7–23 mS/cm). SCW exhibits acidic pH values within the range 3–6 and possesses lower level of fat (0.5–8 g/L), total suspended solids (≈ 8.0 g L−1), and protein (≈ 0.5–8 g/L) than CW. Moreover, it is normally free of amino acids and vitamins (Carvalho et al. 2013). It has been estimated that 15–20 L CW are needed to obtain 1 kg of ricotta cheese and produce 14–19 L of ricotta SCW (Mills 1986).
Currently, the treatment of SCW is considered more essential than that of CW, as the latter is mainly used in ricotta and cottage cheese production. SCW is partially used as supplement feed for livestock, while most are not used or recycled by dairy industries; SCW disposal, then, remains a significant problem for the dairy industry. If SCW is incorporated into the wastewater, it increases the organic content, so the wastewater treatment becomes too expensive, particularly for small cheese plants. Considering that lactose is the major SCW constituent, the search for alternatives to minimize its environmental impact could be promising.
In this perspective, fermentative processes converting it into value-added products will allow both to reduce the pollution potential and to valorize SCW. However, only few studies were focused on SCW treatment to obtain value-added products (Sansonetti et al. 2009).
The management and valorization of CW and SCW are mainly based on physicochemical and biological treatments. Physicochemical processes (i.e., protein precipitation and membrane separation) are useful to produce whey powder, whey protein concentrate (WPC), whey protein isolate (WPI), whey permeate (WP), lactose, and minerals. Biological treatments, instead, involve the microbial conversion of lactose, present in CW, SCW, or cheese whey permeate, into organic acids, bioalcohols, greenhouse gases (e.g., hydrogen, methane), and bioplastics (Prazeres et al. 2012; Yadav et al. 2015; Lappa et al. 2019).
Many of these products are currently marketed worldwide. Information and update about market price of whey and whey derivatives (e.g., liquid whey, whey powder, whey protein concentrates, whey-derived lactose) are available on CLAL website (https://www.clal.it/en/index.php). In Italy, the income from the market of CW and its derivatives has still a small impact on dairy sector. According to data obtained from private dairy industries/factories, the average market prices in North Italy are as follows: CW is 25–30 €/ton; CW powder for both animal husbandry and human nutrition is 1000–1200 €/ton; food-grade lactose is 1600–1700 €/ton; whey permeate (WP) is 700–800 €/ton; whey protein concentrate (WPC) 35 powder is 3200 €/ton; WPC60 powder is 4900–5200 €/ton; WPC80 powder is 8500–12,000 €/ton; CW DEMI50 is 1500–1700 €/ton; CW DEMI70 is 1800–1900 €/ton; CW DEMI90 is 2300–2600 €/ton.
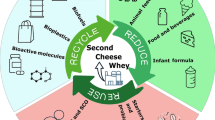
In this paper, the microbial processes useful to promote the bioremediation of CW and SCW are discussed, and an overview on the main whey-derived products is provided (Table 1). Special focus was paid to the production of health-promoting whey drinks (from lactic and acetic fermentations), vinegar, and biopolymers (i.e., poly-hydroxyalkanoates, PHAs; bacterial cellulose, BC) (Fig. 1), which may be exploited as value-added products in different segments of food and pharmaceutical industries (e.g., functional beverages and bio-packaging). An estimation of costs and potential market of the above products has been also provided.
Main value-added compounds obtained by microbial fermentations of cheese whey
The main products obtained by microbial fermentations of whey-based media and the involved microbial groups are reported in Table 1. Many of these products (e.g., lactic acid, bioalcohols, and biogases) have been extensively studied and reviewed over time as possible solution for CW valorization because of their high industrial interest. Others received lesser attention, but their production could provide sustainability and economical boost for several food-related applications.
The bioconversion of CW into functional beverages and biopolymers ranks in the objectives of current European policies driven to promote human health and environmental sustainability. The market of functional beverages recently gains interest because of increasing consumer demand for foods that enhance health and wellbeing. The synthesis of biopolymers for production of bioplastics may have great potential in food, biomedical, and agricultural applications because of biodegradability, thermo-plasticity, biocompatibility, and non-toxicity features.
Bio-valorization of CW and whey derivatives in fermented lactic and acetic beverages as well as in PHAs and BC will be addressed in this review.
Whey-based beverages
The use of CW and WP for the production of beverages, with or without microbial conversion, is one of the most attractive possibilities for the valorization and utilization of whey for human consumption.
The industrial production of whey-based drinks dates back to 1970s and different products (e.g., unfermented and fermented beverages, alcoholic beverages, diet beverages, high-protein sport drinks) have been developed and are currently available on the market (Chavan et al. 2015; Skryplonek and Jasińska 2017). Whey proteins are today the best protein source for the ready-to-drink (RTD) protein beverages, an expanding market that is expected to reach $ 17.67 billion by 2025 (www.globenewswire.com). On the other hand, “Rivella,” a sparkling and flavored whey-based beverage, is the second soft drink in Switzerland, after Coca-Cola, and is expanding in other European countries.
Whey drinks are produced with simple technologies and are characterized by a high nutritional value for the presence of proteins and peptides with several biological and health-promoting functions (e.g., antioxidant, anti-inflammatory, anticancer, immunomodulatory, cardioprotective, and hypotensive activities) (Patel 2015).
Despite this, whey beverages are sometimes perceived as unattractive products with poor sensory quality. The high lactose-glucose ratio, acidity level, and mineral content, in fact, may result in sweet, dairy/sour, and salty/sour flavors, with reduced palatability. The high lactose concentration, additionally, makes these products highly perishable. To overcome these drawbacks, several technological solutions, including pH adjustment, flavor supplementation, and microbial fermentation have been developed.
Lactic-fermented whey beverages
Fermentation is one of the cheapest ways for preserving foods, improving nutritional value, and enhancing sensory properties. CW or milk enriched with CW, WPC, or WPI is a suitable substrate for the production of fermented beverages by using yeasts and lactic acid bacteria (LAB).
As for other dairy-fermented drinks, LAB may improve the shelf life (e.g., prevention of spoilage microorganisms through the lowering of pH), nutritional (e.g., protein degradation, production of bioactive peptides), and sensory (e.g., production lactic acid and aroma compounds) properties of whey-based beverages. Some strains, moreover, are able to degrade β-lactoglobulin, the main allergenic protein in milk and whey-based products (Pescuma et al. 2012). LAB mostly used for the production of whey-based beverages belong to the Lactobacillus and Streptococcus genera. Combinations of yoghurt-derived L. delbrueckii subsp. bulgaricus and S. thermophilus cultures were extensively tested for their capability to reduce the lactose content, and for the acidifying and proteolytic activities (Gallardo-Escamilla et al. 2007; Pescuma et al. 2008; Almeida et al. 2009; Pescuma et al. 2012; Saeed et al. 2013; Sohrabi et al. 2016; Skryplonek 2018), demonstrating to be promising starters also for CW fermentation. Other authors demonstrated the capability of many LAB to produce flavoring compounds (Mauriello et al. 2001; Ricciardi et al. 2019) and to scavenge radicals (Virtanen et al. 2007) when cultivated in whey-based media.
The challenge in whey-beverage segment, however, is certainly the use of probiotic strains. The species mainly used for the production of functional whey beverages are L. acidophilus, L. casei, L. rhamnosus, and L. reuteri (Turkmen et al. 2019). Several authors (Tripathi and Jha 2004; Castro et al. 2013a; Bulatović et al. 2014) demonstrated that different probiotic lactobacilli (i.e., L. acidophilus NCDC-15, L. acidophilus NCDC-15, L. casei NCDC-12, L. casei RTS, L. rhamnosus ATCC 7469), alone or in combination with other LAB cultures, allowed to produce whey drink with satisfactory sensory properties and, in some cases, with antimicrobial effects on different foodborne pathogens (e.g., E. coli, Klebsiella pneumoniae, Salmonella typhi, and Staphylococcus aureus) (Tripathi and Jha 2004).The effect of several bifidobacteria has been also investigated. The probiotic B. animalis subsp. lactis Bb-12 was recently selected for the pilot-plant scale production of carbonate whey beverage (Silva e Alves et al. 2018). Previously, several combinations of probiotic LAB and bifidobacteria (e.g., B. animalis subsp. lactis Bb-12/L. acidophilus La-5/S. thermophilus; B. animalis subsp. lactis Bb-12/L. rhamnosus GG; B. lactis Bl-07/L. acidophilus La-14; B. bifidum NCFB271/L. reuteri NRRL1417) have been used to produce milk and/or fruit-supplemented beverages with satisfactory flavor and significant level of survived probiotics (Hernandez-Mendoza et al. 2007; Yerlikaya et al. 2012; Castro et al. 2013b; AbdulAlim et al. 2019).
Fruit-whey drinks (mixture of whey and fruit juice/pulp) may be attractive products in the segment of functional beverages since they merge the healthy properties of whey to the beneficial effects of vitamin C, β-carotene, mineral salts, dietary fibers, and phenolic compounds from fruits. Fruit-whey beverages, moreover, may promote probiotic delivery. Zoellner et al. (2009) inoculated whey containing acai pulp with B. longum BI-05 and L. acidophilus La-14, demonstrating that the presence of fruit increased (up to 2 log cycles) the survival of the two probiotics. A mixed culture of L. acidophilus (La-5) and B. animalis (Bb-12) was also used to develop an orange-flavored whey beverage (Faisal et al. 2017) with satisfying organoleptic properties. Shukla (2012) used L. acidophilus NCDC-015 to ferment acid whey and pineapple juice, reaching a high sensory score and probiotic survival. The same results were obtained using B. bifidus NCDC-255 for the production of functional drinks formulated with different ratio of whey and Aloe vera juice (Kumar 2015). L. fermentum PH5 was recently used to inoculate beverages containing different concentrations of orange juice and CW (Thakkar et al. 2018), reaching an overall sensory acceptability and maintaining a satisfactory level of probiotic viability. Several authors (de Castro et al. 2009; Yerlikaya et al. 2012; da Silveira et al. 2015), moreover, demonstrated that prebiotic supplementation (e.g., inulin, polydextrose, oligofructose, and resistant starch) may improve survival of LAB and bifidobacteria in whey-based beverages.
Combination of LAB and yeasts has been used for the production of kefir-like whey beverages using kefir grains (including Lactobacillus, Lactococcus, Leuconostoc, Streptococcus spp., and Kluyveromyces, Candida, and Saccharomyces spp.) for fermentation process (Koutinas et al. 2007; Magalhães et al. 2010; Sabokbar and Khodaiyan 2016). These products had interesting aroma profile, sensory properties, and antioxidant capability, suggesting that kefir grains may be potential starters for the production of whey-based beverages.
Although fermented whey drinks may offer greater benefits than unfermented ones, to date, the marketed products containing LAB cultures and/or probiotics are very limited. To our knowledge, “Gefilus” (Valio Ltd. Company, Finland), containing L. rhamnosus GG, lactose-hydrolyzed and demineralized whey or whey protein concentrates, fruit juices or fruit aromas, and fructose as sweetening agent, is the only probiotic drink commercially available.
Compared with whey-based products, fermented milks with probiotic supplementation (see a list in Turkmen et al. 2019) remain the mainstay of functional beverage market. The currently marketed whey drinks are still mainly recognized as energy-sport drinks with specific functions (e.g., recovery of muscle and muscle cramps, increase in lean weight, and neurostimulant). On the contrary, the fermented whey beverages can be used to formulate different products with multiple applications and functionalities, allowing to retain different groups of health-conscious consumers. Furthermore, the production costs of fermented whey drinks would be comparable with those of fermented milks, since CW and derivatives are cost-effective substrates.
Bioconversion into fermented beverages would allow cheese whey valorization also in small and medium scale cheese plants, which cannot sustain the operational and equipment costs for the production of other whey-derived products (e.g., whey protein isolates, whey protein concentrates, purified organic acids).
Alcoholic and low-acetic beverages
The bioconversion of whey and derivatives into low-alcohol and acetic beverages, including vinegar, is an interesting alternative to lactic drinks for producing new food commodities from the dairy waste.
Alcoholic fermentation
Ethanol production worldwide has strongly increased since the oil crises in 1970. Its market grew from less than a billion liters in 1975 to more than 39 billion liters in 2006, and reached 100 billion liters in 2015. Significant amounts of renewable ethanol are produced not only as biofuel but also for beverage and industrial end uses. In 2018, the EU produced 5.81 billion liters of bioethanol, 9% of which was food grade ethanol (www.epure.org). The biological production of ethanol from whey requires microorganisms, generally yeasts, suitable to assimilate lactose into ethanol.
The species Kluyveromyces lactis and Kluyveromyces marxianus (synonyms Kluyveromyces fragilis nom. inval. and Candida pseudotropicalis) are lactose-fermenting yeasts, thanks to the genes LAC12 and LAC4, encoding for lactose permease and intracellular β-galactosidase, respectively (Varela et al. 2017). Both yeasts are commonly isolated from food, fruits, and plants, as well as from fermented dairy products; thus, they gained the European Food Safety Authority (http://www.efsa.europa.eu/) Qualified Presumption of Safety (QPS) status and are Generally Regarded as Safe (GRAS) organisms (Coenen et al. 2000). Despite their phylogenetic closeness, K. lactis and K. marxianus differ in sugar metabolisms. K. marxianus engages better in fermentative metabolism than the respiring yeast K. lactis even at high temperature (45–50 °C), and therefore it is preferred over K. lactis for bioethanol conversion (van Dijken et al. 1993; Siso 1996). However, CW fermentation by K. marxianus suffers of low ethanol yield. The maximum theoretical yield of ethanol from lactose is 0.538 g ethanol/g lactose; thus, the fermented product contains approximately 3–5% ethanol, depending upon strain and fermentation technology adopted. The fermentation product is then centrifuged to remove the biomass, and sent to a distillation column where the ethanol content is increased to 95% (v/v). K. marxianus exhibits a great strain variability in lactose utilization, so an accurate strain selection is essential to optimize ethanol yields. Selected strains should exhibit ethanol- and thermo-tolerance in order to avoid inhibitory effects on yeast growth due to catabolite repression and reduce cooling cost in ethanol production bioprocesses, respectively. Furthermore, high lactose-utilizing yeasts should possess a functional KmLac12 transport which efficiently catalases the lactose uptake (Fonseca et al. 2008).
Feeding, oxygen, temperature, and fermentative modes also strongly contribute to ethanol productivity (Sansonetti et al. 2009). In batch processes, high lactose, relatively low temperature, and low oxygen levels generally increased alcoholic fermentation (Sansonetti et al. 2009; Sansonetti et al. 2010; Sansonetti et al. 2011). Lactose amount in the range of 50–200 g/L enhanced ethanol productivity, while values higher than 200 g/L negatively affected yeast growth (Ferreira et al. 2015). Empirical models indicated 32.3 °C as the best operating temperature (Sansonetti et al. 2010), whereas temperature higher than 35–37 °C increased the lag phase (Christensen et al. 2011). Oxygen depletion reduced biomass and glycerol (required for NADH oxidation) production in favor of ethanol (Sansonetti et al. 2009). Cell immobilization in batch bioreactor (Roohina et al. 2016), fed-batch processes (Brady et al. 1997; Kourkoutas et al. 2002), and continuous cultivation (Kourkoutas et al. 2002; Sansonetti et al. 2011; Gabardo et al. 2014; Hadiyanto et al. 2014) modes coupled with immobilized K. marxianus cells overcame free cells in batch bioreactor in alcohol productivity, as new substrate was available without any catabolite repression.
Differently from Kluyveromyces spp., the best alcohol-producing yeast Saccharomyces cerevisiae is unable to assimilate lactose, and thus it cannot be exploited to produce ethanol from CW and other derivatives (SCW and whey permeate) without any preliminary enzymatic hydrolysis of lactose into glucose and galactose (Guimarães et al. 2010; Das et al. 2016). Exogenous β-galactosidase enzyme from K. lactis and S. cerevisiae cells can be employed in two-step sequential process or, alternatively, in co-immobilized state to avoid enzyme and cell washing out. Bioreactors with direct contact membrane distillation allowed the continuous removal of ethanol and increased the efficiency of sugar conversion to ethanol, by-passing catabolite repression (Tomaszewska and Białończyk 2016). Alternatively, S. cerevisiae strains can be engineered for lactose consumption by the heterologous expression of Kluyveromyces LAC12 and LAC4 genes (Domingues et al. 2010). These engineered strains generally exhibited more ethanol yield than K. marxianus, but should be discarded for the production of food grade ethanol. Furthermore, in presence of glucose and galactose, S. cerevisiae preferentially consumes glucose due to catabolic repression of enzymes necessary for galactose uptake. Diauxic shift to galactose imposes the synthesis of novel enzymes for galactose catabolism, leading to a sluggish fermentation.
Data on economic sustainability of bioethanol conversion from whey permeate are generally poorly available. Cost-benefit analysis performed by Utama et al. (2017) showed that ethanol production from cheese whey and Napa cabbage covered the waste disposal costs, leading to a financial benefit up to US$ 3816.96 per month, and attained the breakeven point in 3.53 months. Conversely, da Silva et al. (2015) suggested that production of WPC was more economically sustainable when coupled with lactose powder production than with ethanol bioconversion of whey permeate due to high production cost for ethanol.
Acetic acid fermentation
CW and derivatives after alcoholic fermentation can reach an ethanol content around 6% (v/v) which allow the production of both vinegar and low-acetic beverages. This way to valorize CW is in tune with consumer’s demand for high value-added products and government initiatives promoting healthy foods and drinks. Furthermore, health-based recommendations include reducing alcohol consumption, calories from added sugars, and limiting the consumption of foods with refined grains, especially those that contain added sugars and sodium. Foods and beverages with added sugars are higher in energy and low in essential nutrients or dietary fiber. Moreover, the safe use of non-nutritive sweeteners, like aspartame, is currently under on-going scientific debate, opening the avenue for alternative low-calorie sweeteners.
Overall, these issues raise the opportunity for the beverage industry with fermentation background to make a dynamic comeback with the production of new whey-based and alcohol-free fermented beverages.
Although now, for these emerging products, there is a lack of information on the economic feasibility of processes, and a number of available studies demonstrated the potential of bioprocesses and the possibility of expanding the whey-based beverages.
From the biotechnological point of view, the conversion of whey-derived ethanol into acetic acid by acetic acid bacteria (AAB) is highly feasible. AAB are able to produce acetic acid in fermenting liquids, in which ethanol content ranges from 2–3% to 15–18%, according to the fermentation system and the microbial strain used (Gullo et al. 2014). This wide range allows to design versatile bioprocesses obtaining vinegars and drinks at variable acetic acid content and residual ethanol. Besides the protective action of residual ethanol or acids on acetic drinks, the fermentation process can have additional roles. Secondary metabolism of AAB, in fact, may improve functional properties and modulate metabolic profile of product.
In this scenario, the exploitation of selective AAB fermentations for production of ethanol-free drinks, containing fructans, low amount of acetic acid, and reduced sugar content may be a challenge in the formulation of whey-based beverages.
Among fructans, levan-type exopolysaccharides (LT-EPS) are synthesized by the extracellular enzyme levansucrase (or sucrose 6-fructosyltransferase), which catalyzes the transfer of D-fructosyl residues from sucrose to a growing fructan chain by trans-fructosylation (Donot et al. 2012). After sucrose depletion, levansucrase cleaves the ß(2-6) linkages of the newly-formed levan chain, causing the consecutive release of the terminal fructose units until a branching point is reached (Méndez-Lorenzo et al. 2015).
The interest for bacterial fructans arises from some of their properties, like biocompatibility, biodegradability, and biomedical properties such as antioxidant, anti-inflammatory, antitumor, and cholesterol-lowering agents. Moreover, they are considered prebiotic molecules since their hydrolysis products, which are short-chain fructooligosaccharides, show the ability to preferentially stimulate the growth of intestinal bifidobacteria (Roberfroid et al. 1998).
Although some studies focused on fructan production by AAB for food and beverage formulation, they are rarely applied in the food industry due to the lack of defined commercially preparations. However, they are used for some non-alcoholic beverages (e.g., in some ultra-high-fructose syrups) as sweetener or dietary fiber (La China et al. 2018).
Among AAB, the strain Gluconacetobacter diazotrophicus SRT4 highlighted the ability to synthetize high amount of branched LT-EPS with a molecular weight above 2 × 106 Da. The ability to synthetize LT-EPS was also found among Komagataeibacter xylinus strains (see La China et al. 2018 for references). Recently, Jakob and co-workers quantified and characterized the LT-EPS produced by strains of the species Gluconobacter frateurii, G. cerinus, Neoasaia chiangmaiensis, and Kozakia baliensis by a combination of NMR and AF4-MALS-RI analysis (Jakob et al. 2013). This latter study showed that the molecular weight of LT-EPS has a high variability among AAB species, ranging from 4 MDa (G. frateurii) to 2,000 MDa (K. baliensis). This aspect deeply affects the physiochemical properties (i.e., different rheology) and function (i.e., changes in antitumor and antiviral activities) of LT-EPS. Although a number of studies emphasized the high potential of AAB in the production of both acetic acid and LT-EPS, no commercial products are still available in the market.
Whey vinegars
The conversion of CW into whey vinegars represents a valuable option to recycle whey in traditional fermented food chain and to circumvent the main disadvantage of low productivity found in bioethanol production from whey. The basic process is the bioconversion of sugars into ethanol by lactose-fermenting Kluyveromyces yeasts, which is further converted into acetic acid by AAB (Parrondo et al. 2009).
The FAO/WHO defines vinegar as any liquid, fit for human consumption, obtained exclusively by the biological process of double fermentation, alcoholic and acetous, from liquids or other substances of agricultural origin (Joint FAO/WHO Food Standards Programme 1998). In the USA, the Food and Drug Administration (FDA) requires that vinegar products must contain a minimum acidity of 4 g per 100 g. There are currently no standards to identify vinegars, but the FDA has established “Compliance Policy Guides” that the Agency follows regarding labeling of vinegars, such as cider, wine, malt, sugar, spirit, and vinegar blends (Food and Drug Amministration 2007). In the EU, each country has specific regional standards for vinegar produced or sold in the national area. Unlike the USA law, the EU has established a minimum threshold of 5% (w/v) and a maximum threshold of 0.5% (v/v) for acidity and ethanol, respectively, when the raw material for acetic acid fermentation is not wine.
The overall vinegar market has reached values worth around USD 1.26 billion in 2017 growing at a rate of 2.1% during 2010–2017 and is further expected to reach a value of USD 1.50 billion by 2022 (www.imarcgroup.com/vinegar-manufacturing-plant).
The most famous vinegars are from wine or cider; however, vinegars can be produced from other non-conventional sources containing sugars, like lactose-rich CW and SCW. Actually, vinegars from CW and its derivatives are produced mainly in Switzerland, but they are poorly known.
As ethanol amount higher than 5–6% could inhibit AAB, Kluyveromyces yeasts grown on whey permeate with lactose up to 200 g/L assure enough alcohol for the subsequent acetic acid production. Parrondo et al. (2003) produced vinegar with acetic acid content between 5 and 6% (v/v) by sequential fermentation of K. marxianus and Acetobacter pasteurianus. Using whey permeate with 135 g/L of lactose, K. marxianus produced whey liquor with a final concentration of ethanol around 55 g/L within 48 h at optimal temperature of 30 °C. Ethanol was converted into acetic acid by A. pasteurianus in 4 days with an efficiency of around 84%. Similarly, K. marxianus strains fermented threefold concentrated whey, producing a whey liquor containing 8% ethanol (Tamura 2000). It was twofold diluted before oxidization of ethanol to acetic acid by A. pasteurianus IFO 14814. The resulting whey vinegar contained 5.2% acetic acid and exhibited a faint odor of cow milk as well as a mellow acidic taste. Whey vinegar was also proposed as stable nutrient ingredient in dairy cattle diet (Lustrato et al. 2013). Sequential fermentation of K. marxianus and A. aceti led to average lactose consumption of 56%, ethanol yield of 6.7 g/L/day, and acetic acid production of 4.35 g/L/day.
The current vinegar market offers several products with peculiar attributes, especially those containing healthy and functional compounds, with number continuously increasing. These vinegars originate from different raw materials such as fermentable fruits and vegetables. CW and its derivatives are suitable raw materials to design innovative bioprocesses conducted by selected yeasts an AAB strains to produce added value vinegars.
Distilled whey-based spirit (whey vodka)
Once produced, bioethanol should be distilled and/or concentrated for food or biofuel usage. Developed since 1940 in Ireland, the so called Carbery’s process represents the first and most common mode to produce potable whey spirits from whey permeate on industrial scale. It relies on batch or fed-batch fermentations by K. marxianus coupled with continuous extractive distillation. The resulting distillate (95% by volume ethanol) is further diluted with water and redistilled to remove impurities and to produce potable whey spirits. The Carbery’s process is currently used in Ireland to produce about 11,000 tons of ethanol per year. Also, in New Zealand, over 18 million liters of ethanol were produced annually from whey through Carbery’s process and exported to Asian market (Hughes et al. 2018).
The concept of producing whey-based spirits has been recently shifted to small craft distilleries which used pot distillation instead of extractive method. Based on life cycle analysis, production of distilled whey-based spirit resulted more sustainable than the conventional method for unaged spirit production from malted barley in terms of carbon dioxide-equivalent (CO2) emissions and water usage (Risner et al. 2018). Volatilome of distillates changed depending upon CW, with sweet whey distillates enriched in alcohols, acids, esters, and ketones, whereas acid whey distillates in aldehydes, terpenes, and terpenoids (Risner et al. 2019).
Although this is a means to reduce CW waste, it cannot be considered among healthy strategies to valorize dairy wastes, since alcoholic beverages are not in tune with healthy recommendations.
Biopolymers
The environmental problems associated with the accumulation of traditional petrol-derived plastics make urgent to find new alternatives (European Commission 2013). The real opportunity to overcome the state of emergency caused by environmental pollution related to the dispersion of plastics and issues relating to their disposal results in the use of biopolymers of bacterial origin. In order to reconcile food security, and natural resource scarcity and environmental sustainability, the side-products of cheese production can also be used to produce biopolymers such as PHAs and BC. These biopolymers are promising candidates for industry to substitute the traditional fossil fuel–derived plastics. However, the industrial production of PHA and BC, by fermentation, is a challenge in terms of economic sustainability of the process. However, the production cost of plastics from petrochemical product is still more competitive and the chemical synthesis is preferred by industrial companies compared with biopolymer production. In fact, to be competitive with petrol-derived plastics, the selling price of biopolymers should not exceed 2000 €/ton. At present, a prediction on the revenues obtainable from biopolymer-derived products is still difficult. This depends both on the type of raw material used, which is 20 to 80% higher than the cost of raw materials of conventional plastics, and on the type of products obtained from biopolymers. It is expected that advancement in the industrialization process of PHA would drive the cost of PHA and make it an effective alternative for conventional plastic (www.marketsandmarkets.com/Market-Reports/pha-market).
Microbial polyhydroxyalkanoates
The PHAs are carbon polymers accumulated in the cytoplasm of many bacterial species under particular conditions of excess of carbon availability, while some other factor is limiting (i.e., N, P, S, etc). CW can be considered as suitable substrate for PHA production, due to its relatively high organic load (Colombo et al. 2016). The use of CW, among several carbon source (Koller et al. 2017; Anjum et al. 2016), for microbial PHA synthesis has the dual function of reducing both PHA production costs and waste management costs, and it has been recently extensively reviewed by Amaro et al. (2019). PHAs are the only “bio-plastics” with a whole “green” life-cycle: renewable resources act as feedstock of the production (bio-based), living cells are responsible for both synthesis of their monomeric building blocks and their subsequent polymerization (bio-synthesized), no adverse effects on the biosphere (biocompatibility), and, lastly, they endure degradation by the action of living organisms (biodegradability) (Koller et al. 2013; Verlinden et al. 2007), such as gram-positive and gram-negative bacteria, Bacillus spp., Pseudomonas spp., Streptomyces spp., and fungi as Aspergillus fumigates (Bugnicourt et al. 2014).
These polymers can be synthesized in different types of PHA that microorganisms accumulate as insoluble inclusion bodies. The first PHA identified was the homopolymer poly-3-hydroxybutyrate (PHB; Lemoigne (1927), a semi-crystalline isotactic polymer that endures surface erosion due to the hydrophobicity of the backbone and its crystallinity. Moreover, hydrolytic degradation of PHB ends in the formation of D-(−)-3-hydroxybutyric acid, a normal blood constituent, making it an excellent candidate for use in long-term tissue-engineering applications being biocompatible, workable, and degradable (Ulerly et al. 2011).
The functionalities of PHAs are different. PHAs can be applied in many types of implant applications including orthopedic, craniomaxillofacial, dental, and cardiovascular, as well as in cardiology, plastic and reconstructive surgery, general surgery, ear, nose, throat surgery, and oral surgery. While the PHAs offer a wide range of mechanical properties, which are potentially useful in medical applications, their use particularly in vivo as bioresorbable polymers has been limited by their slow hydrolysis (Niaounakis 2015). Other general characteristics of PHAs are as follows: water insoluble and relatively resistant to hydrolytic degradation; good ultraviolet resistance but poor resistance to acids and bases; soluble in chloroform and chlorinated hydrocarbons; sinks in water, facilitating its anaerobic biodegradation in sediments; nontoxic; less “sticky” than others polymers when melted (Bugnicourt et al. 2014). These properties make PHAs also good candidates for food packaging. Koller (2014) reviewed the main aspects to be considered when PHAs are used for this purpose: purity and sensory quality, in which a specific role is played by the extraction and purification methods in order to avoid typical rancid odor and smell of the material that can easily and negatively affect the quality of the packaged food. Moreover, particular attention has to be given to the removal of remaining lipids and pyrolytic lipopolysaccharides (endotoxins) that are frequently spotted attached to PHAs from gram-negative strains (Furrer et al. 2007); the oxygen barrier of the film depends on the composition of PHAs on the monomeric level; water barrier, PHA polyesters show the advantage of substantial hydrophobicity in comparison with other biopolymers of natural origin (starch). Generally, values for PHB are similar to petrochemical opponents (PET) and poly(vinyl chloride) (PVC); high barrier for flavoring substances to protect the flavor of the food; chemical resistance, PHAs are easily subjected to acid-catalyzed hydrolytic degradation, so the performance and the suitability of biopolymers stored with common food packaging solution as a function of time has to be assessed. Bugnicourt et al. (2014) and Koller (2014) summarized the most known commercially available PHAs.
According to Research and Markets data, the global polyhydroxyalkanoates market is accounted for $78.20 million in 2017 and is expected to reach $135.78 million by 2026 growing at a CAGR of 6.3% (www.marketsandmarkets.com/Market-Reports/pha-market).
Since the first discovery by Lemoigne (1927), a number of studies indicated many microbial species able to synthesize PHAs, and the most important are E. coli (engineered culture) and Cupriavidus necator. These bacterial species are the most used for industrial applications since they associate high productivity to reduced times of accumulation, ranging from 0.02 to 5.2 g/L/h (Amaro et al. 2019). Unfortunately, as reviewed by Amaro et al. (2019), despite CW is a rich media that support microbial growth, some of the best-described PHA-producing microbial species have been shown to be unable to directly produce PHA from whey, due to its carbon source, the lactose. Alcaligenes latus, Bacillus spp., Bacillus megaterium, Sinorhizobium meliloti, Sinorhizobium spp., Bacillus cereus, Pseudomonas aeruginosa, Hydrogenophaga pseudoflava, Pseudomonas hydrogenovora, Haloferax mediterranei, Thermus thermophiles, Methylobacterium spp., and Halomonas halophila were the species used until now to produce PHAs from whey and a comparison among used substrate, type of culture, microorganism, culture method, productivity, and type of PHA has been reported in Amaro et al. (2019). Alternatively, mixed microbial cultures (MMCs) enriched in PHA-storing bacteria within the classes of Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria have been exploited (Morgan-Sagastume 2016; Amaro et al. 2019). MMCs are used using feast and famine cycles in order to enrich the strains that are able to accumulate PHAs. While MMCs were associated with lower yields of PHA production compared with the pure strains (0.0035–0.56 g/L/h vs 0.0039–0.17 g/L/h), they have the advantage of not requiring sterile condition (Amaro et al. 2019).
Bacterial cellulose
Cellulose is the most abundant biopolymer on earth, recognized as the major component of plant biomass, and also as a representative of microbial extracellular polymers. BC is a highly pure form of cellulose with the same chemical structure as plant cellulose, but having superior physical and chemical properties (e.g., stability at high temperature, purity, biodegradability, and water holding capacity (Gullo et al. 2017). These properties result from a higher degree of polymerization and ultrafine network architecture. Moreover, BC does not contain hemicellulose or lignin and it shows a more crystalline structure with respect to plant cellulose. Because of its unique properties, BC has found a multitude of applications in food, paper, and textile industries, as well as in cosmetic and medicine fields (Gullo et al. 2018). Many BC-based scaffolds are approved by the Food and Drug Administration (FDA) because of the low proteins and endotoxic unit content (Petersen and Gatenholm 2011).
The global BC market is valued at 207.36 million USD in 2016 and is estimated to reach 497.76 million USD by the end of 2022 (https://www.marketresearch.com/QYResearch-Group-v3531/). However, until now, the industrial production of BC suffers from the low production yield. BC production can be properly optimized to overcome these limitations. The design of a rational selection strategy to recover suitable producing strains is the first step to obtain functionalized BC for different applications.
The use of biodegradable BC-based material can be an outstanding alternative to substitute materials currently used in food packaging (Umaraw and Verma 2015).
BC, as eco-friendly polymer, has received considerable attention especially to produce composite materials aimed to increase the shelf life of foods. Due to its specific properties, BC can be functionalized to fabricate innovative materials for the development of new active packaging systems, in which antimicrobial agents are combined into the packaging material creating a protective layer.
Highly interesting seems the development of biodegradable active food packaging with improved physical, mechanical, barrier, and additional bioactive function to ensure food safety and to extend the shelf life of foods. Moreover, BC activated with antimicrobial compounds and probiotics can be effective against bacterial food pathogen infection.
Some studies showed the antimicrobial effect of a BC packaging embedded with sorbic acid in mono- and multilayer BC against E. coli (K12-MG1655) (Jipa et al. 2012). Very recently, the antibacterial activity against gram-positive and gram-negative bacteria was observed in a novel nanocomposite film of BC produced by a K. xylinus strain. The film incorporating GO-CuO nanohybrids could find applications both in the field of active packaging materials and in the biomedical field (Xie et al. 2020).
Among the strictly aerobic AAB, different species may synthetize BC which production yield largely differs, but the most important one is K. xylinus (Chawla et al. 2009; Gullo et al. 2019; La China et al. 2020).
To reduce the costs of feedstock, while contributing to environmental impact reduction, various low-cost alternative carbon sources for producing BC have been valued. In particular, researches focused on cheap agricultural products or waste containing suitable carbon sources (Thompson and Hamilton 2001; Kuo et al. 2010). Although few data are available on the economic feasibility of bioprocesses to produce BC from alternative raw materials, some considerations can be made. In microbial processes, as those required for BC production, the impact of pure substrates accounts for up to 50–60% of the total production cost (Vazquez et al. 2013). The data obtained using low-costs raw materials, such as glycerol from biodiesel production and grape bagasse from wine production, are encouraging for the cost-effective industrial production of BC.
Vazquez and coworkers found that although BC production using analytical grade glycerol was three times higher than production achieved using glycerol from biodiesel, production costs using the biodiesel byproduct could lead to values up to 18-folds lower whereas when using waste wine (grape bagasse) supplemented with nitrogen sources (0.7% w/v diammonium phosphate), production values obtained were only slightly lower than those obtained in presence of commercial glycerol as carbon source.
Few studies evaluate the use of CW and derivatives for BC production. However, recently agri-food waste such the residual liquid of grape in combination with CW was evaluated for BC synthesis (Bekatorou et al. 2019). As for other raw materials, no optimized bioprocesses are available and the main issues are related to the low BC production yield and pros and cons are related to costs and quality of BC produced. In this light, the exploitation of CW and derivatives in producing BC is highly appealing.
Conclusion and perspectives
CW and SCW are the main wastes of dairy industry responsible for a high organic load. Although existing strategies to manage these wastes contribute to reduce their amount, there is a need to further valorize them. Among biological treatments, fermentative approaches using yeasts, LAB, and AAB offer the opportunity to consolidate already used bioprocesses and to introduce innovative bioconversion strategies combining the valorization of these wastes with the need to produce healthy food commodities.
Considering the fermented beverage sector, the selection of appropriate microbial culture of yeasts, LAB, and AAB could reinforce the valorization of CW and its derivatives by increasing the yield of the main fermentation compounds and offering the opportunity to design and produce new functional beverages with healthy attributes.
The production of biopolymers such PHAs and BC from food wastes is of great interest for the biotechnological industry. On the basis of the current knowledge, there is a wide potential but further optimization steps are needed to enhance the industrial feasibility.
References
AbdulAlim TS, Zayan AF, Campelo PH, Bakry AM (2019) Development of new functional fermented product: mulberry-whey beverage. J Nutr Food Res Technol 1:64–69. https://doi.org/10.30881/jnfrt.00013
Ahmadi N, Khosravi-Darani K, Mortazavian AM (2017) An overview of biotechnological production of propionic acid: from upstream to downstream processes. Electronic. J Biotechnol 28:67–75. https://doi.org/10.1016/j.ejbt.2017.04.004
Almeida KE, Tamime AY, Oliveira MN (2009) Influence of total solids contents of milk whey on the acidifying profile and viability of various lactic acid bacteria. LWT - Food Sci Technol 42:672–678. https://doi.org/10.1016/j.lwt.2008.03.013
Amaro TMMM, Rosa D, Comi G, Iacumin L (2019) Prospects for the use of whey for polyhydroxyalcanoate (PHA) production. Front Microbiol 10:992. https://doi.org/10.3389/fmicb.2019.00992
Anjum A, Zuber M, Zia KM, Noreen A, Anjum MN, Tabasum S (2016) Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: a review of recent advancements. Int J Biol Macromol 89:161–174. https://doi.org/10.1016/j.ijbiomac.2016.04.069
Becerra M, Cerdán ME, González-Siso MI (2015) Biobutanol from cheese whey. Microb Cell Factories 14:27–41. https://doi.org/10.1186/s12934-015-0200-1
Bekatorou A, Plioni I, Sparou K, Maroutsiou R, Tsafrakidou P, Petsi T, Kordouli E (2019) Bacterial cellulose production using the corinthian currant finishing side-stream and cheese whey: process optimization and textural characterization. Foods 4:8. E193. https://doi.org/10.3390/foods8060193
Bertrand N, Fliss I, Lacroix C (2001) High nisin-Z production during repeated-cycle batch cultures in supplemented whey permeate using immobilized Lactococcus lactis UL719. Int Dairy J 11:953–960. https://doi.org/10.1016/S0958-6946(01)00129-7
Brady D, Nigam P, Marchant R, McHale AP (1997) Ethanol production at 45°C by alginate-immobilized Kluyveromyces marxianus IMB3 during growth on lactose-containing media. Bioprocess Eng 16:103–104. https://doi.org/10.1007/s004490050295
Bugnicourt E, Cinelli P, Lazzeri A, Alvarez V (2014) Polyhydroxyalkanoate (PHA): review of synthesis, characteristics, processing and potential applications in packaging. Express Polym Lett 8:791–808. https://doi.org/10.3144/expresspolymlett.2014.82
Bulatović ML, Krunić T, Vukašinović-Sekulić MS, Zarić DB, Rakin MB (2014) Quality attributes of a fermented whey-based beverage enriched with milk and a probiotic strain. RSC Adv 4:55503–55510. https://doi.org/10.1039/C4RA08905G
Cardoso T, Marques C, Dagostin JLA, Masson ML (2019) Lactobionic acid as a potential food ingredient: recent studies and applications. J Food Sci 84:1672–1681. https://doi.org/10.1111/1750-3841.14686
Carvalho F, Prazeres AR, Rivas J (2013) Cheese whey wastewater: characterization and treatment. Sci Total Environ 445–446:385–396. https://doi.org/10.1016/j.scitotenv.2012.12.038
Castro WF, Cruz AG, Bisinotto MS, Guerreiro LMR, Faria JAF, Bolini HMA, Cunha RL, Deliza R (2013a) Development of probiotic dairy beverages: rheological properties and application of mathematical models in sensory evaluation. J Dairy Sci 96:16–25. https://doi.org/10.3168/jds.2012-5590
Castro WF, Cruz AG, Rodrigues D, Ghiselli G, Oliveira CAF, Faria JAF, Godoy HT (2013b) Short communication: effects of different whey concentrations on physicochemical characteristics and viable counts of starter bacteria in dairy beverage supplemented with probiotics. J Dairy Sci 96:96–100. https://doi.org/10.3168/jds.2012-5576
Chatzipaschali AA, Stamatis AG (2012) Biotechnological utilization with a focus on anaerobic treatment of cheese whey: current status and prospects. Energies 5:3492–3525. https://doi.org/10.3390/en5093492
Chavan RS, Shradda R, Kumar A, Nalawade T (2015) Whey based beverage: its functionality, formulations, health benefits and applications. J Food Process Technol 6:10. https://doi.org/10.4172/2157-7110.1000495
Chawla PR, Bajaj IB, Survase S, Singhal RS (2009) Microbial cellulose: fermentative production and applications. Food Technol Biotechnol 47:107–124
Christensen AD, Kádár Z, Oleskowicz-Popiel P, Thomsen MH (2011) Production of bioethanol from organic whey using Kluyveromyces marxianus. J Ind Microbiol Biotechnol 38:283–289. https://doi.org/10.1007/s10295-010-0771-0
Chwialkowska J, Duber A, Zagrodnik R, Walkiewicz F, Łężyk M, Oleskowicz-Popiel P (2019) Caproic acid production from acid whey via open culture fermentation – Evaluation of the role of electron donors and downstream processing. Biores Technol 279:74-83. https://doi.org/10.1016/j.biortech.2019.01.086
Coenen TMM, Bertens AMC, De Hoog SCM, Verspeek-Rip CM (2000) Safety evaluation of a lactase enzyme preparation derived from Kluyveromyces lactis. Food Chem Toxicol 38:671–677. https://doi.org/10.1016/S0278-6915(00)00053-3
Colombo B, Sciarria TP, Reis M, Scaglia B, Adani F (2016) Polyhydroxyalkanoates (PHAs) production from fermented cheese whey by using a mixed microbial culture. Bioresour Technol 218:692–699. https://doi.org/10.1016/j.biortech.2016.07.024
da Silva AN, Perez R, Minim VPR, Martins DDSA, Minim LA (2015) Integrated production of whey protein concentrate and lactose derivatives: what is the best combination? Food Res Int 73:62–74. https://doi.org/10.1016/j.foodres.2015.03.009
da Silveira EOD, Lopes Neto JH, da Silva LA, AES R, Magnani M, Cardarelli HR (2015) The effects of inulin combined with oligofructose and goat cheese whey on the physicochemical properties and sensory acceptance of a probiotic chocolate goat dairy beverage. LWT - Food Sci Technol 62:445–451. https://doi.org/10.1016/j.lwt.2014.09.056
Das B, Sarkar S, Maiti S, Bhattacharjee S (2016) Studies on production of ethanol from cheese whey using Kluyveromyces marxianus. In: Materials Today: Proceedings. Elsevier Ltd, pp 3253–3257
de Castro FP, Cunha TM, Ogliari PJ, Teófilo RF, Ferreira MMC, Prudêncio ES (2009) Influence of different content of cheese whey and oligofructose on the properties of fermented lactic beverages: study using response surface methodology. LWT - Food Sci Technol 42:993–997. https://doi.org/10.1016/j.lwt.2008.12.010
Domingues L, Guimarães PMR, Oliveira C (2010) Metabolic engineering of Saccharomyces cerevisiae for lactose/whey fermentation. Bioeng Bugs 1:164–171. https://doi.org/10.4161/bbug.1.3.10619
Donot F, Fontana A, Baccou JC, Schorr-Galindo S (2012) Microbial exopolysaccharides: main examples of synthesis, excretion, genetics and extraction. Carbohydr Polym 87:951–962. https://doi.org/10.1016/j.carbpol.2011.08.083
Enan G, Aziz A, Amri A (2006) Novel plantaricin UG1 production by Lactobacillus plantarum UG1 in enriched whey permeate in batch fermentation processes. J Food Agric Environ 4:85–88
European Commission (2013) Analysis of the public consultation on the green paper “European strategy on plastic waste in the environment”. EU DG ENV
Faisal S, Chakraborty S, Devi WE, Hazarika MK, Puranik V (2017) Sensory evaluation of probiotic whey beverages formulated from orange powder and flavor using fuzzy logic. Int Food Res J 24:703–710
Fernández-Gutiérrez D, Veillette M, Giroir-Fendler A, Avalos Ramirez A, Faucheux N, Heitz M (2017) Biovalorization of saccharides derived from industrial wastes such as whey: a review. Rev Environ Sci Biotechnol 16:147–174. https://doi.org/10.1007/s11157-016-9417-7
Ferreira PG, da Silveira FA, dos Santos RCV, Genier HLA, Diniz RHS, Ribeiro JI, Fietto LG, Passos FML, da Silveira WB (2015) Optimizing ethanol production by thermotolerant Kluyveromyces marxianus CCT 7735 in a mixture of sugarcane bagasse and ricotta whey. Food Sci Biotechnol 24:1421–1427. https://doi.org/10.1007/s10068-015-0182-0
Fonseca GG, Heinzle E, Wittmann C, Gombert AK (2008) The yeast Kluyveromyces marxianus and its biotechnological potential. Appl Microbiol Biotechnol 79:339–354. https://doi.org/10.1007/s00253-008-1458-6
Food and Drug Administration (2017) FDA/ORA Compliance Policy Guides Sec. 525.825 Vinegar, Definitions - Adulteration with Vinegar Eels (CPG 7109.22)
Furrer P, Panke S, Zinn M (2007) Efficient recovery of low endotoxin medium-chain-length poly ([R]-3-hydroxyalkanoate) from bacterial biomass. J Microbiol Meth 69:206–213. https://doi.org/10.1016/j.mimet.2007.01.002
Gabardo S, Rech R, Rosa CA, Ayub MAÔZ (2014) Dynamics of ethanol production from whey and whey permeate byimmobilized strains of Kluyveromyces marxianus in batch andcontinuous bioreactors. Renew Energy 69:89–96. https://doi.org/10.1016/j.renene.2014.03.023
Gallardo-Escamilla FJ, Kelly AL, Delahunty CM (2007) Mouthfeel and flavour of fermented whey with added hydrocolloids. Int Dairy J 17:308–315. https://doi.org/10.1016/j.idairyj.2006.04.009
Guerra NP, Rus ML, Pastrana L (2001) Nutritional factors affecting the production of two bacteriocins from lactic acid bacteria on whey. Int J Food Microbiol 70:267–281. https://doi.org/10.1016/S0168-1605(01)00551-7
Guimarães PMR, Teixeira JA, Domingues L (2010) Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnol Adv 28:375–384. https://doi.org/10.1016/j.biotechadv.2010.02.002
Gullo M, Verzelloni E, Canonico M (2014) Aerobic submerged fermentation by acetic acid bacteria for vinegar production: process and biotechnological aspects. Process Biochem 49:1571–1579. https://doi.org/10.1016/j.procbio.2014.07.003
Gullo M, Sola A, Zanichelli G, Montorsi M, Messori M, Giudici P (2017) Increased production of bacterial cellulose as starting point for scaled-up applications. Appl Microbiol Biotechnol 101:8115–8127. https://doi.org/10.1007/s00253-017-8539-3
Gullo M, La China S, Falcone PM, Giudici P (2018) Biotechnological production of cellulose by acetic acid bacteria: current state and perspectives. Appl Microbiol Biotech 102:6885–6898. https://doi.org/10.1007/s00253-018-9164-5
Gullo M, La China S, Petroni G, Di Gregorio S, Giudici P (2019) Exploring K2G30 genome: a high bacterial cellulose producing strain in glucose and mannitol based media. Front Microbiol 10:6885–6898. https://doi.org/10.3389/fmicb.2019.00058
Gutierrez NA, Debarr AD, Maddox IS (1996) Production of diacetyl from whey permeate using Lactoccoccus lactis subsp. lactis. J Ferment Bioeng 81:183–184. https://doi.org/10.1007/s10295-009-0617-9
Hadiyanto, Ariyanti D, Aini AP, Pinundi DS (2014) Optimization of ethanol production from whey through fed-batch fermentation using Kluyveromyces marxianus. In: Energy Procedia. Elsevier Ltd, pp 108–112. doi: https://doi.org/10.1016/j.egypro.2014.01.203
Hassan AN, Nelson BK (2012) Invited review: anaerobic fermentation of dairy food wastewater. J Dairy Sci 95:756–774. https://doi.org/10.3168/jds.2012-5732
Hernandez-Mendoza A, Robles VJ, Angulo JO, De La Cruz J, Garcia HS (2007) Preparation of a whey-based probiotic product with Lactobacillus reuteri and Bifidobacterium bifidum. Food Technol Biotechnol 45:27–31
Hughes P, Risner D, Meunier Goddik L (2018) Whey to vodka chapter in whey - biological properties and alternative uses. In: Gigli (ed) . IntechOpen. https://doi.org/10.5772/intechopen.81679
Jakob F, Pfaff A, Novoa-Carballal R, Rübsam H, Becker T, Vogel RF (2013) Structural analysis of fructans produced by acetic acid bacteria reveals a relation to hydrocolloid function. Carbohydr Polym 92:1234–1242. https://doi.org/10.1016/j.carbpol.2012.10.054
Jipa IM, Stoica-Guzun A, Stroescu M (2012) Controlled release of sorbic acid from bacterial cellulose based mono and multilayer antimicrobial films. LWT-Food Sci Technol 47:400–406. https://doi.org/10.1016/j.lwt.2012.01.039
Joint FAO/WHO Food Standards Programme (1998) Conversion of European regional standard for vinegar into world-wide standard. Report No: FAO-ESN–CX/PFV-98/8. Codex committee on processed fruits and vegetables. Sess 19. Washington, DC, 16–18 March 1998. FAO, Rome
Jozala AF, Silva DP, Vincente AA, Teixeira JA, Pessoa AJ, Penna CV (2011) Processing of byproducts to improve nisin production by Lactococcus lactis. J Biotechnol 10:14920–14925. https://doi.org/10.5897/AJB11.979
Koller M (2014) Poly(hydroxyalkanoates) for food packaging: application and attempts towards implementation. Appl Food Biotechnol 1(1):1–13. https://doi.org/10.22037/afb.v1i1.7127
Koller M, Salerno A, Muhr A, Reiterer A, Braunegg G (2013) Polyhydroxyalkanoates: Biodegrad-able polymers and plastics from renewable resources. Mater Technol 47:5–12 eid: 2-s2.0-84875258409
Koller M, Maršálek L, de Sousa Dias MM, Braunegg G (2017) Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol 37:24–38. https://doi.org/10.1016/j.nbt.2016.05.001
Kourkoutas Y, Dimitropoulou S, Kanellaki M, Marchant R, Nigam P, Banat IM, Koutinas AA (2002) High-temperature alcoholic fermentation of whey using Kluyveromyces marxianus IMB3 yeast immobilized on delignified cellulosic material. Bioresour Technol 82:177–181. https://doi.org/10.1016/S0960-8524(01)00159-6
Koutinas AA, Athanasiadis I, Bekatorou A, Psarianos C, Kanellaki M, Agouridis N, Blekas G (2007) Kefir-yeast technology: industrial scale-up of alcoholic fermentation of whey, promoted by raisin extracts, using kefir-yeast granular biomass. Enzym Microb Technol 41:576–582. https://doi.org/10.1016/j.enzmictec.2007.05.013
Kumar RS (2015) Development, quality evaluation and shelf life studies of probiotic beverages using whey and Aloe vera juice. J Food Process Technol 6:9. https://doi.org/10.4172/2157-7110.1000486
Kuo CH, Lin PJ, Lee CK (2010) Enzymatic saccharification of dissolution pretreated waste cellulosic fabrics for bacterial cellulose production by Gluconacetobacter xylinus. J Chem Technol Biotechnol 85:1346–1352. https://doi.org/10.1002/jctb.2439
La China S, Zanichelli G, De Vero L, Gullo M (2018) Oxidative fermentations and exopolysaccharides production by acetic acid bacteria: a mini review. Biotechnol Lett 40:1289–1302. https://doi.org/10.1007/s10529-018-2591-7
La China S, Bezzecchi A, Moya F, Petroni G, Di Gregorio S, Gullo M (2020) Genome sequencing and phylogenetic analysis of K1G4: a new Komagataeibacter strain producing bacterial cellulose from different carbon sources. https://doi.org/10.1007/s10529-020-02811-6
Lappa IK, Papadaki A, Kachrimanidou V (2019) Cheese whey processing : integrated biorefinery. Foods 8:347. https://doi.org/10.3390/foods8080347
Lemoigne M (1927) Microbial autolysis and autolytic origin of β-hydroxybutyric acid. Ann Inst Pasteur 41:148–165
Liao C, Yousef AE, Richter ER, Chism GW (1993) Pediococcus acidilactici PO2 bacteriocin production in whey permeate and inhibition of Listeria monocytogenes in foods. J Food Sci 58:430–434. https://doi.org/10.1111/j.1365-2621/1993.tb042291.x
Liu X, Chung YK, Yang ST, Yousef AE (2005) Continuous nisin production in laboratory media and whey permeate by immobilized Lactococcus lactis. Process Biochem 40:13–24. https://doi.org/10.1016/j.procbio.2003.11.032
Liu L, Zhu Y, Li J, Wang M, Lee P, Du G, Chen J (2012) Microbial production of propionic acid from propionibacteria: current state, challenges and perspectives. Crit Rev Biotechnol 32:374–381. https://doi.org/10.3109/07388551.2011.651428
Longanesi L, Frascari D, Spagni C, DeWever H, Pinelli D (2018) Succinic acid production from cheese whey by biofilms of Actinobacillus succinogenes: packe d bed bioreactor tests. J Chem Technol Biotechnol 93:246–256. https://doi.org/10.1002/jctb.5347
Lule VK, Singh R, Pophaly S, Poonam, Tomar SK (2016) Production and structural characterisation of dextran from an indigenous strain of Leuconostoc mesenteroides BA08 in whey. Int J Dairy Technol 69:520–531. https://doi.org/10.1111/1471-0307.12271
Lustrato G, Salimei E, Alfano G, Belli C, Fantuz F, Grazia L, Ranalli G (2013) Cheese whey recycling in traditional dairy food chain: effects of vinegar from whey in dairy cow nutrition. Acetic Acid Bact 2:47. https://doi.org/10.4081/aab.2013.s1.e8
Macedo MG, Lacroix C, Gardner N, Champagne CP (2002) Effect of medium supplementation on exopolysaccharide production by Lactobacillus rhamnosus RW-9595M in a whey permeate. Int Dairy J 12:419–426. https://doi.org/10.1016/S0958-6946(01)00173-X
Magalhães KT, Pereira MA, Nicolau A, Dragone G, Domingues L, Teixeira JA, Batista De Almeida Silva J, Schwan RF (2010) Production of fermented cheese whey-based beverage using kefir grains as starter culture: evaluation of morphological and microbial variations. Bioresour Technol 101:8843–8850. https://doi.org/10.1016/j.biortech.2010.06.083
Mauriello G, Moio L, Moschetti G, Piombino P, Addeo F, Coppola S (2001) Characterization of lactic acid bacteria strains on the basis of neutral volatile compounds produced in whey. J Appl Microbiol 90:928–942. https://doi.org/10.1046/j.1365-2672.2001.01327.x
Méndez-Lorenzo L, Porras-Domínguez JR, Raga-Carbajal E, Olvera C, Rodríguez-Alegría ME, Carrillo-Nava E, Costas M, Munguía AL (2015) Intrinsic levanase activity of Bacillus subtilis 168 levansucrase (SacB). PLoS One 10. https://doi.org/10.1371/journal.pone.0143394
Mills O (1986) Sheep dairying in Britain — a future industry. Int J Dairy Technol 39:88–90. https://doi.org/10.1111/j.1471-0307.1986.tb02378.x
Mollea C, Marmo L, Bosco F (2013) Valorisation of cheese whey, a by-product from the dairy industry. 549-588. https://doi.org/10.5772/53159
Morgan-Sagastume F (2016) Characterisation of open, mixed microbial cultures for polyhydroxyalkanoate (PHA) production. Rev Environ Sci Bio 15:593–625. https://doi.org/10.1007/s11157-016-9411
Nadal I, Rico J, Pérez-Martínez G, Yebra MJ, Monedero V (2009) Diacetyl and acetoin production from whey permeate using engineered Lactobacillus casei. J Ind Microbiol Biotechnol 36:1233–1237. https://doi.org/10.1007/s10295-009-0617-9
Nath A, Verasztó B, Basak S, Koris A, Kovács Z, Vatai G (2016) Synthesis of lactose-derived nutraceuticals from dairy waste whey-a review. Food Bioprocess Technol 9:16–48. https://doi.org/10.1007/s11947-015-1572-2
Niaounakis M (2015) Medical, dental, and pharmaceutical applications. Biopolymers: applications and trends, (1st). Elsevier, pp. 291-405. ISBN: 9780323353991
Niknezhad SV, Asadollahi MA, Zamani A, Biria D, Doostmohammadi M (2015) Optimization of xanthan gum production using cheese whey and response surface methodology. Food Sci Biotechnol 24:453–460. https://doi.org/10.1007/s10068-015-0060-9
O’Brien DJ, Panzer C, Eisele W (1990) Biological production of acrylic acid from cheese whey by resting cells of Clostridium propionicum. Biotechnol Prog 6(4):237–242. https://doi.org/10.1012/bp00004a001
Pagliano G, Ventorino V, Panico A, Romano I, Pirozzi F, Pepe O (2019) Anaerobic process for bioenergy recovery from dairy waste: meta-analysis and enumeration of microbial community related to intermediates production. Front Microbiol 9:3229. https://doi.org/10.3389/fmicb.2018.03229
Pandey A, Srivastava S, Rai P, Duke M (2019) Cheese whey to biohydrogen and useful organic acids: a non-pathogenic microbial treatment by L. acidophilus. Sci Rep 9:8320. https://doi.org/10.1038/s41598-019-42752-3
Panesar PS, Panesar R, Singh RS, Kennedy JF, Kumar H (2006) Microbial production, immobilization and applications of β-D-galactosidase. J Chem Technol Biotechnol 81:530–543. https://doi.org/10.1002/jctb.1453
Parrondo J, Herrero M, García LA, Díaz M (2003) A note – production of vinegar from whey. J Inst Brew 109:356–358. https://doi.org/10.1002/j.2050-0416.2003.tb00610.x
Parrondo J, Garcia LA, Diaz M (2009) Whey vinegar. In: Vinegars of the World. Springer Milan, pp 273–288
Patel S (2015) Emerging trends in nutraceutical applications of whey protein and its derivatives. J Food Sci Technol 52:6847–6858. https://doi.org/10.1007/s13197-015-1894-0
Pescuma M, Hébert EM, Mozzi F, Font De Valdez G, Heert EM, Mozzi F, Font De Valdez G (2008) Whey fermentation by thermophilic lactic acid bacteria: evolution of carbohydrates and protein content. Food Microbiol 25:442–451. https://doi.org/10.1016/j.fm.2008.01.007
Pescuma M, Hébert EM, Bru E, de Valdez GF, Mozzi F (2012) Diversity in growth and protein degradation by dairy relevant lactic acid bacteria species in reconstituted whey. J Dairy Res 79:201–208. https://doi.org/10.1017/S0022029912000040
Pescuma M, Font de Valdez G, Mozzi F (2015) Whey-derived valuable products obtained by microbial fermentation. Appl Microbiol Biotechnol 99:6183–6196. https://doi.org/10.1007/s00253-015-6766-z
Petersen N, Gatenholm P (2011) Bacterial cellulose-based materials and medical devices: current state and perspectives. Appl Microbiol Biotechnol 91:1277–1286. https://doi.org/10.1007/s00253-011-3432-y
Prazeres AR, Carvalho F, Rivas J (2012) Cheese whey management: a review. J Environ Manag 110:48–68. https://doi.org/10.1016/j.jenvman.2012.05.018
Ricciardi A, Zotta T, Ianniello RG, Boscaino F, Matera A, Parente E (2019) Effect of respiratory growth on the metabolite production and stress robustness of Lactobacillus casei N87 cultivated in cheese whey permeate medium. Front Microbiol 10. https://doi.org/10.3389/fmicb.2019.00851
Risner D, Shayevitz A, Haapala K, Meunier-Goddik L, Hughes P (2018) Fermentation and distillation of cheese whey: carbon-dioxide equivalent emissions and water use in the production of whey spirits and white whiskey. J Dairy Sci 101:2963–2973. https://doi.org/10.3168/jds.2017-13774
Risner D, Tomasino E, Hughes P, Meunier-Goddik L (2019) Volatile aroma composition of distillates produced from fermented sweet and acid whey. J Dairy Sci 102:202–210. https://doi.org/10.3168/jds.2018-14737
Roberfroid MB, Van Loo JAE, Gibson GR (1998) The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr 128:11–19. https://doi.org/10.1093/jn/128.1.11
Roohina F, Mohammadi M, Najafpour GD (2016) Immobilized Kluyveromyces marxianus cells in carboxymethyl cellulose for production of ethanol from cheese whey: experimental and kinetic studies. Bioprocess Biosyst Eng 39:1341–1349. https://doi.org/10.1007/s00449-016-1610-0
Sabokbar N, Khodaiyan F (2016) Total phenolic content and antioxidant activities of pomegranate juice and whey based novel beverage fermented by kefir grains. J Food Sci Technol 53:739–747. https://doi.org/10.1007/s13197-015-2029-3
Saeed M, Anjum FM, Khan MR, Khan MI, Nadeem M (2013) Isolation, characterization and utilization of starter cultures for the development of wheyghurt drink. Br Food J 115:1169–1186. https://doi.org/10.1108/BFJ-10-2011-0274
Sansonetti S, Curcio S, Calabrò V, Iorio G (2009) Bio-ethanol production by fermentation of ricotta cheese whey as an effective alternative non-vegetable source. Biomass Bioenergy 33:1687–1692. https://doi.org/10.1016/j.biombioe.2009.09.002
Sansonetti S, Curcio S, Calabrò V, Iorio G (2010) Optimization of ricotta cheese whey (RCW) fermentation by response surface methodology. Bioresour Technol 101:9156–9162. https://doi.org/10.1016/j.biortech.2010.07.030
Sansonetti S, Hobley TJ, Calabrò V, Villadsen J, Sin G (2011) A biochemically structured model for ethanol fermentation by Kluyveromyces marxianus: a batch fermentation and kinetic study. Bioresour Technol 102:7513–7520. https://doi.org/10.1016/j.biortech.2011.05.014
Savvides AL, Katsifas EA, Hatzinikolaou DG, Karagouni AD (2012) Xanthan production by Xanthomonas campestris using whey permeate medium. World J Microbiol Biotechnol 28:2759–2764. https://doi.org/10.1007/s11274-012-1087-1
Shukla M (2012) Development of probiotic beverage from whey and pineapple juice. J Food Process Technol 04:4–7. https://doi.org/10.4172/2157-7110.1000206
Silva e Alves A, Spadoti L, Zacarchenco P, Trento F (2018) Probiotic functional carbonated whey beverages: development and quality evaluation. Beverages 4:49. https://doi.org/10.3390/beverages4030049
Siso MIG (1996) The biotechnological utilization of cheese whey: a review. Bioresour Technol 57:1–11
Skryplonek K (2018) The use of acid whey for the production of yogurt-type fermented beverages. Mljekarstvo 68:139–149. https://doi.org/10.15567/mljekarstvo.2018.0207
Skryplonek K, Jasińska M (2017) Whey-based beverages. Electr J Polish Agr Univ 20(4). https://doi.org/10.30825/5.EJPAU.36.2017.20.4
Sohrabi Z, Eftekhari MH, Eskandari MH, Rezaeianzadeh A, Sagheb MM (2016) Development and characterization of fermented and unfermented whey beverages fortified with vitamin E. J Agric Sci Technol 18:1511–1521
Tamura Y (2000) Manufacture of the vinegar from cheese whey. Milk Sci 49:15–20. https://doi.org/10.11465/milk.49.15
Thakkar P, Vaghela B, Patel A, Modi HA, Prajapati JB (2018) Formulation and shelf life study of a whey-based functional beverage containing orange juice and probiotic organisms. Int Food Res J 25:1675–1681
Thompson DN, Hamilton MA (2001) Production of bacterial cellulose from alternate feedstocks. Appl Biochem Biotechnol 91-93:503–513. https://doi.org/10.1385/ABAB:91-93:1-9:503
Tomaszewska M, Białończyk L (2016) Ethanol production from whey in a bioreactor coupled with direct contact membrane distillation. Catalysis Today. Elsevier, pp 156–163. https://doi.org/10.1016/j.cattod.2016.01.059
Tripathi V, Jha YK (2004) Development of whey beverage with antagonistic characteristics and probiotics. Int J Food Prop 7:261–272. https://doi.org/10.1081/JFP-120030037
Turkmen N, Akal C, Özer B (2019) Probiotic dairy-based beverages: a review. J Funct Foods 53:62–75. https://doi.org/10.1016/j.jff.2018.12.004
Ulerly BD, Nair LS, Laurencin CT (2011) Biomedical applications of biodegradable polymers. J Polym Sci B Polym Phys 49(12):832–864. https://doi.org/10.1002/polb.22259
Umaraw P, Verma A (2015) Comprehensive review on application of edible film on meat and meat products: an eco-friendly approach. Crit Rev Food Sci 57(6):1549–7852. https://doi.org/10.1080/10408398.2014.986563
Utama GL, Kurnani TBA, Sunardi S, Cahyandito MF, Balia RL (2017) Joint cost allocation of cheese-making wastes bioconversions into ethanol and organic liquid fertilizer. Bulg J Agric Sci 23:1016–1020
van Dijken JP, Weusthuis RA, Pronk JT (1993) Kinetics of growth and sugar consumption in yeasts. Antonie Van Leeuwenhoek 63:343–352. https://doi.org/10.1007/BF00871229
Vaningelgem F, Zamfir M, Adriany T, De Vuyst L (2004) Fermentation conditions affecting the bacterial growth and exopolysaccharide production by Streptococcus thermophilus ST 111 in milk-based medium. J Appl Microbiol 97:1257–1273. https://doi.org/10.1111/j.1365-2672.2004.02418.x
Varela JA, Montini N, Scully D, Van der Ploeg R, Oreb M, Boles E, Hirota J, Akada R, Hoshida H, Morrissey JP (2017) Polymorphisms in the LAC12 gene explain lactose utilisation variability in Kluyveromyces marxianus strains. FEMS Yeast Res 17. https://doi.org/10.1093/femsyr/fox021
Vazquez A, Foresti ML, Cerrutti P, Galvagno M (2013) Bacterial cellulose from simple and low cost production media by Gluconacetobacter xylinus. J Polym Environ 21:545–554. https://doi.org/10.1007/s10924-012-0541-3
Ventkiteshwaran K, Bocher B, Maki J, Zitomer D (2015) Relating anaerobic digestion microbial community and process function. Microbiol Insights 8:37–44. https://doi.org/10.4137/MBI.S33593
Verlinden RA, Hill DJ, Kenward MA, Williams CD, Radecka I (2007) Bacterial synthesis of biodegradable polyhydroxyalkanoates. J Appl Microbiol 102:1437–1449. https://doi.org/10.1111/j.1365-2672.2007.03335.x
Virtanen T, Pihlanto A, Akkanen S, Korhonen H (2007) Development of antioxidant activity in milk whey during fermentation with lactic acid bacteria. J Appl Microbiol 102:106–115. https://doi.org/10.1111/j.1365-2672.2006.03072.x
Wan C, Li Y, Shahbazi A, Xiu S (2008) Succinic acid production from cheese whey using Actinobacillus succinogenes 130 Z. Appl Biochem Biotechnol 145:111–119. https://doi.org/10.1007/s12010-007-8031-0
Xie YY, Hu XH, Zhang YW, Wahid F, Chu LQ, Jia SR, Zhong C (2020) Development and antibacterial activities of bacterial cellulose/graphene oxide-CuO nanocomposite films. Carbohydr Polym 229:115456. https://doi.org/10.1016/j.carbpol.2019.115456
Yadav JSS, Yan S, Pilli S, Kumar L, Tyagi RD, Surampalli RY (2015) Cheese whey: a potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol Adv 33:756–774. https://doi.org/10.1016/j.biotechadv.2015.07.002
Yerlikaya O, Akpinar A, Torunoglu FA, Kinik O, Akbulut N, Uysal H (2012) Effect of some prebiotic combination on viability of probiotic bacteria in reconstituted whey and milk beverages. Agro Food Ind Hi Tech 23:XXVII–XXIX
Zisu B, Shah NP (2003) Effects of pH, temperature, supplementation with whey protein concentrate, and adjunct cultures on the production of exopolysaccharides by Streptococcus thermophilus 1275. J Dairy Sci 86:3405–3415. https://doi.org/10.3168/jds.S0022-0302(03)73944-7
Zoellner SS, Cruz AG, Faria JAF, Bolini HMA, Moura MRL, Carvalho LMJ, Sant’ana AS (2009) Whey beverage with acai pulp as a food carrier of probiotic bacteria. Aust J Dairy Technol 64:177–181
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zotta, T., Solieri, L., Iacumin, L. et al. Valorization of cheese whey using microbial fermentations. Appl Microbiol Biotechnol 104, 2749–2764 (2020). https://doi.org/10.1007/s00253-020-10408-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10408-2