Abstract
In the present study, the modulatory effects of bifidobacterial spp. (Bifidobacterium breve NCIM 5671, Bifidobacterium longum NCIM 5672 and Bifidobacterium bifidum NCIM 5697) on adjuvant induced arthritis in rats were evaluated. Arthritis was induced in male Wistar rats by injecting 250 μg of Freund’s adjuvant directly into the paw. Fifteen days before and 15 days after the induction of arthritis, suspended cultures of bifidobacteria (109 cfu/ml) were administered by oral gavage. Paw volume, bone mineral content, oxidative stress markers, antioxidant enzymes, cytokines, eicosanoids and expression of COX2, as well as bone hydrolytic enzymes, were assessed by RT PCR. Although piroxicam-treated groups (drug control) had better effects than bifidobacteria-treated groups, bifidobacteria probiotics administration exhibited significant (P < 0.05) prophylactic effects in terms of downregulating arthritis markers. Parameters including paw volume, bone mineral content, cytokines, and eicosanoids level were significantly (p < 0.05) modulated in bifidobacteria administered groups compared to arthritic control group. Among the three strains tested, B. breve NCIM 5671 exhibited superior prophylactic effects as assessed in the experimental rat model of arthritis. In conclusion, bifidobacteria probiotics administration can downregulate the markers of arthritis and hence can be a potential therapeutic regimen in the treatment of arthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic degenerative disease that affects joints causing permanent disability if not prevented through early treatments. The disease symptoms include joint pain, swellings and movement restrictions that can be generally countered using over the counter anti-inflammatory treatments (Yadav et al. 2016). RA affects 1% of the population, and the aetiology of the disease remains untraced (Firestein and McInnes 2017). Cytokines are known to play a key role in the progress of the disease. Pro-inflammatory cytokines interleukin-1 beta (IL-1β), interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α) and interleukin-17 (IL-17) are some of the major cytokines indicated in the pathogenesis of arthritis. These cytokines activate leukocytes, synovial fibroblasts, the release of other cytokines and chemokines, expression of adhesion molecules, matrix enzymes, osteoclasts, resorption of bone and suppression of T-regulatory (Treg) cells (McInnes et al. 2016). T cells and B cells are also implicated in the pathophysiology of RA. T-helper17 (Th 17) cells are known to secrete IL-17 which promote synovitis. Absence of Treg cells or defective function of Treg cells responsible for controlling and suppressing effector T cells and lymphocytes has also been recognised for the progress of RA (Alunno et al. 2015; Choy 2012). Cure for RA has not yet been identified and the current treatment strategies involve the use of non steroid anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drug (DMARD) and cytokine inhibitors which have several side effects (Grosser et al. 2010; Smolen and Aletaha 2015; Tarp et al. 2017).
Although the exact cause of RA has not been identified, loss of self-tolerance is considered to elicit such immune dysregulation (McInnes et al. 2016). The relation between gut mucosal immune system and microbes is also being considered for the origin of RA (Firestein and McInnes 2017). Several clinical and pre-clinical studies have documented the relationship between RA and the gut microbiome. Infectious aetiology, molecular mimicry, microbial components and metabolites produced have been proposed to increase predisposition to RA (Taneja 2014). Gut commensals are known to maintain homeostasis and are capable of modulating immune system. Any variation or changes in the microbial composition of the gut can alter the immune system and thus lead to autoimmune disorders. Studies have reported gut dysbiosis in case of RA patients wherein counts of Bifidobacterium, Bacteroides, Porphyromonas, Prevotella, Eubacterium rectal and Clostridium coccoides were scarce (Taneja 2014).
Bifidobacteria is one of the predominant gut commensals, known to promote the health of the host (Achi and Halami 2017). Various clinical, in vivo and in vitro studies have documented the health beneficial effects of bifidobacteria in case of diseases such as inflammatory bowel disease, irritable bowel syndrome, cancer, diarrhoea, lactose intolerance, etc. (Lee and O'Sullivan 2010). Studies have reported the anti-inflammatory activity of bifidobacteria. In vivo studies have demonstrated the immune modulating potential of bifidobacteria in gastrointestinal and non-gastrointestinal diseases (Groeger et al. 2013). Bifidobacteria also induce development of reg dendritic cells, Treg cells and Tr1 cells (Fu et al. 2017; Kwon et al. 2010; López et al. 2011; O'Mahony et al. 2008; Shi et al. 2018; Verma et al. 2018; Zheng et al. 2014). Due to their health beneficial effects, they are widely acclaimed as a probiotic. Several studies have reported the preventive and therapeutic effect of probiotic lactobacillus in RA (Amdekar et al. 2013; Kim et al. 2015; Kwon et al. 2010; Lee et al. 2015; So et al. 2008). However, to the best of our knowledge, there are no reports documenting the beneficial effects of bifidobacteria in RA. Hence, in the present study, three strains of bifidobacteria viz. B. breve NCIM 5671, B. longum NCIM 5672 and B. bifidum NCIM 5697 previously characterised for their probiotic properties (Achi and Halami 2019) have been used to evaluate the prophylactic effect in in vivo model of RA.
Materials and methods
Materials
Microbiological media and chemicals were supplied by Hi Media Pvt. Ltd., Mumbai, India. All fine chemicals were purchased from Sigma-Aldrich Inc. Other chemicals used were of AR grade unless otherwise specified. Cytokine ELISA kits IL-1β (Cat no: ab100767), IL-4 (Cat no: ab100770), IL-6 (Cat no: ab119548), IL-10 (Cat no: ab100764) and TNF-α (Cat no: ab46070) were procured from Abcam, Cambridge, U.K; LTB4 (Cat no: 514010), LTC4 (Cat no: 501070) and PGE2 (Cat no: 514010) were purchased from Cayman Chemicals, Michigan USA; Rat MCP-1 (Cat no: 900-M59) was procured from Peprotech, USA. Immunoassay kits for Rf factors and C-reactive protein were acquired from Coral clinical systems-Tulip diagnostics, Goa, India. Freund’s complete adjuvant for induction of arthritis was purchased from Sigma-Aldrich Inc.
Preparation of bifidobacteria cultures
Bifidobacteria strains B. breve NCIM 5671, B. longum NCIM 5672 and B. bifidum NCIM 5697 were cultured in de Man, Rogosa and Sharpe medium supplemented with 0.05% cysteine hydrochloride (MRS-C). Anaerobic conditions for bifidobacteria cultures during incubation were maintained using Anaerogas pack (Hi Media). The cultures were activated from frozen glycerol stock by two successive sub-culturing in MRS-C medium and incubating under anaerobic condition at 37 °C for 24 h. The cultures were then centrifuged and resuspended in skim milk to obtain a count of 108–109 cells/0.5 ml for feeding rats as a probiotic dosage.
Animals and arthritic induction
A total of 48 male Wistar rats (120 ± 5 g) were acquired from animal house facility of CSIR-Central Food Technological Research Institute, Mysuru, India. The animals were housed four per cage under standard conditions with 12-h dark and light cycle at animal house facility. The animals had free access to food and water. All the procedures followed in the study were in accordance with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India guidelines and approved by the Institute Animal Ethical Committee (IAEC No. 387/15). After acclimatisation, the rats were randomly assigned into six groups (n = 8/ group). Groups I, II, III and V received B. breve NCIM 5671, B. longum NCIM 5672, B. bifidum NCIM 5697 and standard anti-inflammatory drug (piroxicam) respectively, groups IV and VI served as arthritic control (AIA) and normal control respectively. The feeding of probiotic strains by oral gavage at abovementioned dosage started 15 days prior to induction of arthritis and continued after arthritis induction for 15 days. Arthritis was induced by injecting (subplantar) 250 μL of complete Freund’s adjuvant into the hind footpad of the rats. The rats were observed for any morphological or physiological changes, and their body weight was recorded weekly. The paw inflammation was examined by measuring the paw volume by mercury displacement method. After the study period, the animals were sacrificed by isoflurane anaesthesia, and blood and tissues were isolated for histopathology analysis.
Faecal microflora analysis
Faecal samples were collected freshly once in a week in sterile containers. The faecal samples were homogenised, serially diluted and plated on BIM25 (for Bifidobacteria), MRS agar (for Lactobacillus), MacConkey’s agar (for E. coli), Clostridium agar (for Clostridia) and Mannitol salt agar (for Staphylococcus). The plates were incubated at 37 °C under anaerobic conditions for Bifidobacteria and Clostridia and aerobic conditions for Escherichia coli, Staphylococcus, and Lactobacillus.
Estimation of arthritis markers in serum
Levels of C-reactive protein (CRP) and rheumatoid factor (RF) in blood are indicative of the inflammation due to rheumatoid arthritis. The CRP and RF levels in serum were measured by turbidimetric method following the instructions provided in the commercial kit.
Measurement of inflammatory markers and cytokines in serum
Prostaglandins (PGE2), leukotrienes (LTB4 and LTC4), interleukin-1 beta (IL-1β), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), tumour necrosis factor (TNF-α) and monocyte chemoattractant protein 1 (MCP-1) were estimated in serum sample by sandwich ELISA per the manufacturer’s recommendation.
Measurement of oxidative stress markers and antioxidant enzyme activity
Serum lipid peroxidation was measured by estimating thiobarbituric acid (TBA) reactivity (Yagi 1984). Nitric oxide (NO) level was determined using Griess reagent (Green et al. 1982). Protein carbonyls were estimated as per the method described by Mesquita et al. (2014). Antioxidant enzymes catalase, glutathione reductase and glutathione peroxidase were measured as per the protocols described by Aebi (1984), Carlberg and Mannervik (1985), Flohé and Günzler (1984) respectively. The protein concentration was determined as per the method described by Lowry et al. (1951).
Radiographic analysis and bone mineral composition
The rats were anaesthetized, and hind limbs were subjected to X-ray examination in the local veterinary hospital. The radiographic images were analysed, and the extent of damage to the bones was scored with the help of a radiologist. The bone sample for mineral analysis was prepared as per the method described by Yadav et al. (2016). Briefly, the joint bones were removed, adherent tissues were separated and bone weight was determined. The bone samples were kept at 180 °C in an oven, overnight for drying. These samples were then kept for charring in a muffle furnace at 440 °C for 6 h followed by cooling to room temperature. One millilitre of deionised water, 2 ml of nitric acid and perchloric acid each were added and then kept on boiling water bath till complete evaporation. Further 10 ml of deionised water was added and filtered through Whatman No. 40 filter paper. The final volume was made to 100 ml using deionised water containing 5% nitric acid in a standard volumetric flask. The samples were suitably diluted and mineral content was determined by using atomic emission spectroscopy (AES).
Quantitative PCR analysis
Expression of cyclooxygenase-2(Cox-2) hydrolytic enzymes collagenase, elastase, hyaluronidase, matrix metalloproteinase 3 and cell receptors ICAM-1, TNF-α and EP-1 were analysed in joint tissue by qPCR. To evaluate the level of transcript, total RNA was isolated from the joint tissue using TRIzol reagent. Reverse transcription to cDNA was performed using iscript reverse transcriptase (Bio-Rad). The synthesised cDNAs were subjected to qPCR using iTaq Universal SYBR Green supermix (Bio-Rad) and primers. The details of the primers are presented in supplementary (Table S1). The amplification was performed using RT PCR system (Thermal cycler CFX96, Bio-Rad). The data was normalised using the expression of GAPDH. The results are presented as relative expression of gene of interest in experimental group compared to that of the control group.
Statistical analysis
Data are expressed as means ± SD, analysed by one-way ANOVA and Sidak’s multiple comparisons test. P of < 0.05 was considered statistically significant.
Results
Faecal microflora analysis
Faecal microflora analysis revealed differences in microbial count of AIA, bifidobacteria and piroxicam fed groups. The counts of Clostridium, coliforms and Staphylococcus had increased in AIA group than the control. Bifidobacteria and Lactobacillus counts were higher in bifidobacteria fed groups whereas counts of Clostridium, coliforms and Staphylococcus were found to be lower compared to the AIA, piroxicam and control groups (Fig. S1).
Arthritis score
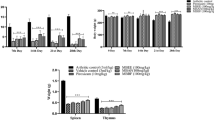
All the rat groups showed symptoms of arthritis upon injection of adjuvant on day 15 with gradual progress in inflammation. However, in case of bifidobacteria-treated groups, the paw volume was found to be lower than the AIA group indicating slower progress of inflammation (Figs. 1a and S2).
Serum arthritis markers
The arthritis markers in serum Rf and CRP were measured. The level of CRP among the adjuvant induced arthritic rats were 5- and 3-fold higher than the control group and the piroxicam group, respectively. However, groups fed with different bifidobacteria had lower CRP levels than the adjuvant induced arthritic rats, with lowest being recorded for B. breve. There was 4.3- and 3-fold increase in Rf levels of adjuvant induced arthritic rats compared to the control and piroxicam administered group, respectively. B. breve fed group had 1.8-fold reduced Rf value than the adjuvant induced arthritic rats whereas 1.5-fold decline was observed in B. longum and B. bifidum treated groups (Fig. 2).
Inflammatory markers and cytokine levels in serum
The effect of bifidobacteria on serum levels of eicosanoids (PGE2, LTB4 and LTC4) and cytokines (IL-1β, IL-4, IL-6, IL-10, TNF-α and MCP-1) was determined. Adjuvant induced arthritic rats showed significantly higher level of PGE2, LTB4 and LTC4 compared to that of control rats. Administration of B. breve, B. longum and B. bifidum demonstrated significant reduction in PGE2, LTB4 and LTC4 levels compared to the arthritic control group. AIA rats showed 6.04-, 6.3-, 13-fold increase in PGE2, LTB4 and LTC4 respectively when compared to control group. However, only 2.7-, 2.1- and 3.9-fold increase in PGE2, LTB4 and LTC4 respectively were observed for group treated with B. breve NCIM 5671.
The levels of pro-inflammatory cytokines IL-1β, IL-6 and MCP-1 increased significantly in arthritic rats compared to control rats. The piroxicam and bifidobacteria-treated groups displayed significant reduction in these cytokines compared to arthritic group with the former being comparatively better. Among the bifidobacteria-treated groups, group I treated with B. breve had shown higher reduction with 43%, 33%, 56% and 33% in levels of pro-inflammatory cytokines IL-1β, IL-6, TNF-α and MCP-1, respectively compared to diseased group. Anti-inflammatory cytokine levels were significantly lower in case of arthritic rats compared to the control group. However, piroxicam-treated groups and bifidobacteria-treated groups had higher levels of cytokine compared to the arthritic rats. Group fed with B. breve, B. longum and B. bifidum had demonstrated 62%, 36% and 39% increase in case of IL-10 respectively and 56%, 49% and 50% increase in case of IL-4 respectively when evaluated against AIA group (Fig. 3).
Effect of bifidobacterial probiotic administration on inflammatory cytokine and eicosanoid levels in serum. Pro-inflammatory cytokines:IL-1B, TNF-α, IL-6, MCP-1. Anti-inflammatory cytokines: IL-4, IL-10. Eicosanoids: PGE2, LTB4, LTC4. Data shown are the means ± SD; “*, **, ***, ****” are significantly different at P < 0.05, P < 0.01, P < 0.001, P < 0.0001 respectively compared to the AIA control rats
Measurement of oxidative stress markers and antioxidant enzyme activity
Serum levels of lipid peroxide were determined and AIA group exhibited 2.8-fold increase compared to control groups. However, an increase of 2.19-, 2.47-, 2.45-, and 1.6-folds were observed in B. breve, B. longum, B. bifidum and piroxicam fed groups, respectively. The levels of protein carbonyls in serum were found to be significantly altered in AIA group compared to control. B. breve fed group showed 1.45-fold increase whereas AIA group had 2.34-fold increase. Elevated levels of NO were observed in AIA group, wherein an increase of 4.44-fold was observed compared to control. Feeding bifidobacteria reduced the levels of NO and increase of 2.5-, 3.8- and 3.6-folds were detected in B. breve, B. longum and B. bifidum groups which were lower than the arthritic group (Table 1).
Activities of antioxidant enzymes catalase, glutathione peroxidase and glutathione reductase were measured in serum. There was a decrease of 52.7%, 31.19% and 50% in activity of catalase, glutathione peroxidase and glutathione reductase respectively in AIA groups compared to control. B. breve-fed groups demonstrated improvement in activity with an increase of 65.92%, 18.60% and 88.19% in activity of catalase, glutathione peroxidase and glutathione reductase respectively, compared to arthritic rats (Table 2).
Radiographic analysis and bone mineral composition
Radiographic analysis of arthritic rats clearly revealed destruction of bone in AIA group. However, such effect was not observed in case of bifidobacteria-fed groups as well as the piroxicam-treated group (Fig. 1b). The mineral compositions of the joints were determined using AES. A significant decrease in calcium content was detected in case of AIA group compared to the control. The groups treated with bifidobacteria and piroxicam had higher calcium levels compared to AIA. No significant difference was observed in the levels of potassium among the groups whereas significant decline in the levels of magnesium was observed for AIA group compared to the control (Table 3).
Quantitative PCR analysis
The effect of bifidobacteria on cycloxygenase was monitored by analysing the expression levels of COX2 in synovial tissue. The groups treated with bifidobacteria and piroxicam had reduced expression levels of COX2 compared to the arthritic rats. The expression levels of arthritic rats was found to be fivefold higher than the control group. The expression levels of cytokine receptors TNF-α R, ICAM1 and prostaglandin receptor EP1 in synovial tissue were also evaluated. AIA groups showed 30-fold increase in TNF-α R and 2-fold increase in expression levels of both ICAM1 and EP1 compared to the control, whereas the probiotic-treated groups and piroxicam-treated groups had decreased expression levels.
The activities of major bone hydrolytic enzymes were determined by examining their expression levels in synovial tissue. AIA group had higher levels of collagenase (MMP2 14-fold, MMP3 3.76-fold and MMP13 6.76-fold) compared to control group. However, the bifidobacteria fed groups had lower expression levels of collagenase than arthritic group with B. breve fed groups showing the lowest expression levels (MMP2 2.4-fold, MMP3 0.11-fold and MMP13 0.08-fold). Expression levels of hyaluronidase and elastase were also higher in case of AIA group (hyal1 1.57-fold and elan 5.2-fold) compared to the control group. The genes for hyal1 and elan was found to be downregulated in case of B. breve-fed groups whereas B. longum and B. bifidum-fed groups had lower expression levels of hyal1 and elan than the AIA group (Fig. 4). The qPCR results were additionally validated by visualising the reaction products on gel (Fig. S3).
Effect of bifidobacterial probiotic administration on mRNA expression levels in joint tissue: Cyclooxygenase: Cox2; receptors for inflammatory mediators: EP-1, TNF-αR, ICAM; bone hydrolytic enzymes: Elan, Hyal, MMP2, MMP3, MMP13. Data shown are the means ± SD; “*, **, ***, ****” are significantly different at P < 0.05, P < 0.01, P < 0.001, P < 0.0001 respectively compared to the AIA control rats
Discussion
Bifidobacteria positively contribute to the health of the host, and decline in counts of these bacteria are often co-related with various diseases. Hence, they are exploited as a probiotic for their functional properties. However, these properties of probiotic are highly species and strain specific. In the present study, three different species of probiotic bifidobacteria, B. breve NCIM 5671, B. longum NCIM 5672 and B. bifidum NCIM 5697 were evaluated for the prophylactic effect against chronic inflammatory disease, rheumatoid arthritis in rat model. The rats were fed with probiotic bifidobacteria throughout the study period. A gradual increase in the counts of bifidobacteria was observed in the faecal samples collected from the groups administered with bifidobacteria signifying the colonisation of these bacteria. The increase in count of bifidobacteria has also been associated with increased lactic acid bacteria count which was observed in bifidobacteria-fed groups. The counts of pathogenic microbes were found to decline in the bifidobacteria-fed groups which might be due to the various metabolites produced, antimicrobial activity, competition for adherence site, etc. by lactobacilli and bifidobacteria.
Oral gavaging of AIA rats with bifidobacteria probiotics reduced the symptoms of arthritis which was evident with the arthritic score of the paws. The increased levels of free radicals generated during RA are known to induce oxidative stress which may affect the tissue proteins and lipids modifying them, initiating synovial inflammation and articular degeneration (Abbas and Monireh 2008). Hence, the ability of probiotic bifidobacteria for reducing the levels of oxidative stress markers was evaluated. Lipid peroxidation occurs due to oxidative stress and is known to induce tissue damage by altering membrane fluidity, permeability and activity (Quiñonez-Flores et al. 2016). The high oxidative stress in RA results in protein carbonyl formation which is considered as a significant biomarker (Datta et al. 2014). The decreased levels of lipid peroxides, nitric oxides and protein carbonyls in bifidobacteria-treated groups than the AIA are indicative of reduced oxidative stress in these groups. Several studies have documented the antioxidant potential and the ability of bifidobacteria in controlling the oxidative stress (Awney 2011; Shen et al. 2011; Xu et al. 2011).
Antioxidants are known to scavenge free radicals and counter the oxidative damage induced during arthritis. Enzymes such as glutathione peroxidase, glutathione reductase and catalase are known for antioxidant response (Quiñonez-Flores et al. 2016). Hence, the antioxidant activity of these enzymes in serum was evaluated. In the case of AIA group, the activities of these enzymes were found to be affected and decreased. Feeding bifidobacteria was found to improve the activities of these enzymes. Bifidobacteria have been reported to enhance the activities of antioxidant enzymes (Shen et al. 2011; Xu et al. 2011).
Cytokines play an important role in the pathophysiology of RA. They are implicated in the progression of inflammatory synovitis and destruction of joint tissues. Pro-inflammatory cytokines IL-1β, IL-6, TNF-α and MCP-1 are known to increase the production of various other chemokines, cytokines, antibodies, eicosanoids, reactive oxygen intermediates, metalloproteinases and cell adhesion molecules, as well as activate various other immune cells which help in progress of RA (McInnes and Schett 2011). The rats treated with bifidobacteria had lower levels of pro-inflammatory cytokines. Absence or low levels of anti-inflammatory cytokines IL-4 and IL-10 have also been established in cases of RA, and similar results were observed in case of AIA group. However, the levels of IL-4 and IL-10 were restored in case of bifidobacteria fed groups. IL-10 is known to inhibit the activation of effector T cells and dendritic cells, decrease metalloproteinases and control the progress of inflammatory response (McInnes and Schett 2007). Probiotics and commensal bacteria are known to induce reg dendritic cells and Treg cells which may secrete regulatory cytokines and thus control the progress of inflammation (Kwon et al. 2010; Marietta et al. 2016).
Eicosanoids are inflammatory mediators that play a major role in pathogenesis of RA. PGE2 is known to be related with edema, joint bone and cartilage destruction (McCoy et al. 2002) and expression of cytokines. LTB4 is held responsible for recruitment of leukocytes to the joint due to its chemotactic properties and can act as suppressor of Treg cells (Korotkova and Jakobsson 2014). LTB4 and LTC4 are produced by 5-lipoxygenase pathway. The serum levels LTB4 and LTC4 were found to be significantly reduced in bifidobacteria-treated groups. PGE2 is produced by COX2 pathway (Korotkova and Jakobsson 2014), the expression levels of COX2 in synovial tissues were found to be lowered in case of bifidobacteria-fed groups. Consequently, reduction in the levels of PGE2 produced was observed. Cell adhesion molecules such as ICAM1 are known to be involved in trafficking of leukocytes to synovium and retain them in the tissue by anchoring them to extracellular matrix (Brown et al. 2016; Sweeney and Firestein 2004). The expression levels of ICAM are found to be elevated in case of RA and are controlled by cytokines IL-1 and TNF-α (Sweeney and Firestein 2004). In our studies, the expression of ICAM1 was downregulated in case of probiotic-treated groups.
Matrix metalloproteinases, MMP2, MMP3 and MMP13, are incriminated for degrading aggrecan, collagen of joint cartilage and thus promoting joint destruction in RA (Mohammed et al. 2003). An imbalance of pro-inflammatory to anti-inflammatory cytokines has been reported to be the cause with IL-1β and TNF-α being the major pro-inflammatory cytokines responsible for the induction and secretion of these enzymes (Mohammed et al. 2003). The expression levels of MMP2 were decreased and repressed in case of MMP3 and MMP13 among the bifidobacteria-treated groups. Similarly, hyaluronidase and elastase act on their substrates and degrades the joint. The expression levels of hyal1 and elan were downregulated and decreased in bifidobacteria-fed groups. The decrease in expression levels of these enzymes can be attributed to the lower levels of pro-inflammatory cytokines in the bifidobacteria-administered groups. Bifidobacteria have also been reported to improve bone mass density and prevent bone loss by influencing the expression of sparc and bmp-2 genes in ovariectomized mice (Parvaneh et al. 2015). Although these genes have not been included in the current study, such an effect cannot be neglected and may possibly be responsible for the improvement observed in the joint bones of bifidobacteria-administered rats.
The drug control (piroxicam treated rats) was found to be better when compared to bifidobacteria-fed rats in downregulating the symptoms of arthritis. Similar results have also been observed by Lee et al. (2015) in case of collagen II-induced arthritis in DMA/1J mice treated with ibuprofen and probiotic strain Lactobacillus sakei OK67. Lactobacillus strains with comparable or better effects than drug control (Indomethacin) have also been documented by Amdekar et al. (2011, 2013) respectively in Wistar rats with collagen-induced arthritis. Based on the arthritis model, drug and the probiotic strains used considerable variation has been observed in the results; hence, a direct correlation is not possible. Although synthetic drugs (NSAIDs) may offer better protection, the side effects associated with it serve as a limitation. Hence, probiotics can serve as an alternative natural remedy in the prevention/treatment of such diseases.
In conclusion, we have demonstrated that bifidobacteria are capable of reducing the severity and progress of arthritis and the effects are strain dependent. Our study suggests that among the bifidobacterial species studied, B. breve NCIM 5671 had better anti-arthritic effects in the rat model. Such strains should be explored further for their positive effects in treatment of rheumatoid arthritis.
References
Abbas M, Monireh M (2008) The role of reactive oxygen species in immunopathogenesis of rheumatoid arthritis. Iran J Allergy Asthma Immunol 7(4):195–202 doi: 07.04/ijaai.195202
Achi SC, Halami PM (2017) Bifidobacterial probiotics through fermented foods. In: Kalia VC, Shouche Y, Purohit HJ, Rahi P (eds) Mining of Microbial Wealth and MetaGenomics. Springer, pp 267–285. https://doi.org/10.1007/978-981-10-5708-3_16
Achi SC, Halami PM (2019) In vitro comparative analysis of probiotic and functional attributes of indigenous isolates of bifidobacteria. Curr Microbiol 76:1–8. https://doi.org/10.1007/s00284-018-1615-9
Aebi H (1984) Catalase in vitro. In: Packer L (ed) Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Alunno A, Manetti M, Caterbi S, Ibba-Manneschi L, Bistoni O, Bartoloni E, Valentini V, Terenzi R, Gerli R (2015) Altered immunoregulation in rheumatoid arthritis: the role of regulatory T cells and proinflammatory Th17 cells and therapeutic implications. Mediat Inflamm 2015:1–12. https://doi.org/10.1155/2015/751793
Amdekar S, Singh V, Singh R, Sharma P, Keshav P, Kumar A (2011) Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines. J Clin Immunol 31(2):147–154. https://doi.org/10.1007/s10875-010-9457-7
Amdekar S, Singh V, Kumar A, Sharma P, Singh R (2013) Lactobacillus casei and Lactobacillus acidophilus regulate inflammatory pathway and improve antioxidant status in collagen-induced arthritic rats. J Interf Cytokine Res 33(1):1–8. https://doi.org/10.1089/jir.2012.0034
Awney HA (2011) The effects of Bifidobacteria on the lipid profile and oxidative stress biomarkers of male rats fed thermally oxidized soybean oil. Biomarkers 16(5):445–452. https://doi.org/10.3109/1354750X.2011.590228
Brown PM, Pratt AG, Isaacs JD (2016) Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat Rev Rheumatol 12(12):731–742. https://doi.org/10.1038/nrrheum.2016.175
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490. https://doi.org/10.1016/S0076-6879(85)13062-4
Choy E (2012) Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 51(suppl_5):v3–v11. https://doi.org/10.1093/rheumatology/kes113
Datta S, Kundu S, Ghosh P, De S, Ghosh A, Chatterjee M (2014) Correlation of oxidant status with oxidative tissue damage in patients with rheumatoid arthritis. Clin Rheumatol 33(11):1557–1564. https://doi.org/10.1007/s10067-014-2597-z
Firestein GS, McInnes IB (2017) Immunopathogenesis of rheumatoid arthritis. Immunity 46(2):183–196. https://doi.org/10.1016/j.immuni.2017.02.006
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–120. https://doi.org/10.1007/s10067-014-2597-z
Fu L, Song J, Wang C, Fu S, Wang Y (2017) Bifidobacterium infantis potentially alleviates shrimp tropomyosin-induced allergy by tolerogenic dendritic cell-dependent induction of regulatory T cells and alterations in gut microbiota. Front Immunol 8:1536. https://doi.org/10.3389/fimmu.2017.01536
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126(1):131–138. https://doi.org/10.1016/0003-2697(82)90118-X
Groeger D, O’Mahony L, Murphy EF, Bourke JF, Dinan TG, Kiely B, Shanahan F, Quigley EM (2013) Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 4(4):325–339. https://doi.org/10.4161/gmic.25487
Grosser T, Yu Y, FitzGerald GA (2010) Emotion recollected in tranquility: lessons learned from the COX-2 saga. Annu Rev Med 61:17–33. https://doi.org/10.1146/annurev-med-011209-153129
Kim J-E, Chae CS, Kim G-C, Hwang W, J-s H, Hwang S-M, Kim Y, Ahn Y-T, Park S-G, Jun C-D (2015) Lactobacillus helveticus suppresses experimental rheumatoid arthritis by reducing inflammatory T cell responses. J Funct Foods 13:350–362. https://doi.org/10.1016/j.jff.2015.01.002
Korotkova M, Jakobsson P-J (2014) Persisting eicosanoid pathways in rheumatic diseases. Nat Rev Rheumatol 10(4):229–241. https://doi.org/10.1038/nrrheum.2014.1
Kwon H-K, Lee C-G, So J-S, Chae C-S, Hwang J-S, Sahoo A, Nam JH, Rhee JH, Hwang K-C, Im S-H (2010) Generation of regulatory dendritic cells and CD4+ Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci 107(5):2159–2164. https://doi.org/10.1073/pnas.0904055107
Lee JH, O'Sullivan DJ (2010) Genomic insights into bifidobacteria. Microbiol Mol Biol Rev 74(3):378–416. https://doi.org/10.1128/MMBR.00004-10
Lee S-Y, Jeong J-J, Kim K-A, Kim D-H (2015) Lactobacillus sakei OK67 ameliorates collagen-induced arthritis in mice by inhibiting NF-κB activation and restoring Th17/Treg cell balance. J Funct Foods 18:501–511. https://doi.org/10.10106/j.jff.2015.08.006
López P, González-Rodríguez I, Gueimonde M, Margolles A, Suárez A (2011) Immune response to Bifidobacterium bifidum strains support Treg/Th17 plasticity. PLoS One 6(9):e24776. https://doi.org/10.1371/journal.pone.0024776
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Marietta EV, Murray JA, Luckey DH, Jeraldo PR, Lamba A, Patel R, Luthra HS, Mangalam A, Taneja V (2016) Suppression of inflammatory arthritis by human gut-derived Prevotella histicola in humanized mice. Arthritis Rheum 68(12):2878–2888. https://doi.org/10.1002/art.39785
McCoy JM, Wicks JR, Audoly LP (2002) The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest 110(5):651–658. https://doi.org/10.1172/JCI15528
McInnes IB, Schett G (2007) Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 7(6):429–442. https://doi.org/10.1038/nri2094
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. New Engl J Med 365(23):2205–2219. https://doi.org/10.1056/NEJMra1004965
McInnes IB, Buckley CD, Isaacs JD (2016) Cytokines in rheumatoid arthritis—shaping the immunological landscape. Nat Rev Rheumatol 12(1):63–68. https://doi.org/10.1038/nrrheum.2015
Mesquita CS, Oliveira R, Bento F, Geraldo D, Rodrigues JV, Marcos JC (2014) Simplified 2, 4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal Biochem 458:69–71. https://doi.org/10.1016/j.ab.2014.04.034
Mohammed F, Smookler D, Khokha R (2003) Metalloproteinases, inflammation, and rheumatoid arthritis. Ann Rheum Dis 62(suppl 2):ii43–ii47. https://doi.org/10.1136/ard.62.suppl_2.ii43
O'Mahony C, Scully P, O'Mahony D, Murphy S, O'Brien F, Lyons A, Sherlock G, MacSharry J, Kiely B, Shanahan F (2008) Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-κB activation. PLoS Pathog 4(8):e1000112. https://doi.org/10.1371/journal.ppat.1000112
Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei ANM, Abdul-Majeed S, Ahmad Z, Ibrahim Z, Jamaluddin R (2015) Probiotics (Bifidobacterium longum) increase bone mass density and upregulate sparc and bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int 2015:1–10. https://doi.org/10.1155/2015/897639
Quiñonez-Flores CM, González-Chávez SA, Del Río Nájera D, Pacheco-Tena C (2016) Oxidative stress relevance in the pathogenesis of the rheumatoid arthritis: a systematic review. Biomed Res Int 2016:1–14. https://doi.org/10.1155/2016/6097417
Shen Q, Shang N, Li P (2011) In vitro and in vivo antioxidant activity of Bifidobacterium animalis 01 isolated from centenarians. Curr Microbiol 62(4):1097–1103. https://doi.org/10.1007/s00284-010-9827-7
Shi P, Qu H, Nian D, Chen Y, Liu X, Li Q, Li Q, Wang C, Ye M, Ma B (2018) Treatment of Guillain-Barré syndrome with Bifidobacterium infantis through regulation of T helper cells subsets. Int Immunopharmacol 61:290–296. https://doi.org/10.1016/j.intimp.2018.06.015
Smolen JS, Aletaha D (2015) Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol 11(5):276–289. https://doi.org/10.1038/nrrheum.2015.8
So J-S, Kwon H-K, Lee C-G, Yi H-J, Park J-A, Lim S-Y, Hwang K-C, Jeon YH, Im S-H (2008) Lactobacillus casei suppresses experimental arthritis by down-regulating T helper 1 effector functions. Mol Immunol 45(9):2690–2699. https://doi.org/10.1016/j.molimm.2007.12.010
Sweeney SE, Firestein GS (2004) Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol 36(3):372–378. https://doi.org/10.1016/S1357-2725(03)00259-0
Taneja V (2014) Arthritis susceptibility and the gut microbiome. FEBS Lett 588(22):4244–4249. https://doi.org/10.1016/j.febslet.2014.05.034
Tarp S, Eric Furst D, Boers M, Luta G, Bliddal H, Tarp U, Heller Asmussen K, Brock B, Dossing A, Schjødt Jørgensen T (2017) Risk of serious adverse effects of biological and targeted drugs in patients with rheumatoid arthritis: a systematic review meta-analysis. Rheumatology 56(3):417–425. https://doi.org/10.1093/rheumatology/kew442
Verma R, Lee C, Jeun E-J, Yi J, Kim KS, Ghosh A, Byun S, Lee C-G, Kang H-J, Kim G-C (2018) Cell surface polysaccharides of Bifidobacterium bifidum induce the generation of Foxp3+ regulatory T cells. Sci Immunol 3(28):eaat6975. https://doi.org/10.1126/sciimmunol.aat6975
Xu R, Shang N, Li P (2011) In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe 17(5):226–231. https://doi.org/10.1016/j.anaerobe.2011.07.010
Yadav NV, Ramaiyan B, Acharya P, Belur L, Talahalli RR (2016) Sesame oil and Rice bran oil ameliorates adjuvant-induced arthritis in rats: distinguishing the role of minor components and fatty acids. Lipids 51(12):1385–1395. https://doi.org/10.1007/s11745-016-4203-4
Yagi K (1984) Assays of glutathione peroxidase. Methods Enzymol 105:328–331. https://doi.org/10.1016/S0076-6879(84)05042-4
Zheng B, van Bergenhenegouwen J, Overbeek S, van de Kant HJ, Garssen J, Folkerts G, Vos P, Morgan ME, Kraneveld AD (2014) Bifidobacterium breve attenuates murine dextran sodium sulfate-induced colitis and increases regulatory T cell responses. PLoS One 9(5):e95441. https://doi.org/10.3920/bm2015.0037
Acknowledgements
SA would like to acknowledge CSIR for granting Senior Research Fellowship. We would like to thank Dharna J and Chris Cherita for their assistance during the animal studies.
Funding
This work was funded by Council of Scientific and Industrial Research (CSIR), New Delhi, under XIIth 5-year plan project (BSC0202). The Director CSIR-Central Food Technological Research Institute (CFTRI), Mysuru, India, provided the necessary the funds and facilities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines and approved by the ethical committee of the institute.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 632 kb)
Rights and permissions
About this article
Cite this article
Achi, S.C., Talahalli, R.R. & Halami, P.M. Prophylactic effects of probiotic Bifidobacterium spp. in the resolution of inflammation in arthritic rats. Appl Microbiol Biotechnol 103, 6287–6296 (2019). https://doi.org/10.1007/s00253-019-09864-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09864-2