Abstract
Rheumatoid arthritis (RA) is a debilitating autoimmune disease whose etiology remains unknown, but studies have consistently implicated a plethora of inflammatory mechanisms culminating in chronic symmetric and erosive synovitis. Importantly, reactive oxygen species (ROS) have been attributed to directly contribute towards the destructive, proliferative synovitis evident in RA. Accordingly, this study aimed to establish whether the degree of oxidative stress and disease activity score (DAS28) correlated with the downstream effects of oxidative damage. The redox status of neutrophils sourced from synovial fluid (SF) was measured by flow cytometry in terms of total ROS and hydroxyl radicals. Among the molecular damage markers, protein carbonylation and lipid peroxidation were detected by spectrophotometry and S-nitrosothiols by fluorimetry. Neutrophils constituted the major cellular component of the SF of patients with RA and their levels of ROS and hydroxyl radicals correlated strongly with protein carbonylation and lipid peroxidation. However, all the oxidative damage markers correlated positively with DAS28. Taken together, in patients with RA, the strong correlation between levels of ROS and DAS28 with markers of oxidative damage suggests that measurement of oxidative stress could serve as a biomarker for monitoring disease severity in RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
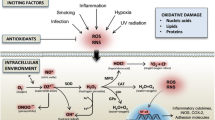
Rheumatoid arthritis (RA) is a debilitating autoimmune disease of global prevalence whose etiology is basically unknown. Several studies have implicated a combination of genetic factors and environmental triggers, and current literature indicates that a host of inflammatory mechanisms including oxidative stress contributes towards disease pathogenesis [1]. It has been proposed that along with inflammation, there is activation of neutrophils, macrophages, and lymphocytes which extravasate from blood vessels into the inflammatory area leading to increased secretion of proinflammatory mediators that include kinins, reactive oxygen, and nitrogen species (ROS and RNS), eicosanoids, proinflammatory cytokines (IL-12, TNF-α, IFN-γ, IL-6, and IL-1β) and proinflammatory enzymes that mediate production of prostaglandins (PGE2) and leukotrienes (e.g., lipooxygenase), together with expression of adhesion molecules, matrix metalloproteases (MMPs), and hyperproliferation of synovial fibroblasts [2]. All these factors are regulated by activation of the transcription factor NF-κβ and Iκβ kinase (IKK)s which maintain the cellular oxidative stress [3].
Biomarkers of oxidative stress have helped to explore the relation between oxidative damage to macromolecules (proteins, lipids, and DNA) and disease progression [4]. Increased production of superoxide and hydrogen peroxide initiates the proinflammatory events in RA [1] along with peroxynitrite that mediates many cytotoxic effects that impact upon cellular thiols, lipids, proteins, and DNA [1, 5]. Although these reactive species directly contribute towards the destructive, proliferative synovitis evident in RA [2], studies correlating the degree of oxidative stress with downstream effects of oxidative damage in synovial joints have not been undertaken. Accordingly, this study aimed to establish this correlation, if any, between pro-oxidants generated in the synovial joint and markers of oxidative damage, with a view to facilitate monitoring of disease progression in patients with RA.
Materials and methods
Reagents
All chemicals were obtained from Sigma Aldrich (St. Louis, MO, USA) except 3′-p-hydroxyphenyl fluorescein (HPF) from Molecular Probes (Carlsbad, CA, USA), thiobarbituric acid (TBA), ammonium sulfamate, 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB), trichloroacetic acid (TCA) from Sisco Research Laboratories (Mumbai, India), cell lysis buffer from Cell Signaling Technology (Danvers, MA, USA), enzyme-linked immunosorbent assay (ELISA) kits for TNF-α, IL-6, and IL-8 (Immunotools, Friesoythe, Germany), and 2,2′-azino-bis[3-ethylbenzthiazoline-6-sulfonic acid] (ABTS) (Roche Applied Science, Penzberg, Germany).
Study population
The study population included patients with RA who fulfilled the American College of Rheumatology criteria for RA [6, 7]. They were recruited from the outpatient unit of the Rheumatology clinic at Seth Sukhlal Karnani Memorial Hospital, Kolkata and were unresponsive to both DMARDs and NSAIDs. Patients with psoriatic arthritis, scleroderma, reactive arthritis, viral polyarthritis, and systemic lupus erythematosus were excluded as were patients with RA having renal and/or hepatic impairment as also if they were receiving steroids or any antioxidants. All knee joints demonstrated signs of active synovitis at the time of aspiration; from each patient, synovial fluid was collected. The study protocol received prior approval from the Institutional Ethics Committee, and informed consent was obtained from all participants.
Disease activity assessment
Disease activity status was determined using disease activity score evaluating 28 joints, erythrocyte sedimentation rate (ESR), and visual analog score [8]. According to the American College of Rheumatology (ACR) criteria [6, 7], patients were classified according to their disease activity into four different groups, namely, (1) group 1: DAS28 < 2.6 (remission); (2) group 2: 2.6 ≤ DAS28 ≤ 3.2 (low disease activity); (3) group 3: 3.2 < DAS28 ≤ 5.1 (moderate disease activity); and (4) group 4: DAS28 > 5.1 (high disease activity). Additionally, C-reactive protein (CRP) and ESR were measured using commercially available kits.
Isolation of cellular components in the synovial fluid
Synovial fluid from the knee joint was collected by aspiration; following centrifugation (300×g, 15 min), the resultant pellet was considered as the cellular infiltrate and is stated as “synovial cells” along with the supernatant, stated as “cell-free fraction” (stored at −20 °C). The cell pellets were washed twice with RPMI 1640 PR− medium (250×g for 5 min) and cell viability (>85 %) confirmed by trypan blue exclusion. Neutrophils were gated and acquired in a flow cytometer.
Generation of free radicals
Intracellular ROS was measured using a membrane-permeable compound 2′, 7′,-dichlorodihydrofluorescein diacetate (H2DCFDA), a nonfluorescent dye which upon enzymatic hydrolysis by esterases and oxidation by intracellular ROS produces a fluorescent compound dichlorofluorescein (DCF) [9]. Therefore, the DCF-based fluorescence is directly proportional to the amount of intracellular ROS.
Synovial cells (1 × 106/ml) were preloaded with H2DCFDA (50 μM, 30 min, 37 °C); after two washes with phosphate buffered saline (PBS, 0.02 M, pH 7.2), cells were resuspended in PBS and acquired in a flow cytometer [10]. For hydroxyl radicals, synovial cells (1 × 106/ml) were incubated in the dark with HPF (2.5 μM, 20 min, 37 °C), and acquired in a flow cytometer [10].
Preparation of cellular lysate
Synovial cells (1 × 106/ml) were lysed in a cell lysis buffer (as per manufacturer’s instructions) followed by three freeze thaw cycles using liquid nitrogen; after centrifugation (9,000×g, 10 min, 4 °C), the cell lysate was collected and protein was estimated [11].
Measurement of advanced oxidation of protein products
Advanced oxidation of protein products (AOPP) were assayed by spectrophotometry [12]; briefly, in 96well plates, 200 μl cell-free fraction (diluted 1:20 in PBS) was added followed by acetic acid (20 μl) and potassium iodide (1.16 M, 10 μl). In standard wells, potassium iodide (1.16 M, 10 μl) was added to chloramine-T (0–100 μmol/l, 200 μl) and acetic acid (20 μl). For calibration, chloramine-T (0–100 μmol/l) was used, and absorbances were immediately read at 340 nm on a spectrofluorometer (Spectromax, M2/M2e Multimode plate reader, Molecular Devices, Sunnyvale, CA, USA). AOPP concentrations were expressed as micromoles per liter chloramine-T equivalents.
Measurement of protein carbonylation
To obtain the degree of protein carbonylation, derivatization was initially done with 2, 4-dinitrophenyl-hydrazine (DNPH) [13, 14]. Briefly, protein lysates from synovial cells (50 μg) were incubated with DNPH (10 mM in 2 N HCl, 100 μl for 30 min in dark, 20–25 °C); proteins were precipitated with TCA (20 %, 100 μl) and free DNPH was removed by washing with ethanol-ethyl acetate (1:1). The resultant pellet was dissolved in 350 μl of sodium dodecyl sulfate (2 % SDS) and absorbances of the protein-hydrazone complex measured at 370 nm. The carbonyl concentration was calculated from the specific absorption (relative to the reagent blank), the extinction coefficient of the protein-hydrazone complex being 22,000 M−1 cm−1 and expressed as nanomoles of carbonyl groups per milligram protein [15].
Measurement of S-nitrosothiols
Levels of S-nitrosothiols were measured in the cell-free fraction of patients with RA [16]. Briefly, the cell-free fraction (50 μl) was diluted 1:1 in H2O and incubated with ammonium sulfamate (50 μl, 100 μM in H2O, 10 min) in order to trap the NO2− present. This was followed by addition of a 2, 3-diaminonaphthalene (DAN) reaction mixture containing HgCl2 (1.11 mM) and DAN (158 μM in 0.62 N HCl) in a ratio of 1:4; after incubation at room temperature in the dark for 10 min, the pH was then adjusted to 11.5–12.0 with 1 N NaOH, and fluorescence measured in a spectrofluorometer, excitation/emission wavelengths being 360 and 450 nm, respectively. The standard curve was prepared by incubating 50 μl GSH (0–10 μM in 1 N HCl) with 50 μl NaNO2 (10 μM in H2O) for 15 min at room temperature; this reaction mixture was then incubated with 50 μl ammonium sulfamate (100 μM in H2O), followed by addition of the DAN reaction mixture (50 μl) and processed as described above; results were expressed as micromolars.
Measurement of lipid peroxidation
Lipid peroxidation was measured in terms of malonaldehyde (MDA) in the cell-free fraction using the modified method of Beuge and Aust [17, 18]. Briefly, one volume of cell-free fraction was mixed thoroughly with two volumes of 15 % w/v TCA, 0.375 % w/v TBA, and 0.25 N HCl. The mixture was incubated at 100 °C for 30 min in a shaking water bath; it was then cooled and the flocculent precipitate was removed by centrifugation (1,000×g for 10 min, 20–25 °C). The absorbances were determined at 535 nm and concentration of thiobarbituric acid reactive substances (TBARS) calculated using 1.56 × 105 M−1 cm−1 as molar extinction coefficient of MDA.
Measurement of cytokines in synovial fluid
Levels of TNF-α, IL-6, and IL-8 were determined in the cell-free fraction by ELISA as per the manufacturer’s instructions. Briefly, individual cytokine antibodies were coated on ELISA plates; nonspecific binding sites were blocked with PBS-bovine serum albumin; cytokine standards (TNF-α/IL-6/IL-8) or serum samples were added to designated wells (100 μl/well) and after being incubated for 1 h at room temperature, binding was detected using biotinylated anti-cytokine capture antibodies followed by addition of streptavidin-HRP, with ABTS as the substrate, and absorbances were measured at 405 nm. The cytokine levels were then correlated with the oxidant status of patients with RA.
Flow cytometry
Cells (106) were monitored for their intracellular fluorescence using a flow cytometer (FACS Calibur, Becton Dickinson, San Jose, CA, USA) equipped with an argon-ion laser (15 mW) tuned to 488 nm. The fluorescence of DCF and oxidized HPF were collected in the FL1 channel, equipped with a 530/30 nm band pass filter. Fluorescence was measured in the log mode and expressed as geometrical mean fluorescence channel (GMFC). Cells were gated on the basis of the characteristic forward and side scatter of neutrophils; neutrophils were confirmed with CD66b-FITC and appropriate isotype control. Acquisitions were performed on 5,000 events gated according to morphology, and the fluorescence of DCF or HPF was acquired. Data analysis was carried out using CellQuest Pro software (BD Biosciences, San Jose, CA, USA).
Statistical analysis
Data are expressed as mean ± SD or median and interquartile range (IQR). Correlation was done using Pearson’s correlation coefficient test for parametric data and Spearman rank correlation coefficient for nonparametric data using Graph Pad Prism software, version 5.0, (GraphPad Software Inc, La Jolla, CA, USA); p < 0.05 was considered as significant.
Results
Study population
The study population included 36 patients with RA who fulfilled the ACR criteria [6, 7]. Their ages ranged from 23 to 56 years, median and IQR being 40 (31–50), a female predominance was evident (female:male being 7:2), and duration of disease ranged from 11 months to 24 years (Table 1). Out of 36 patients, only 8 (22 %) belonged to group 3, i.e., moderate activity, while the majority (78 %) belonged to group 4, i.e., high disease activity. Levels of CRP and ESR were raised in all patients but serum rheumatoid factor was positive in 26/36 patients (Table 1); none of them received any antioxidants.
Characterization of synovial cells and levels of oxidative stress in synovial fluid of patients with RA
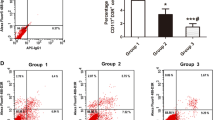
The neutrophil component was gated as R1 based on their characteristic side and forward scatter. Furthermore, neutrophils were confirmed by staining with FITC labeled CD66b whose frequency was found to be 82.11 % (Figs. 1a–c). These cells generated maximum amount of ROS as compared to other cells present in the synovial fluid (SF) [19]. Neutrophils, upon activation of the NADPH oxidase system generate reactive oxygen intermediates which by their propensity to freely react with biomolecules like lipids, proteins, and nucleic acids can cause oxidative damage. High levels of total ROS including hydroxyl radical was generated in the synovial cells (primarily neutrophils), the GMFC of DCF being 1,293.10 ± 648.50 and HPF being 188.05 ± 36.80. In the synovial fluid of patients with rheumatoid arthritis, neutrophils, macrophages, and lymphocytes were the principal cellular components, of which neutrophils were predominated.
Representative profile of gated neutrophils in synovial fluid of a patient with RA. a Representative profile of the characteristic side and forward scatter of the cellular components present in the synovial fluid; based on their morphological characteristics, neutrophils were gated as R1. b Representative profile of % positivity of isotype mouse IgG1 in neutrophils (R1). c Representative profile of % positivity of CD66b+ in neutrophils (R1)
Correlation of protein damage markers with oxidative stress and DAS28
AOPP are considered as a stable marker of oxidative protein damage as they are produced in pro-oxidative conditions by the action of chlorinated oxidants (mainly chloramines and hypochlorous) [20]. The levels of AOPP present in the cell-free fraction was 150.90 ± 82.43 μM/mg protein (Table 2) and showed a positive correlation with disease activity score (r = 0.48, 95 % CI 0.053 to 0.763, p < 0.05, Fig. 2a) but correlated poorly with generation of ROS (data not given).
Correlation between protein damage markers, oxidative stress, and DAS28. a Relationship between DAS28 and levels of AOPP in patients with RA. b Relationship between generation of ROS in neutrophils and protein carbonylation in patients with RA. c Relationship between hydroxyl radicals generated in neutrophils and protein carbonylation in patients with RA. d Relationship between DAS28 and protein carbonylation in patients with RA. e Relationship between DAS28 and level of S-nitrosothiols in patients with RA
ROS are known to react with the amino group of lysine residues and cause carbonyl groups to be introduced into proteins; therefore, the degree of carbonylation is considered as an effective indicator of the extent of ROS-induced oxidative modification of proteins [4]. A substantial amount of protein carbonylation was present in the synovial cells, being 1.54 ± 0.30 nmol/mg protein (Table 2). Additionally, the raised levels of ROS sourced from synovial cells strongly correlated with protein carbonylation (r = 0.73, 95 % CI 0.403 to 0.893, p < 0.001, Fig. 2b). More specifically, the hydroxyl radical which is considered as the most toxic among ROS strongly correlated with protein carbonylation (r = 0.64, 95 % CI −0.112 to 0.927, p < 0.05 Fig. 2c). The extent of protein carbonylation also correlated with the DAS28 (r = 0.67, 95 % CI 0.326 to 0.859, p < 0.001, Fig. 2d).
Another important group of cytotoxic molecules includes the nitrosothiols (RSNO) as they can disrupt the protein structure and/or interfere with the catalytic activity of enzymes [20, 21]. In the cell-free fraction of RA patients, the levels of RSNO were 6.62 ± 1.54 μM and correlated with DAS28 (r = 0.61, 95 % CI 0.102 to 0.872, p < 0.05, Fig. 2e); however, they correlated poorly with intracellular ROS (data not given).
Markers of lipid damage correlated with markers of oxidative stress and DAS28
Lipid peroxidation occurs following an attack on polyunsaturated fatty acids of the membrane by ROS and thereby initiates a self-propagating chain reaction which culminates in destruction of membrane lipids and loss of cell viability [22]. In the synovial fluid of RA patients, lipid peroxidation as measured in terms of MDA was 6.24 ± 1.47 μM. The levels of ROS in terms of GMFC, mean ± SD (1,293.10 ± 648.50), and hydroxyl radicals (188.05 ± 36.80) strongly correlated with levels of MDA, r = 0.80 (95 % CI 0.522 to 0.925, p < 0.001, Fig. 3a) and r = 0.69 (95 % CI 0.057 to 0.929, p < 0.05, Fig. 3b), respectively. Additionally, the levels of MDA also significantly correlated with DAS28 (r = 0.59, 95 % CI 0.207 to 0.821, p < 0.05, Fig. 3c).
Correlation of lipid damage marker with oxidative stress and DAS28. a Relationship between generation of ROS in neutrophils and levels of MDA in patients with RA. b Relationship between hydroxyl radicals generated in neutrophils and levels of MDA in patients with RA. c Relationship between DAS28 and levels of MDA in patients with RA
Correlation of cytokines with the oxidant status
In the cell-free fraction, levels of proinflammatory cytokines TNF-α, IL-6, and IL-8 were 418.40 ± 210.40, 3,503.00 ± 2,118.00, and 1,861.00 ± 1,622.00 pg/ml, respectively, and corroborated with published data [1]. Although levels of all three cytokines were raised, the generation of ROS correlated only with levels of TNF-α, r = 0.56 (p = 0.12, 95 % CI being −0.165 to 0.892, Fig. 4); whereas levels of IL-6 and IL-8 correlated poorly with ROS, r = 0.19 (95 % CI −0.427 to 0.691) and −0.76 (95 % CI −0.930 to −0.337), respectively.
Discussion
In several disease pathologies, there is a large body of evidence implicating the role of ROS that are generated as by-products of cellular metabolism, primarily in the mitochondria [4]. Physiological amounts of ROS are a mandatory cellular requirement as they are involved in signaling pathways, regulating a variety of cellular activities including cytokine secretion, growth, differentiation, and host defense against invading pathogens [23]. However, ROS can be considered as a double-edged sword as it also has the potential to induce significant biological damage by countering the cell’s robust antioxidant system [1, 4]. It has been previously demonstrated that in patients with RA, neutrophils obtained from the synovial fluid generate large amounts of ROS [10, 19] which was corroborated by this study. However, an unanswered yet pertinent question is whether enhanced generation of pro-oxidants directly correlates with the extent of tissue damage responsible for the disease progression.
Proteins are major components of the synovial fluid and one of the main targets of oxidation, wherein the structure and activity of proteins is altered along with prolongation of the half-life of AOPPs. These circulating AOPPS have been reported to be elevated in several diseases including Chagas disease, Type 2 diabetes, neurodegenerative disorders, rheumatoid arthritis, systemic sclerosis, etc., and the concentration of AOPPs ranged from 20.83 to 180.00 μM/mg protein [24–27]. The scenario was similar in this study where levels of AOPP in the synovial infiltrate were elevated (Table 2) and importantly correlated positively with DAS28 (Fig. 2a), strongly suggesting that measurement of AOPPs can serve as a biomarker of tissue damage.
Carbonylation is an irreversible nonenzymatic modification of proteins following the direct oxidation of amino acid side chains (Pro, Arg, Lys, and Thr), resulting in the formation of 2-pyrrolidone [28, 29]. The introduction of carbonyl groups into proteins occurs by the Michael addition reactions of a,b-unsaturated aldehydes, such as 4-hydroxy-2-nonenal, MDA and 2-propenal (acrolein) derived from lipid peroxidation, being inserted into either the amino group of lysine, the imidazole moiety of histidine, or the sulfydryl group of cysteine leading to the generation of AOPPs [28].
As compared to other parameters of oxidative stress such as glutathione disulphide and MDA, protein carbonyls tend to form early and circulate in the blood for longer periods [28]. Among the protein oxidation markers, carbonyls are relatively difficult to induce as compared to methionine sulfoxide and cysteinyl derivatives as also higher levels of oxidative stress are required for their induction. Therefore, the presence of carbonyl groups indicates a higher generation of oxidative stress in the synovial cells [28]. In patients with RA, plasma levels of carbonylated proteins were higher than healthy controls; similarly, in the SF of RA patients, higher degree of carbonylation was evident vis a vis nonarthritic controls, e.g., osteoarthritis, with values ranging from 1.27 to 3.5 nmol/mg of protein [13, 15, 18]. However, unlike our study, none of these studies have concomitantly measured the extent of oxidative stress. A substantial amount of protein carbonylation was evident (Table 2) which correlated positively with levels of ROS (Fig. 2b) and hydroxyl radicals (Fig. 2c) as also with disease severity (Fig. 2d). Taken together, the redox imbalance following enhanced generation of pro-oxidants can be directly linked to the extent of oxidative damage, strongly indicating that antioxidants may well be considered as an additional therapeutic modality in RA.
Nitric oxide (NO) is an important mediator of diverse physiologic and pathologic processes, including arthritis. However, as NO has a relatively short half-life (1 to 10 s), it is technically difficult to quantify [16, 30]. A viable alternative is measurement of S-nitrosothiols being a relatively stable form, whose levels would reflect the amount of NO that forms adducts with cellular SH compounds, such as glutathione, thereby decreases the bioavailability of free thiols in biological fluid, rendering cells to become more susceptible to oxidative stress. This relatively stable complex can also diffuse to remote sites and release bioactive NO. In previous studies, we have shown that relatively low amounts of NO was generated in the synovial cells [19] and showed a poor correlation with DAS28, whereas the levels of S- nitrosothiols correlated with DAS28 (Table 2, Fig. 2e).
Malonaldehyde which arises from the breakdown of lipid peroxyl radicals is an important indicator of oxidative stress and mediates injury by oxidizing protein molecules and by facilitating incorporation of protein carbonyl and sulfydryl groups [18, 31]. Lipid peroxidation is a self-propagating chain reaction wherein the initial oxidation of a few lipid molecules perpetuates significant tissue damage [22]. MDA levels were enhanced in RA patients, both in plasma and SF as also patients with Chagas disease and diabetes mellitus, the values ranged from 0.71 to 4.8 μM [18, 24–27]. In this study, the MDA levels were comparable (Table 2) and importantly correlated with both levels of ROS and OH radicals (Figs. 3a, b) as also the DAS28 (Fig. 3c) which was in concordance with previous studies that indicated a positive correlation between levels of ROS, hydroxyl radical, and DAS28 [10]. Although TNF-α showed a positive correlation with generation of ROS (Fig. 4), other proinflammatory cytokines like IL-6 and IL-8, even though present in higher levels in SF, correlated poorly with levels of ROS, possibly because increased levels of proinflammatory cytokines are contributed by multiple factors, one of them being oxidative stress.
Measurement of oxidative stress in peripheral blood has been shown to mirror the status in the synovial infiltrate and it was proposed that measurement of ROS in peripheral blood can serve as an indirect measure of the degree of inflammation in patients with RA [10]. As this study has established a strong correlation between increased oxidative damage and causative agents such as ROS and OH radicals, it strengthens our contention that measurement of oxidative stress (peripheral blood and/or SF) is an effective biomarker for monitoring disease activity. Administration of methotrexate along with an antioxidant has been shown to suppress disease progression in rat models of arthritis [32]. Moreover, in an adjuvant-induced arthritis model, combinatorial treatment of methotrexate with an antioxidant had beneficial effect in terms of disease progression and attenuation of increased methotrexate-induced liver damage [33, 34]. Taken together, the positive correlation between disease severity and oxidative damage suggests that inclusion of antioxidants in the pharmacotherapy of RA is worthy of future consideration.
References
Filippin LI, Vercelino R, Marroni NP, Xavier RM (2008) Redox signaling and the inflammatory response in rheumatoid arthritis. Clin Exp Immunol 152:415–422
Mirshafiey A, Mohsenzadegan M (2008) The role of reactive oxygen species in immunopathogenesis of rheumatoid arthritis. Iran J Allergy Asthma Immunol 7:195–202
Goldsby RA, Kindt TJ, Osborne BA, Kuby J (2003) Leukocyte migration and inflammation. In: Immunology, 5th edn. WH Freeman, New York, pp 338–358
Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A (2006) Biomarkers of oxidative damage in human disease. Clin Chem 52:601–623
Forman HJ, Fukuto JM, Torres M (2004) Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287:C246–C256
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
van der Linden MP, Batstra MR, Bakker-Jonges LE (2011) Towards a data-driven evaluation of the 2010 American College of Rheumatology/European League Against Rheumatism criteria for rheumatoid arthritis: is it sensible to look at levels of rheumatoid factor? Arthritis Rheum 63:1190–1199
van Gestel AM, Haagsma CJ, van Riel PL (1998) Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum 41:1845–1850
Mandal G, Wyllie S, Singh N, Sundar S, Fairlamb AH, Chatterjee M (2007) Increased levels of thiols protect antimony unresponsive Leishmania donovani field isolates against reactive oxygen species generated by trivalent antimony. Parasitology 134:1679–1687
Kundu S, Ghosh P, Datta S, Ghosh A, Chattopadhyay S, Chatterjee M (2012) Oxidative stress as a potential biomarker for determining disease activity in patients with Rheumatoid Arthritis. Free Radic Res 46:1482–1489
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Guilpain P, Chéreau C, Goulvestre C, Servettaz A, Montani D, Tamas N, Pagnoux C, Hachulla E, Weill B, Guillevin L, Mouthon L, Batteux F (2011) The oxidation induced by anti myeloperoxidase antibodies triggers fibrosis in microscopic polyangiitis. Eur Respir J 37:1503–1513
Firuzi O, Fuksa L, Spadaro C, Bousová I, Riccieri V, Spadaro A, Petrucci R, Marrosu G, Saso L (2006) Oxidative stress parameters in different systemic rheumatic diseases. J Pharm Pharmacol 58:951–957
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Shalfier S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Mantle D, Falkous G, Walker D (1999) Quantification of protease activities in synovial fluid from rheumatoid and osteoarthritis cases: comparison with antioxidant and free radical damage markers. Clin Chim Acta 284:45–58
Marzinzig M, Nussler AK, Stadler J, Marzinzig E, Barthen W, Nussler NC, Beger HG, Morris SM Jr, Brückner UB (1997) Improved methods to measure end products of nitric oxide in biological fluids: nitrite, nitrate, and S-nitrosothiols. Nitric Oxide 1:177–189
Beuge JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Seven A, Güzel S, Aslan M, Hamuryudan V (2008) Lipid, protein, DNA oxidation and antioxidant status in rheumatoid arthritis. Clin Biochem 41:538–543
Kundu S, Bala A, Ghosh P, Mukhopadhyay D, Mitra A, Sarkar A, Bauri AK, Ghosh A, Chattopadhyay S, Chatterjee M (2011) Attenuation of oxidative stress by allylpyrocatechol in synovial cellular infiltrate of patients with Rheumatoid Arthritis. Free Radic Res 45:518–526
Santulli P, Borghese B, Lemaréchal H, Leconte M, Millischer AE, Batteux F, Chapron C, Borderie D (2013) Increased serum oxidative stress markers in women with uterine leiomyoma. 36. PLoS One 8:e72069
Stalmer JS, Singel DJ, Loscalzo J (1992) Biochemistry of nitric oxide and its redox-activated forms. Science 258:1898–1902
Mylonas C, Kouretas D (1999) Lipid peroxidation and tissue damage. In Vivo 13:295–309
Halliwell B (2007) Free radicals in biology and medicine. Oxford University Press, New York
Dhiman M, Coronado YA, Vallejo CK, Petersen JR, Ejilemele A, Nuñez S, Zago MP, Spratt H, Garg NJ (2013) Innate immune responses and antioxidant/oxidant imbalance are major determinants of human Chagas disease. PLoS Negl Trop Dis 7:e2364
Chang D, Zhang X, Rong S, Sha Q, Liu P, Han T, Pan H (2013) Serum antioxidative enzymes levels and oxidative stress products in age-related cataract patients. Oxidative Med Cell Longev. doi:10.1155/2013/587826
Bansal S, Chawla D, Siddarth M, Banerjee BD, Madhu SV, Tripathi AK (2013) A study on serum advanced glycation end products and its association with oxidative stress and paraoxonase activity in type 2 diabetic patients with vascular complications. Clin Biochem 46:109–114
Yang XH, Liu X, Shang J, Liu HG, Xu YJ (2013) Correlation between the serum level of advanced oxidation protein products and the cognitive function in patients with obstructive sleep apnea hypopnea syndrome. Zhonghua Jie He He Hu Xi Za Zhi 36:274–279
Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A (2003) Protein carbonylation in human diseases. Trends Mol Med 9:169–176
Bhattacharya S, Mula S, Gamre S, Kamat JP, Bandyopadhyay SK, Chattopadhyay S (2007) Inhibitory property of Piper betel extract against photosensitization-induced damages to lipids and proteins. Food Chem 100:1474–1480
Mahmoud AA, Ismail MA (2011) Serum protein carbonyl content, total thiol and nitric oxide in patients with rheumatoid arthritis. J Am Sci 7:683–686
Marin DP, Bolin AP, dos Santos RC, Curi R, Otton R (2010) Testosterone suppresses oxidative stress in human neutrophils. Cell Biochem Funct 28:394–402
Roy S, Sannigrahi S, Vaddepalli RP, Ghosh B, Pusp P (2012) A novel combination of methotrexate and epigallocatechin attenuates the overexpression of pro-inflammatory cartilage cytokines and modulates antioxidant status in adjuvant arthritic rats. Inflammation 35:1435–1447
Drafi F, Bauerova K, Kuncirova V, Ponist S, Mihalova D, Fedorova T, Harmatha J, Nosal R (2012) Pharmacological influence on processes of adjuvant arthritis: effect of the combination of an antioxidant active substance with methotrexate. Interdiscip Toxicol 5:84–91
Banji D, Pinnapureddy J, Banji OJ, Saidulu A, Hayath MS (2011) Synergistic activity of curcumin with methotrexate in ameliorating Freund’s complete adjuvant induced arthritis with reduced hepatotoxicity in experimental animals. Eur J Pharmacol 668:293–298
Acknowledgments
Financial assistance was provided by the Department of Biotechnology, Govt. of India. SK is a recipient of Senior Research Fellowship from Indian Council of Medical Research, Govt. of India.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Suhana Datta and Sunanda Kundu contributed equally to this work and are considered as joint first authors.
Rights and permissions
About this article
Cite this article
Datta, S., Kundu, S., Ghosh, P. et al. Correlation of oxidant status with oxidative tissue damage in patients with rheumatoid arthritis. Clin Rheumatol 33, 1557–1564 (2014). https://doi.org/10.1007/s10067-014-2597-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-014-2597-z