Abstract
In this study, a uridine and acetoin co-production pathway was designed and engineered in Bacillus subtilis for the first time. A positive correlation between acetoin and uridine production was observed and investigated. By disrupting acetoin reductase/2,3-butanediol dehydrogenasegenebdhA, the acetoin and uridine yield was increased while 2,3-butanediol formation was markedly reduced. Subsequent overexpression of the alsSD operon further improved acetoin yield and abolished acetate formation. After optimization of fermentation medium, key supplementation strategies of yeast extract and soybean meal hydrolysate were identified and applied to improve the co-production of uridine and acetoin. With a consumption of 290.33 g/L glycerol, the recombinant strain can accumulate 40.62 g/L uridine and 60.48 g/L acetoin during 48 h of fed-batch fermentation. The results indicate that simultaneous production of uridine and acetoin is an efficient strategy for balancing the carbon metabolism in engineered Bacillus subtilis. More importantly, co-production of value-added products is a possible way to improve the economics of uridine fermentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uridine is a pyrimidine nucleoside with various bioactive functions and applications in multiple fields, including health care, drug manufacturing, and the food industry (Connolly and Duley 1999). Uridine has sleep-promoting and anti-epileptic effects, and can affect moods, improve memory function, and influence neuronal plasticity (Dobolyi et al. 2011). In addition, uridine is an important nutritional supplement for the modulation of unwanted toxicity of antiviral and anticancer drugs (Cheng et al. 2016; Jordheim et al. 2013).
Traditionally, uridine is prepared by condensation reaction of uracil and d-ribose or by enzymatic hydrolysis of ribonucleic acid (Moffatt and Ashihara 2002). However, these methods cannot be widely used because of associated limitations such as expensive raw materials, harsh reaction conditions, and environmental pollution. Therefore, microbial fermentation has been employed for producing uridine from engineered or mutated Bacillus subtilis. Reports have shown that B. subtilis can synthesize uridine monophosphate (UMP) through the de novo pyrimidine biosynthesis pathway, which is encoded by the pyr operon with 10 cistrons. UMP can be further converted to uridine through catalysis by nucleotide phosphoesterase. However, the expression of the pyr operon is regulated by a transcriptional attenuation mechanism (Hobl and Mack 2007; Lu et al. 1996), and the activity of carbamoyl phosphate synthetase (CPSase) encoded by pyrAA/pyrAB is strongly inhibited by UMP and stimulated by phosphoribosyl pyrophosphate (PRPP) (Braxton et al. 1999; Pierrat and Raushel 2002). To improve the uridine yield of B. subtilis, some researchers have focused on genetic engineering to enhance the metabolic flux through the UMP biosynthesis pathway by introducing mutations to CPSase and overexpressing the PRPP synthetase gene prs, as well as by blocking competing metabolic pathways (Wang et al. 2018; Zhu et al. 2015). The best engineered B. subtilis produced 11 g/L uridine with a yield of 0.24 g/g glucose (Wang et al. 2018). Moreover, pyrimidine analog-resistant mutants of B. subtilis can also accumulate large amounts of uridine (Doi et al. 1989; Fan et al. 2017). In a previous study, we used atmospheric and room temperature plasma (ARTP) mutagenesis to improve the uridine production of engineered B. subtilis TD12np. The final mutant, B. subtilis F126, produced 30.3 g/L uridine with a yield of 0.11 g/g glucose (Fan et al. 2017).
In addition to uridine, B. subtilis F126 also generates a considerable amount of acetoin. Interestingly, we observed that after disruption of acetoin metabolism, both acetoin and uridine yield were markedly reduced. Acetoin is a natural physiological metabolite excreted by several Bacillus strains (Dai et al. 2015; Liu et al. 2011). It participates in the regulation of the NAD/NADH ratio and in that of carbon storage (Xiao and Xu 2007). Aspartate, glutamate, and PRPP have been known as the important precursors for uridine synthesis and involve in different carbon metabolism. Therefore, the excess accumulation of uridine may cause carbon flux overflow in vivo and inhibit cell growth. Acetoin accumulation during uridine fermentation can rebalance carbon metabolism and restore the optimal cell physiology. Moreover, acetoin is a well-known flavor and platform chemical, which has been widely used in foods and the chemical industry (Xiao and Lu 2014). Co-production of acetoin may be an effective approach to improving the economics of uridine fermentation.
In this study, we described the positive correlation between uridine and acetoin metabolism in engineered B. subtilis. To increase acetoin accumulation, the bdhA gene, which is responsible for the conversion of acetoin to 2,3-butanediol, was disrupted, and the alsSD operon, which is responsible for acetoin synthesis, was overexpressed. To our knowledge, this is the first report that using engineered B. subtilis to simultaneously produce acetoin during uridine fermentation. After optimization of fermentation medium by statistical analysis, key supplementation strategies of yeast extract and soybean meal hydrolysate were identified and applied to improve the co-production of uridine and acetoin.
Materials and methods
Strains, primers, and media
The bacterial strains used in this study are listed in Table S1. Primers used are listed in Table S2. All B. subtilis were derived from the wild-type B. subtilis 168. DNA isolation and manipulations were carried out using standard protocols.
The seed medium contained 25 g/L glucose, 5 g/L yeast extract, 10 g/L tryptone, 2 g/L MgSO4, 2 g/L K2HPO4, and1 g/L KH2PO4. The flask medium contained 80 g/L glucose, 5 g/L yeast extract, 5 g/L (NH4)2SO4, 5 g/L sodium glutamate, 10 mL/L corn steep liquor, 5 g/L urea, 10 mL/L soybean meal hydrolysate, 2 g/L MgSO4, 2 g/L K2HPO4, and 2 g/L KH2PO4. The initial fermentor medium contained 80 g/L glycerol, 5 g/L yeast extract, 2.5 g/L (NH4)2SO4, 5 g/L sodium glutamate, 5 g/L urea, 2 g/L K2HPO4, and 1 g/L KH2PO4.
Deletion of the bdhA gene
The used method of marker-free gene deletion was previously described by Liu et al. (2008). The 0.8 kb upstream homologous region (UP), 1.2 kb downstream homologous region (DN), and 0.7 kb homologous region (G) selected in the bdhA gene were separately amplified from the B. subtilis 168 genome using primers bdhA-UP1, bdhA-UP2, bdhA-DN1, bdhA-DN2, bdhA-G1, and bdhA-G2, respectively. The 2.1 kb cat-araR selective marker cassette (CR) was obtained by the pDM19-T (CR) plasmid using primers bdhA-CR1 and bdhA-CR2. To construct the recombinant fragments for transformation, these four fragments were ligated in the order UP-DN-CR-G by overlapped extension PCR using primers bdhA-UP1 and bdhA-G2. The overlapped PCR products were transformed into competent cells of the B. subtilis strain and were selected on Luria–Bertani (LB) agar plates supplemented with 6 μg/mL chloramphenicol. The chloramphenicol-resistant transformants were confirmed by colony PCR. The correct mutants were cultured for 12 h in LB liquid medium without antibiotics and were then selected on an LB agar plate containing 30 μg/mL neomycin. Lastly, the neomycin-resistant mutants were verified by PCR.
Chromosomal integration of alsSD operon
The 0.8 kb UP, 3.5 kb alsSD operon gene fragment (Z), 0.9 kb DN, and 0.6 kb G were selected in the nprE gene and were separately amplified from the B. subtilis 168 genome using primers als-UP1, als-UP2, als-A, als-S, als-DN1, als-DN2, als-G1, and als-G2, respectively. The 2.1 kb cat-araR CR was obtained by the pDM19-T (CR) plasmid using primers als-CR1 and als-CR2. To construct the recombinant fragments for transformation, these five fragments were ligated in the order UP-Z-DN-CR-G by overlapped extension PCR, using primers als-UP1 and als-G2. The recombinants were selected by the method described above.
Medium optimization performed in shake-flask
To evaluate the effect of nutritional factors on the co-production of uridine and acetoin, medium optimization was performed in a shake-flask. Single colony of B. subtilis cells was transferred into 5 mL seed medium and cultivated at 37 °C for 6 h. Then, 3 mL seed culture were transferred into a 500 mL shake-flask containing 30 mL flask medium, and incubated at 37 °C, 200 rpm on a rotating shaker for cultivation. The pH was maintained at 7.0 by adding 4 M ammonium hydroxide using a micro-injector, according to the color change of phenol red in the culture.
For the selection of an optimal carbon resource, the glucose in the flask fermentation medium was replaced by equal amounts of fructose, xylose, glycerol, maltose, sucrose, and molasses, respectively. For the selection of significant variables, the composition of the flask fermentation medium was tested and identified via the Plackett-Burman design experiment (Plackett and Burman 1946). The principal effects of each variable on uridine and acetoin production were estimated as the difference between both averages of measurements made at the higher level and at the lower level. The significance of each variable was determined via t test, using the Minitab 18 software.
Fed-batch fermentation in 5-L fermentor
To evaluate the potential advantages of the engineered strain, fed-batch fermentation was performed in a 5-L fermentor (Baoxing, Shanghai, China) containing 2.7-linitial fermentor medium. For this, 300 mL seed culture from the shake-flask cultivation was transferred into the fermentor and the reaction conditions were set as follows: the pH was automatically controlled to 6.4 by addition of sterilized sodium hydroxide and soybean meal hydrolysate, respectively; dissolved oxygen was maintained at ~30% of air saturation by varying the stirrer speed and the aeration rate; the temperature was kept constant at 37 °C. When the glycerol in the initial fermentor medium was consumed, a mixed solution containing 800 g/L glycerol and 60 g/L yeast extract was added automatically, at an appropriate rate, to maintain the residual glycerol at ~5 g/L. The total amount of soybean meal hydrolysate and yeast extract used in the fed-batch fermentation was controlled to ~20 mL/L and ~20 g/L, respectively.
Analytical methods
Cell density and the concentration of uridine were determined using previously described methods (Fan et al. 2017). Acetoin, 2,3-butanediol, acetate, and sugar concentration were measured by HPLC (Shimadzu, Japan), using an Aminex HPX-87H column (Bio-Rad, Hercules, CA, USA) at 30 °C with a refractive index detector. As the mobile phase, 5 mM sulfuric acid was supplied at a flow rate of 0.5 mL/min.
Results
Positive correlation between uridine and acetoin metabolism in engineered B. subtilis
The construction of highly efficient biosynthesis pathways is essential for improving pyrimidine nucleoside synthesis in microorganisms. In order to increase uridine production in B. subtilis168, combinatorial strategies have been employed previously (Fan et al. 2017; Zhu et al. 2015) (Fig. 1): (1) the pyrR gene was deleted to increase the expression of the pyrimidine operon; (2) the pdp gene was deleted to block uridine degradation; (3) the nupC gene was deleted to reduce uridine uptake; (4) thehom gene was deleted to improve the supply of precursor aspartate supply; (5) a second copy of the prs gene was integrated into the xylR locus to improve the supply of precursor PRPP; (6) a pyrAB mutant (E949*) resistant to UMP feedback inhibition was obtained by ARTP mutagenesis. The resulting strain, F126,accumulated 5.73 g/L uridine and 15.83 g/L acetoin in shake-flask fermentation, which represents a 19.76- and 1.57-fold increase over the wild strain B. subtilis 168 (Table 1). Moreover, relatively small amounts of 2,3-butanediol (3.45 g/L) and acetate (2.81 g/L) were also formed.
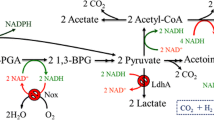
Considering that 20% of glucose is consumed to produce the primary byproduct of acetoin, we attempted to knockout the alsSD gene to block acetoin metabolism and increase uridine production. Unfortunately, the uridine and acetoin yield decreased significantly when the alsS or alsD gene was deleted in B. subtilis F126 (Fig. 2). Furthermore, a large amount of acetate was formed when the alsS gene was deleted (Fig. 2), suggesting that alsS gene deficiency can increase the carbon flux from pyruvate to acetate.
In summary, disruption of acetoin metabolism had a negative effect on uridine production. We inferred that acetoin plays an important role on the balance of carbon metabolism and acts as an energy-storing substance in B. subtilis. The conversion of excess pyruvate to uncharged acetoin instead of acetate can prevent overacidification of the intracellular environment and culture medium, which is necessary for normal growth and metabolite accumulation (Xiao and Xu 2007).
Modification of acetoin metabolism in B. subtilis F126
The biosynthesis of acetoin in B. subtilis requires α-acetolactate synthase (ALS) and α-acetolactate decarboxylase (ALDS), both of which are encoded by the alsSD operon (Renna et al. 1993). Acetoin can be converted into 2,3-butanediol reversibly by acetoin reductase/2,3-butanediol dehydrogenase (AR/BDH), which is encoded by the bdhA gene (Nicholson 2008). To improve acetoin production and eliminate undesired byproducts such as 2,3-butanediol, the bdhA gene was knocked out firstly. As shown in Table 1, the acetoin titer of engineered strain F126-1 increased from 15.83 to 17.46 g/L, while 2,3-butanediol production was reduced by 74%. Although the AR/BDH encoding gene was successfully knocked out, a small amount of 2,3-butanediol (0.91 g/L) was still detected, which may be due to the presence of another AR enzyme in B. subtilis (Nicholson 2008; Zhang et al. 2014b). Furthermore, the uridine titer of F126-1 also increased from 5.73 to 6.71 g/L, suggesting that disruption of 2,3-butanediol may rebalance the pyruvate metabolism and improve the supplement of precursors aspartate and glutamate for uridine synthesis.
Subsequently, an additional copy of the alsSD operon was inserted into the F126-1 chromosome, at the nprE locus, to yield F126-2. As shown in Table 1, the acetoin titer of engineered strain F126-2 increased from 17.46 to 20.32 g/L, while acetate production was reduced by 54%. The results are consistent with previous studies which showed that overexpression of the alsSD operon can improve ALS activities and increase the availability of pyruvate for acetoin biosynthesis (Liu et al. 2015; Wang et al. 2012). Furthermore, the uridine and 2,3-butanediol titer of F126-2 were very similar to that of F126-1, suggesting that the overexpression of ALS and ALDS cannot induce a significant change in uridine and 2,3-butanediol synthesis.
Optimization of fermentation medium for the co-production of uridine and acetoin
Various carbon sources, including glucose, fructose, xylose, glycerol, maltose, sucrose, and molasses, were selected among the commonly used industrial bacterial feeds, and their effects on the co-production of uridine and acetoin were investigated. As shown in Fig. 3, maximum growth of F126-2 was obtained when glucose was used as the major carbon source. However, the highest uridine titer of 7.37 g/L and acetoin titer of 22.04 g/L were achieved when glycerol was used as the major carbon source. Furthermore, it was difficult for F126-2 to utilize xylose as the sole carbon source, due to the absence of a specific xylose uptake system (Chen et al. 2013; Zhang et al. 2015). Also, oligomeric forms of monosaccharides (maltose, sucrose, and molasses) were utilized less efficiently than the monomer itself (glucose and fructose) for the co-production of uridine and acetoin.
To further enhance the growth of engineered strain F126-2 and to improve the production of uridine and acetoin, a total of ten nutritional factors were analyzed by the Plackett-Burman design experiment. The design matrix selected for the screening of significant variables and the corresponding responses are shown in Table 2. The principal effects of each variable on uridine and acetoin co-production were estimated by t test. Factors evidencing P values of less than 0.05 were considered to have a significant impact. As shown in Table 3, yeast extract, with a probability value of 0.017, was determined to be the most significant factor for uridine production, followed by soybean meal hydrolysate (0.018), glycerol (0.020), and corn steep liquor (0.044). One of the four significant variables screened, corn steep liquor, exerted a negative effect, whereas all the other variables exerted positive effects on uridine production. Furthermore, yeast extract (0.027) and glycerol (0.028) also had significant positive effects on acetoin production, suggesting that these two nutritional factors can stimulate cell growth and regulate the cellular metabolic activities of engineered B. subtilis (Cho et al. 2015; Doi et al. 1994).
Co-production of uridine and acetoin by fed-batch fermentation
Glucose and glycerol were respectively used as the major carbon source for the fed-batch fermentation based on the results of the carbon source selection experiment. As shown in Fig. 4a, b, lower biomass and sugar consumption were achieved when glycerol was used as the major carbon resource, but the uridine and acetoin titer increased to 33.73 and 48.08 g/L, respectively. This means that more than 30% of glycerol consumption was directed toward uridine and acetoin synthesis, whereas only 22% of glucose was consumed for this purpose.
Effect of different fed-batch fermentation conditions on the co-production of uridine and acetoin. a Glucose was used as major carbon resource, and 20 g/L yeast extract and 20 ml/L soybean meal was added into the initial medium. b Glycerol was used as major carbon resource, and 20 g/L yeast extract and 20 ml/L soybean meal was added into the initial medium. c Glycerol was used as major carbon resource, and 5 g/L yeast extract and 20 ml/L soybean meal was added into the initial medium, and 15 g/L yeast extract was replenished during the fermentation. d Glycerol was used as major carbon resource, and 5 g/L yeast extract was added into the initial medium, and 15 g/L yeast extract and 20 ml/L soybean meal was replenished during the fermentation
Yeast extract is another important nutritional factor based on the results of the Plackett-Burman experiment. Two different control strategies were applied to improve the co-production of uridine and acetoin in the fed-batch fermentation. The first involves adding 20 g/L yeast extract into the initial medium. The second involves adding 5 g/L yeast extract into the initial medium and replenishing 15 g/L yeast extract intermittently within 12–36 h of the fermentation. The results were shown in Fig. 4b, c, respectively. Acetoin accumulated rapidly at the beginning under the first control strategy, but the acetoin production rate dropped drastically at the late stage. Instead, acetoin accumulated at relatively stable rate under the second control strategy. Furthermore, the decrease in biomass under the second control strategy was much lower than that under the first control strategy, suggesting that yeast extract replenishment can stimulate cell growth by prolonging the stable growth phase. Because specific uridine production rate was coupled to cell growth (Fan et al. 2017), more uridine was produced under the second control. To sum up, when 15 g/L yeast extract was replenished, the uridine and acetoin titer reached to 35.84 and 53.45 g/L, respectively, being 6 and 11% higher than those obtained without yeast extract replenishment.
Besides yeast extract, soybean meal hydrolysate also had a positive effect on uridine and acetoin production (Xiao et al. 2007). Industrial soybean meal hydrolysate is always prepared by acid hydrolysis, so we tried to use it instead of hydrochloric acid as a pH-neutralizing reagent for fed-batch fermentation. As shown in Fig. 4d, when 20 mL/L sterilized soybean meal hydrolysate was replenished to control pH at 6.4, the uridine titer increased from 35.84 to 40.62 g/L, and the acetoin titer increased from 53.45 to 60.48 g/L. However, further rising pH value led to an increase of acetate formation, while reducing pH value led to a decrease in biomass (data not shown). These results are consistent with previous studies which showed that high external pH favors catabolism-generating acids, whereas low external pH upregulates acetoin production (alsSD) (Wilks et al. 2009).
Discussion
B. subtilis, which is generally regarded as safe, is often utilized as a platform organism for the industrial production of nucleoside compounds (Asahara et al. 2010; Duan et al. 2010; Wang et al. 2018; Zhu et al. 2015). In our previous study, we obtained the uridine-producing strain B. subtilis F126 by genetic manipulation and mutagenesis (Fan et al. 2017). However, we observed that a considerable amount of acetoin was also generated during uridine fermentation. To minimize the accumulation of byproducts and maximize the yield of uridine, we attempted to block acetoin metabolism by disrupting the alsSD operon. To our surprise, the deficiency of alsSD operon resulted in a drastic decline in both acetoin and uridine production (Fig. 2). Meanwhile, the amount of acetate was greatly improved (Fig. 2). In B. subtilis, PRPP is one of the important precursors for uridine biosynthesis (Fig. 1). The most significant influence on uridine accumulation is exerted by the constraint that PRPP is strictly coupled to HMP flux in vivo. It is well known that HMP flux is in the range of 20 to 30% of the total glucose uptake in bacteria. Excess uridine accumulation can lead to carbon overflow via glycolysis pathway, which eventually resulted in the increase of byproducts of pyruvate metabolism, including lactate, acetate, and acetoin. The conversion of excess pyruvate to uncharged acetoin in B. subtilis has the advantage of preventing overacidification and storing carbon (Vivijs et al. 2014; Xiao and Xu 2007), which is a step that is essential for continued glycolysis and other precursors aspartate and glutamate synthesis. Therefore, acetoin metabolism is indispensable for uridine over-production in engineered B. subtilis.
Genetic engineering is an effective strategy for improving acetoin production in Bacillus strains. Common approaches include overexpression of the acetoin biosynthetic alsSD operon and the transcriptional regulator AlsR, elimination of byproduct, as well as modulation of the NADH level. Among the tested methods, moderate expression of AlsR inhibited cell growth, while deletion of the pta gene caused little variation in acetate and acetoin yield (Wang et al. 2012; Zhang et al. 2013). Moreover, introduction of a NAD+ regeneration system led to dramatic changes in the synthesis of NADH-dependent metabolites (Zhang et al. 2014b). Considering the previous results, we first knocked out the bdhA gene of F126 to block the main flux of acetoin to 2,3-butanediol. Then, a copy of the alsSD operon mediated by its native promoter was integrated into the chromosome to strengthen the acetoin pathway. The uridine and acetoin titers of the resulting strain F126-2 sharply increased to 6.85 and 20.32 g/L, respectively, which were 19 and 28% higher than those of F126 (Table 1). Meanwhile, the production of 2,3-butanediol and acetate was reduced by 70 and 58%, respectively (Table 1). Thus, we successfully improved the co-production of uridine and acetoin and reduced other byproducts accumulation by modification of acetoin metabolism in engineered B. subtilis. P43 and PHpaII are well-characterized constitutive promoters that have been wildly used to strengthen gene transcription (Liu et al. 2015; Shi et al. 2009). Our experiments indicated that the native promoter of the alsSD operon can also improve acetoin production, without affecting normal cell growth (Frädrich et al. 2012).
Glycerol is an inevitable byproduct of biodiesel production that has become an attractive carbon source for fermentation processes because of its low price and high degree of reduction. When glycerol was used as the major carbon source, a high acetoin titer of 48.08 g/L was obtained, representing an approximate 1.34-fold increase compared to that obtained with glucose as the major carbon source (Fig. 4b). Meanwhile, the uridine titer also increased from 30.06 to 33.73 g/L (Fig. 4b). As proved in previous studies, more reducing equivalents can be generated upon the dissimilation of glycerol compared to glucose (Blankschien et al. 2010; Durnin et al. 2009). Therefore, enough ATP is gained through oxidative phosphorylation resulting from the reducing equivalents generated when using glycerol, which is subsequently used for glutamine and pyrimidine nucleoside synthesis (Eisenberg et al. 2000; Moffatt and Ashihara 2002).
In addition to glycerol, yeast extract and soybean meal hydrolysate also had positive effects on uridine and acetoin production, as proved by the Plackett-Burman experiment (Table 3). However, when these two nutritional factors were directly added into the fermentation medium, the cell growth and product accumulation were not favorable. Therefore, we replenished the yeast extract and used soybean meal hydrolysate as a pH-neutralizing reagent during the entire period of fermentation. Under the optimal supplementation strategies, the uridine and acetoin titers increased to 40.62 and 60.48 g/L, with a productivity of 0.85 and 1.26 g/(L h), respectively (Fig. 4d). The titer and production rate were comparable to those corresponding to acetoin and uridine production (Table 4). The highest reported titer of acetoin was 76.0 g/L with a productivity of 1.00 g/(L h), while that of uridine was 65.0 g/L with a productivity of 0.90 g/L. Although the acetoin and uridine titer in this study were lower compared to the reported results using glucose as substrate, acetoin productivity was the highest, and strain F126-2 exhibited the potential to simultaneously produce acetoin and uridine using glycerol as substrate. The successful co-production of bulk chemicals with a value-added product improves the economics of the fermentation process. As uridine and acetoin can be easily separated by distillation due to the difference in volatility, the overall biological co-production process is much more cost-effective and commercially feasible than a chemical process. This study provides valuable insights into the role of acetoin metabolism in uridine over-producing strain of B. subtilis and should be highly useful for the development of industrial bioproduction of uridine and acetoin.
References
Asahara T, Mori Y, Zakataeva NP, Livshits VA, Yoshida K, Matsuno K (2010) Accumulation of gene-targeted Bacillus subtilis mutations that enhance fermentative inosine production. Appl Microbiol Biotechnol 87(6):2195–2207
Blankschien MD, Clomburg JM, Gonzalez R (2010) Metabolic engineering of Escherichia coli for the production of succinate from glycerol. Metab Eng 12(5):409–419
Bo Z, Li X, Jing F, Ning L, Wang Z, Tang Y, Tao C (2016) Production of acetoin through simultaneous utilization of glucose, xylose, and arabinose by engineered Bacillus subtilis. PLoS One 11(7):e0159298
Braxton BL, Mullins LS, Raushel FM, Reinhart GD (1999) Allosteric dominance in carbamoyl phosphate synthetase. Biochemistry 38(5):1394–1401
Chen T, Liu WX, Fu J, Zhang B, Tang YJ (2013) Engineering Bacillus subtilis for acetoin production from glucose and xylose mixtures. J Biotechnol 168(4):499–505
Cheng KG, Su CH, Huang JY, Liu J, Zheng YT, Chen ZF (2016) Conjugation of uridine with oleanolic acid derivatives as potential antitumor agents. Chem Biol Drug Des 88(3):329–340
Cho S, Kim T, Woo HM, Kim Y, Lee J, Um Y (2015) High production of 2,3-butanediol from biodiesel-derived crude glycerol by metabolically engineered Klebsiella oxytoca M1. Biotechnol Biofuels 8(1):1–12
Connolly GP, Duley JA (1999) Uridine and its nucleotides: biological actions, therapeutic potentials. Trends Pharmacol Sci 20(5):218–225
Dai JY, Cheng L, He QF, Xiu ZL (2015) High acetoin production by a newly isolated marine Bacillus subtilis strain with low requirement of oxygen supply. Process Biochem 50(11):1730–1734
Dobolyi A, Juhász G, Kovács Z, Kardos J (2011) Uridine function in the central nervous system. Curr Top Med Chem 11(8):1058–1067
Doi M, Asahi S, Tsunemi Y, Akiyama SI (1989) Mechanism of uridine production by Bacillus subtilis mutants. Appl Microbiol Biotechnol 30(3):234–238
Doi M, Tsunemi Y, Asahi S (1994) Opimization of conditions for production of uridine by a mutant of Bacillus subtilis. Biosci Biotechnol Biochem 58(9):1608–1612
Duan YX, Chen T, Chen X, Zhao XM (2010) Overexpression of glucose-6-phosphate dehydrogenase enhances riboflavin production in Bacillus subtilis. Appl Microbiol Biotechnol 85(6):1907–1914
Durnin G, Clomburg J, Yeates Z, Alvarez P, Zygourakis K, Campbell P, Gonzalez R (2009) Understanding and harnessing the microaerobic metabolism of glycerol in Escherichia coli. Biotechnol Bioeng 103(1):148–161
Eisenberg D, Gill HS, Pfluegl GM, Rotstein SH (2000) Structure–function relationships of glutamine synthetases 1. Biochim Biophys Acta 1477(1):122–145
Fan X, Wu H, Li G, Yuan H, Zhang H, Li Y, Xie X, Chen N (2017) Improvement of uridine production of Bacillus subtilis by atmospheric and room temperature plasma mutagenesis and high-throughput screening. PLoS One 12(5):e0176545
Frädrich C, March A, Fiege K, Hartmann A, Jahn D, Härtig E (2012) The transcription factor AlsR binds and regulates the promoter of the alsSDoperon responsible for acetoin formation in Bacillus subtilis. J Bacteriol 194(5):1100–1112
Hobl B, Mack M (2007) The regulator protein PyrR of Bacillus subtilis specifically interacts in vivo with three untranslated regions within pyr mRNA of pyrimidine biosynthesis. Microbiology 153(3):693–700
Jordheim LP, Durantel D, Zoulim F, Dumontet C (2013) Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat Rev Drug Discov 12(6):447–464
Liu S, Endo K, Ara K, Ozaki K, Ogasawara N (2008) Introduction of marker-free deletions in Bacillus subtilis using the AraR repressor and the ara promoter. Microbiology 154(9):2562–2570
Liu Y, Zhang S, Yong YC, Ji Z, Ma X, Xu Z, Chen S (2011) Efficient production of acetoin by the newly isolated Bacillus licheniformis strain MEL09. Process Biochem 46(1):390–394
Liu D, Chen Y, Ding F, Guo T, Xie J, Zhuang W, Niu H, Shi X, Zhu C, Ying H (2015) Simultaneous production of butanol and acetoin by metabolically engineered Clostridium acetobutylicum. Metab Eng 27:107–114
Lu Y, Turner RJ, Switzer RL (1996) Function of RNA secondary structures in transcriptional attenuation of the Bacillus subtilispyr operon. Proc Natl Acad Sci U S A 93(25):14462–14467
Moffatt BA, Ashihara H (2002) Purine and pyrimidine nucleotide synthesis and metabolism. Arabidopsis Book 1(2002):e0018
Nicholson WL (2008) The Bacillus subtilisydjL (bdhA) gene encodes acetoin reductase/2,3-butanediol dehydrogenase. Appl Environ Microbiol 74(22):6832–6838
Pierrat OA, Raushel FM (2002) A functional analysis of the allosteric nucleotide monophosphate binding site of carbamoyl phosphate synthetase. Arch Biochem Biophys 400(1):34–42
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33(4):305–325
Renna MC, Najimudin N, Winik LR, Zahler SA (1993) Regulation of the Bacillus subtilisalsS, alsD, and alsR genes involved in post-exponential-phase production of acetoin. J Bacteriol 175(12):3863–3875
Shi S, Chen T, Zhang Z, Chen X, Zhao X (2009) Transcriptome analysis guided metabolic engineering of Bacillus subtilis for riboflavin production. Metab Eng 11(4):243–252
Vivijs B, Moons P, Aertsen A, Michiels CW (2014) Acetoin synthesis acquisition favors Escherichia coli growth at low pH. Appl Environ Microbiol 80(19):6054–6061
Wang M, Fu J, Zhang X, Chen T (2012) Metabolic engineering of Bacillus subtilis for enhanced production of acetoin. Biotechnol Lett 34(10):1877–1885
Wang Y, Ma R, Liu L, He L, Ban R (2018) Improvement of uridine production in Bacillus subtilis by metabolic engineering. Biotechnol Lett 40(1):151–155
Wilks JC, Kitko RD, Cleeton SH, Lee GE, Ugwu CS, Jones BD, Bondurant SS, Slonczewski JL (2009) Acid and base stress and transcriptomic responses in Bacillus subtilis. Appl Environ Microbiol 75(4):981–990
Xiao Z, Lu JR (2014) Strategies for enhancing fermentative production of acetoin: a review. Biotechnol Adv 32(2):492–503
Xiao Z, Xu P (2007) Acetoin metabolism in bacteria. Crit Rev Microbiol 33(2):127–140
Xiao ZJ, Liu PH, Qin JY, Xu P (2007) Statistical optimization of medium components for enhanced acetoin production from molasses and soybean meal hydrolysate. Appl Microbiol Biotechnol 74(1):61–68
Zhang X, Yang T, Lin Q, Xu M, Xia H, Xu Z, Li H, Rao Z (2011) Isolation and identification of an acetoin high production bacterium that can reverse transform 2,3-butanediol to acetoin at the decline phase of fermentation. World J Microbiol Biotechnol 27(12):2785–2790
Zhang X, Zhang R, Bao T, Yang T, Xu M, Li H, Xu Z, Rao Z (2013) Moderate expression of the transcriptional regulator ALsR enhances acetoin production by Bacillus subtilis. J Ind Microbiol Biotechnol 40(9):1067–1076
Zhang X, Bao T, Rao Z, Yang T, Xu Z, Yang S, Li H (2014a) Two-stage pH control strategy based on the pH preference of acetoin reductase regulates acetoin and 2,3-butanediol distribution in Bacillus subtilis. PLoS One 9(3):e91187
Zhang X, Zhang R, Bao T, Rao Z, Yang T, Xu M, Xu Z, Li H, Yang S (2014b) The rebalanced pathway significantly enhances acetoin production by disruption of acetoin reductase gene and moderate-expression of a new water-forming NADH oxidase in Bacillus subtilis. Metab Eng 23(2):34–41
Zhang B, Li N, Wang ZW, Tang YJ, Chen T, Zhao XM (2015) Inverse metabolic engineering of Bacillus subtilis for xylose utilization based on adaptive evolution and whole-genome sequencing. Appl Microbiol Biotechnol 99(2):885–896
Zhu H, Yang SM, Yuan ZM, Ban R (2015) Metabolic and genetic factors affecting the productivity of pyrimidine nucleoside in Bacillus subtilis. Microb Cell Factories 14(1):1–12
Acknowledgements
We gratefully acknowledge the generous support of Dr. Rui Ban from Tianjin University. This work was financially supported by the National Natural Science Foundation of China (31700037), Key Technologies Research and Development Program of Tianjin (16YFZCSY00770), China Postdoctoral Science Foundation(2018M631747) and Research Project of Tianjin Education Commission (2017KJ006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This paper does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 63 kb)
Rights and permissions
About this article
Cite this article
Fan, X., Wu, H., Jia, Z. et al. Metabolic engineering of Bacillus subtilis for the co-production of uridine and acetoin. Appl Microbiol Biotechnol 102, 8753–8762 (2018). https://doi.org/10.1007/s00253-018-9316-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9316-7