Abstract
Despite the large number of bioreporters developed to date, the ability to detect heavy metal(loid)s with bioreporters has thus far been limited owing to the lack of appropriate genetic systems. We here present a novel approach to modulate the selectivity and sensitivity of microbial whole-cell bioreporters (WCBs) for sensing metal(loid)s via the znt-operon from Escherichia coli, which were applied to quantify the bioavailability of these contaminants in environmental samples. The WCB harboring the fusion gene zntAp::egfp was used as a microbial metal(loid) sensor, which was turned on by the interaction between ZntR and metal(loid) ions. This design makes it possible to modulate the selectivity and sensitivity to metal(loid)s simply by changing the metal-binding property of ZntR and by disrupting the metal efflux system of E. coli, respectively. In fact, the E. coli cell-based bioreporter harboring zntAp::egfp showed multi-target responses to Cd(II), Hg(II), and Zn(II). However, the WCBs showed responses toward only Cd(II) and Hg(II) when the amino acid sequence of the metal-binding loop of ZntR was changed to CNHEPGTVCPIC and CPGDDSADC, respectively. Moreover, the sensitivity toward both Cd(II) and Hg(II) was enhanced when copA, which is known to export copper and silver, was deleted. Thus, our findings provide a strong foundation for expanding the target of WCBs from the currently limited number of genetic systems available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diverse materials released to environmental systems in excessive amounts have resulted in substantial environmental problems that ultimately pose a serious risk for human health. Therefore, it is crucial to continuously monitor the level of diverse pollutants in environmental systems. Typically, the amount of pollutants, such as heavy metal(loid)s and diverse chemical compounds are determined with the use of analytical instruments. However, such instrumental analyses are time-consuming and cannot reflect the full bioavailability of contaminants since they typically measure the total amount in the environmental samples (Juhasz et al. 2006; Magrisso et al. 2008; Walker et al. 2003). Although information of the total amount of contaminants is clearly an important aspect required to assess the overall risk, this information could possibly exaggerate the degree of the adverse effects because a large portion of contaminants, such as heavy metal(loid)s will be present in biologically inactive forms. Despite controversy related to the most important data required to accurately assess the risk of contamination, it is generally agreed that microbial cell-based biosensors, otherwise known as whole-cell bioreporters (WCBs), are useful alternative tools to measure the bioavailability of contaminants, which have been intensively studied and developed in recent years (Belkin 2003; Bjerketorp et al. 2006; Harms et al. 2006).
The majority of bacterial cell-based biosensors developed to date have a common working mechanism. The fusion of a set of genes, including a sensing domain, promoter regions of stress-responsive genes, and reporter domains, along with genes encoding enzymes and fluorescent proteins, are introduced to bacterial cells, and the transcription of the reporter genes is controlled by the regulatory proteins for sensing domains (Belkin 2003; Daunert et al. 2000). Accordingly, the presence of stresses, including heavy metal(loid)s, antibiotics, and chemical compounds, can be determined by measuring the expression level of the reporter genes (Close et al. 2009; Hong and Park 2014; Magrisso et al. 2008). In this way, the presence of target contaminants in environmental samples can be determined by WCB systems relatively easily, and quantification can also be achieved if the responses of reporter genes are proportional to the amount of target materials present (Yoon et al. 2016a; Yoon et al. 2016b). Despite these several advantageous aspects of WCBs over traditional instrumental analysis, the practical application of WCBs has thus far been relatively limited because there are not yet a sufficient number of WCB systems to correspond to the number of potential contaminants, and the selectivity and sensitivity of currently available WCBs are insufficient to quantify specific targets. Thus, it is necessary to enhance the properties of WCBs, such as their selectivity and sensitivity toward targets.
The majority of metal(loid)s-sensing WCBs shows broad selectivity for sensing multiple targets (Hynninen and Virta 2009; Magrisso et al. 2008). In fact, the selectivity of WCBs is generally determined by the interaction between the regulatory proteins controlling the sensing domain and targets. In most cases, a regulatory protein plays a role as a repressor to turn off the expression of a reporter gene, and is then released from DNA upon the interaction with targets to then turn on the expression of reporter genes (Branco et al. 2013; Merulla et al. 2013; Yoon et al. 2016c; Yoon et al. 2016d). Therefore, it would be possible to modulate the selectivity of reported WCBs by engineering the target recognition of regulatory proteins. This system would further help to enhance sensitivity by disrupting the bacterial homeostasis system, since it is mainly dependent on the amount of biologically available metal(loid)s in cells. Indeed, previous studies showed that the target metal sensitivity was improved by up to 45 times when deleting a gene encoding a metal-exporting protein (Hynninen et al. 2010; Ibáñez et al. 2015; Kang et al. 2018): disruption of the metal-exporting system resulted in the accumulation of target metals in cells, thereby enhancing the sensitivity. In particular, a WCB driven by the promoter of the zinc/cadmium/lead-transporting P-type ATP-ase (zntA) as a sensing domain and enhanced green fluorescent protein (egfp) as a reporter gene was reported to show relatively broad selectivity toward metal(loid)s, such as Cd, Zn, and Hg (Brocklehurst et al. 1999; Wang et al. 2012). In this system, the expression of the reporter gene is controlled by a regulatory protein corresponding to the sensing domain; thereby, this broad selectivity achieved is likely attributed to the broad selectivity of the regulatory protein, ZntR. From this perspective, we hypothesized that the selectivity of a WCB could be changed by enhancing the selectivity of the regulatory protein. Thus, in the present study, we propose a new approach for enhancing the sensitivity and modulating the metal selectivity of WCBs by employing the promoter of zntA and egfp genes as sensing and reporter domains, respectively. The broad selectivity was modified to respond to a single metal(loid) ion by engineering the regulatory protein ZntR, and the sensitivity was further enhanced by deleting the copA gene, which encodes a metal-exporting protein.
Materials and methods
Construction of mutant E. coli strains and plasmids
Escherichia coli BL21 (DE3) was used as a host strain for the WCBs, and the genes encoding the copper ion exporting channel (copA) and the regulatory protein for zinc-inducible operon (zntR) in the chromosomal DNA of E. coli were deleted by the Quick & Easy E. coli Gene Deletion Kit (Gene Bridges, Heidelberg, Germany) according to the manufacturer instructions. In brief, a pRedET plasmid carrying the recombinase gene was transformed into E. coli BL21 (DE3), and then the FRT-flanked PGK-gb2-neo cassettes targeting zntR and copA were electroporated at 1350 V, 10 μF, and 600 Ω using an Eppendorf Electroporator 2510. The target genes were replaced with the kanamycin resistant gene (kan) by recombinase activity induced with the addition of 10% arabinose. In the case of double gene deletion for BL21-zntR/copA, an additional deletion process was applied to the single gene-deleted E. coli strain. The metal-sensing plasmids pZntA-eGFP and pZntA-mCherry were constructed in a previous study, consisting of the promoter region of zntA (zntAp) in E. coli and egfp or mcherry as a sensing and reporter domain, respectively (Yoon et al. 2016a; Yoon et al. 2016c). The information of the E. coli strains generated in this study as well as the plasmids and WCBs is summarized in Table 1. The pCDF-ZntR plasmid was used as a template for metal-binding loop-exchanged ZntR mutants, as described previously (Yoon et al. 2017), and two-step polymerase chain reaction (PCR) was employed to replace the metal-binding loop region of ZntR with different sequences and lengths of amino acids (Good and Nazar 1992). The sequences of primers used in this study and amino acid sequences of newly introduced loops are listed in Tables 2 and 3, respectively. The DNA polymerase for PCR and the genomic DNA extraction kit were purchased from Qiagen, and the enzymes used for molecular cloning were purchased from Takara Korea Biomedical.
WCB assay
The WCBs tested in this study were generated by introducing the sensing plasmid pZntA-eGFP with a regulatory plasmid carrying the wild-type (WT) and mutant zntR genes into E. coli WT and mutant strains. Only pZntA-eGFP was introduced to WT E. coli BL21(DE3), while the plasmid carrying zntR was co-transformed with pZntA-eGFP to recover the role of ZntR, the regulatory protein for zntAp::egfp, in the case of the E. coli ΔzntR and ∆zntR/∆copA mutant strains.
The metal(loid)s selectivity and sensitivity of the WCBs were tested for AsCl3, CdCl2, CrSO4, NiCl2, HgCl2, PbSO4, ZnCl2, and CuSO4 (Sigma-Aldrich), which were dissolved in distilled water to prepare 1-mM stock solutions, according to previously reported procedures (Yoon et al. 2016a). In brief, the WCB cells were grown overnight and inoculated into fresh Luria-Bertani broth. When the optical density at 600 nm reached 0.3–0.4, the cells were treated with each metal(loid) solution at a final concentration of 1 μM. After 1–2 h of further incubation, the expression level of eGFP was measured by fluorescence spectrophotometry on the FC-2 fluorescence spectrophotometer (Scinco, Korea) equipped with a xenon lamp as a light source and 0.1–5-nm bandwidth filter sets in the emission and excitation wavelengths of 500–600 and 480 nm, respectively. The response of the WCB to each metal(loid) was expressed as the induction coefficient value defined as [arbitrary unit of eGFP fluorescence with metal(loid) treatment]/[arbitrary unit of eGFP fluorescence without metal(loid) treatment].
Characterization of the metal(loid)-sensing properties of WCBs
To confirm the newly obtained metal(loid)-sensing properties of the WCBs BL21-zntR/copA cells harboring pZntA-eGFP/pZntR-loop1 (Cd-sensing WCB) and pZntA-eGFP/pZntR-loop2 (Hg sensing WCB), they were exposed to single and combined Hg and Cd treatment. Specifically, the WCBs were exposed to 1 μM of Cd, Hg, or Cd/Hg, and the expression of eGFP was measured. In addition, the WCBs were exposed to a fixed concentration of Cd or Hg with 2.5 and 0–10 μM of the opposite metal(loid). The expression level of eGFP induced by Cd and Hg was measured by the fluorescence spectrophotometer and expressed as the induction coefficient values.
Simultaneous quantification of Cd and Hg using novel WCB systems
To elucidate the applicability of the novel WCB systems, the amounts of Cd and Hg in contaminated solution were determined using the Cd-sensing WCB and Hg-sensing WCB-Red. Since green and red emission at 510 nm/610 nm represents Cd and Hg, respectively, it was possible to quantify the amount of Cd and Hg simultaneously. To test this proposed approach, the two WCBs were mixed and exposed to 0–5 μM of the Cd/Hg mixture for 1 h. The expression levels of eGFP and mCherry were determined and converted to induction coefficient values. The induction coefficient values of eGFP and mCherry were analyzed by linear regression to obtain standard curves for Cd and Hg, respectively. The amounts of Cd and Hg in artificially contaminated solution samples were then determined based on the equation of standard curves obtained from linear regression analysis.
Results
Genetic engineering to modulate the properties of WCBs
The metal(loid)-sensing properties of WCBs were determined based on monitoring the expression of regulatory proteins, which control the transcription of the reporter genes. In the case of the zntAp::egfp system, ZntR plays a key role in regulating the transcription of egfp. As shown in Fig. 1, two zinc ions interact with the binding loops of the ZntR dimer. Therefore, to modify the metal-binding properties of ZntR, it was replaced with new metal-binding loops consisting of different sequences and lengths. The insertion of new loops was performed by two-step PCR (Good and Nazar 1992), and the DNA sequences encoding new peptides were placed in the primer sequences. Ultimately, we obtained five zntR mutants, named pZntR-loop1–5, and the DNA sequences of the new loop regions were confirmed by DNA sequencing.
Three-dimensional structure of the ZntR homodimer (a) and metal-binding loop region of ZntR with two zinc ions (b). The metal-binding loop regions and zinc ions are indicated in green and magenta, respectively. The structure was downloaded from the Protein Data Bank and visualized using the PyMol software (DeLano 2002)
To elucidate the effects of the new loops of ZntR on the properties of WCBs, the plasmids carrying mutant zntR would normally be introduced with the sensing plasmid, pZntA-eGFP. However, this process would not be sufficient since E. coli expresses endogenous ZntR. Thus, it was necessary to first remove the endogenous zntR from E. coli to test the effect of the mutants. In addition, the metal-exporting gene copA was also deleted to enhance the metal(loid) sensitivity of the WCBs. Accordingly, three mutant E. coli strains were obtained, named BL21-zntR, BL21-copA, and BL21-zntR/copA that lost zntR, copA, and zntR/copA, respectively. These mutants were then used as the host strains to receive the plasmids. Consequently, several WCBs were generated by the combination of plasmids and E. coli strains.
Effect of genetic engineering on the metal(loid)-sensing properties of WCBs
To test the metal(loid)-sensing properties of the newly generated WCBs, they were treated with diverse metal(loid)s, including As, Cd, Cr, Ni, Hg, Pb, Zn, and Cu, at a final concentration of 1 μM for 1 h. The WT E. coli BL21 harboring pZntA-eGFP and the mutant strain BL21-zntR harboring pZntA-eGFP and pCDF-ZntR were first tested to verify whether exogenous ZntR could recover the role of endogenous ZntR. As reported in our previous study, WT E. coli BL21 harboring pZnt-eGFP and BL21-zntR with pZnt-eGFP/pCDF-ZntR showed similar responses toward the tested metal(loid), demonstrating that exogenous ZntR could recover the role as a regulatory protein (Yoon et al. 2017). Since the effects of ZntR mutants should be tested in zntR-deleted E. coli, BL21-zntR harboring pZntA-eGFP/pCDF-eGFP (hereafter, WT WCB) was used as a positive control for further study.
Although exogenous ZntR recovered the deleted endogenous ZntR, the tested WCBs harboring mutant ZntR-loop1–5 based on BL21-zntR showed no significant response toward the tested metal(loid)s. However, WCBs harboring the same plasmids based on BL21-zntR/copA showed enhanced responses from ZntR-loop1 and ZntR-loop2 (Fig. S1). Moreover, disruption of the metal homeostasis system did not affect the properties of the bacterial cell-based bioreporters. Since only loop1 and loop2 among the tested ZntR mutants showed an enhanced response, these two mutants were further investigated to determine the metal(loid) selectivity by comparison to the properties of WT WCB. The WCBs were exposed to 1 μM of metal(loid)s for 1 h and then the expression level of eGFP was measured, represented as the induction coefficients. As shown in Fig. 2, WCB with the pZntR-loop 1 (CNHEPGTVCPIC) showed a Cd-specific response despite a decrease in the arbitrary fluorescent unit. Moreover, ZntR-loop2 (CPGDDSADC) responded to both Cd and Hg with greatly enhanced signals, and the specific responses toward Cd and Hg were opposite to those of WT WCB.
Responses of tested WCBs toward metal(loid)s. a Induction coefficient values of WT WCB (BL21-zntR/copA harboring pZntA-eGFP/pCDF-ZntR) toward tested metal(loid)s. b Induction coefficient values of Cd-sensing WCB (BL21-zntR/copA harboring pZntA-eGFP/pZntR-loop1). c Induction coefficient values of Hg-sensing WCB (BL21-zntR/copA harboring pZntA-eGFP/pZntR-loop2)
Characterization of Cd- and Hg-sensing WCBs
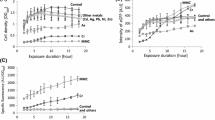
In contrast to WT WCB, which responds to both Cd and Hg, the newly developed WCBs with ZntR-loop1 and -loop2 showed modulated metal(loid) selectivity based on the screening assay. Although ZntR-loop2 responded to both Cd and Hg, the WCB based on BL21-zntR/copA harboring pZntA-eGFP/pZntR-loop1 and pZntA-eGFP/pZntR-loop2 were named Cd-sensing WCB and Hg-sensing WCB, respectively, for convenience. To investigate their metal(loid)s-sensing properties, the WCBs were treated with 1 μM of Cd and Hg independently and in combination. If WCBs are specific to a single metal(loid) ion, the induction coefficient values would be similar for both the independent and combined treatments. As expected, WT WCB showed an induction coefficient value of 5.1 for Cd, 6.1 for Hg, and 8.5 for the combined treatment of Cd and Hg, whereas the Cd-sensing WCB showed values of 4.6, 1, and 4.4, respectively (Fig. 3). Based on these results, it was concluded that WT WCB responded to both Cd and Hg, whereas Cd-sensing WCB was specific to only Cd. Although the Hg-sensing WCB also responded to Cd, the induction coefficient value for Hg was much stronger, and that of the combined treatment was also highly similar to the level observed for the Hg treatment (Fig. 3).
Responses of WCBs toward sole and combined treatment of Cd and Hg. The responses to Cd, Hg, and Cd/Hg treatment were compared. WT WCB showed responses toward Cd and Hg, and the response was increased compared to that of the combined Cd/Hg treatment. Cd-specific WCB responded to only Cd and the response to combined Cd/Hg showed a similar level to that of sole Cd treatment. Hg-specific WCB showed a strong response to Hg and a weak response to Cd
We further verified the metal specificity of the newly generated WCBs. For this purpose, the concentration of a given metal was fixed at 2.5 μM and then the concentration of the other was varied from 0 to 5 μM. As expected, the induction coefficient values for WT WCB were increased upon increasing the concentration of Cd and Hg from WT WCB (Fig. 4a, b), since this WCB responded to both Cd and Hg. Moreover, the eGFP signal clearly increased when the two metals were combined. By contrast, the Cd-sensing WCB showed an increased signal when the Cd concentration was increased (Fig. 4c, d) with similar responses to the combined treatment of Cd plus Hg and no response to Hg. This finding suggested that the WCB obtained Cd selectivity through loop1 exchange in ZntR. Similarly, for the Hg-sensing WCB, the response toward Hg was dominant and the eGFP signal increased upon the increase of Hg concentration, which was the opposite pattern observed from the Cd-sensing WCB (Fig. 4e, f). However, this WCB cannot be fully considered to be Hg-specific since it still showed a response to Cd, even though it was relatively weak. Despite this lack of specific only to Hg, this WCB could still be used to measure Hg under controlled experimental conditions, such as with a shorter exposure time and using a different reporter gene with a longer maturation time.
Concentration-dependent responses of tested WCBs with combined treatment of Cd and Hg. The concentration of Cd and Hg was fixed at 2.5 μM and the concentration of the opposite metal(loid) ranged from 0 to 5 μM. a Induction coefficient values of WT WCB from fixed Cd with 0–5 μM of Hg. b Induction coefficient values of WT WCB from fixed Hg with 0–5 μM of Cd. c Induction coefficient values of Cd-specific WCB from fixed Cd with 0–5 μM of Hg. d Induction coefficient values of Cd-specific WCB from fixed Hg with 0–5 μM of Cd. e Induction coefficient values of Hg-specific WCB from fixed Cd with 0–5 μM of Hg. f Induction coefficient values of Hg-specific WCB from fixed Hg with 0–5 μM of Cd
Effect of reporter genes on the metal-sensing properties of WCBs
Since the so-called Hg-sensing WCB was not a single metal-targeting sensor, it was subject to further modulating processes toward improving the specificity. In our previous study, we found that the sensitivity of WCBs was modulated by changing reporter genes (Yoon et al. 2016c). Thus, in the present study, egfp was replaced with mcherry, which has a relatively longer maturation time, so as to decrease the Hg sensing of the Hg-sensing WCB. To elucidate the effects of reporter genes, the responses of WCBs based on BL21-zntR/copA harboring pZntR-loop2/pZntA-eGFP and pZntRloop2/pZntA-mCherry toward Cd and Hg were tested. The WCBs were exposed to 0–10 μM Cd and Hg for 1 h, and the expression levels of eGFP and mCherry were measured. As shown in Fig. 5, the induction coefficient values of eGFP increased in a concentration-dependent manner with both Cd and Hg. However, the induction coefficient values induced by Hg were much higher (1–40) than those induced by Cd (1–2) from the WCB with mCherry. Therefore, it was confirmed that the sensitivity of WCBs would be varied by changing the properties of reporter genes. Moreover, based on these results, our so-called Hg-specific WCB should be renamed as Hg-sensing WCB-Red, given that it cannot be considered as a universal Hg-sensing WCB since it still showed responses toward Cd at exposure times over 1 h (data not shown). Thus, Hg-sensing WCB-Red indicates that the WBC is specific to sensing Hg under the restricted experimental condition of detection with mCherry as the reporter. Nonetheless, we have shown that the properties of WCBs could be modulated by varying the reporter genes, and were able to obtain the Hg-specific WCB from the zntAp::egfp system by genetic and biochemical engineering.
Simultaneous quantification of Cd and Hg using the new WCB systems
As described above, using our proposed approach, we developed novel WCBs that specifically responded to Cd and Hg through genetic and biochemical engineering processes. Since the detection of Cd and Hg is reflected through the emission of eGFP and mCherry, respectively, with these systems, it should be possible to determine the amount of Cd and Hg in a contaminated sample without interference of emission signals. Therefore, the simultaneous detection of Cd and Hg could be achieved by measuring the fluorescence of the eGFP and mCherry signals. To test this idea, the Cd-specific WCB and Hg-specific WCB-Red were freshly grown and mixed at a 1:1 ratio, and then exposed to a Cd/Hg mixed solution at various concentrations, ranging from 0 to 5 μM. The emission signals of eGFP and mCherry measured at 510 and 610 nm, respectively, were converted to induction coefficient values and plotted against the concentration of Cd and Hg. The results showed that the induction coefficient of eGFP and mCherry increased in proportion to the concentration of Cd and Hg, respectively (Fig. 6a). The correlations between the induction coefficient values and concentration were then used to fit the linear regression equations and used as standard curves for Cd and Hg quantification (Fig. 6b, c). Using this method, artificial sample prepared with 2.50/1.50 μM was found to contain 2.74/1.32 μM that was 91.2/88.0% of accuracy based on standard curves. Conclusively, the novel WCBs obtained in this study show good ability for the simultaneous quantification of Cd and Hg independently.
Simultaneous quantification of Cd and Hg with a mixture of Cd-specific WCB and Hg-specific WCB-Red. a Induction coefficient values of eGFP and mCherry in WCB mixtures determined from an Cd/Hg mixture ranging from 0 to 5 μM. b Standard curves obtained from eGFP and mCherry for Cd (left) and Hg (right) quantification, respectively. The equation for each standard curve fit is shown in the figure with associated R2 values
Discussion
Bacterial cell-based biosensors, so-called WCBs, have been actively developed recently owing to their simplicity, low cost, and convenience. Although WCBs show great potential for monitoring the concentration of contaminants in environmental systems, the primary limitations are the low number of target contaminants and broad selectivity and specificity. In the present study, we sought to overcome these limitations by expanding the inducible genetic systems and broad selectivity of the regulatory proteins of WCBs. Since the working mechanism of WCBs is based on genes related to stress-inducible systems and reporter genes (Belkin 2003; Harms et al. 2006), the expression of reporter gene in WCBs representing the presence of target materials is controlled by regulatory proteins. Therefore, we focused on two main aspects to modulate the properties of WCBs: (1) modifying the metal-binding properties of the regulatory protein ZntR and (2) disrupting the metal homeostasis system in E. coli.
We introduced mutations on ZntR to modulate the properties of WCBs based on pZntA-eGFP. The metal-binding loop region of ZntR was determined in a previous study and the three-dimensional structure was deposited to the Protein Data Bank (Changela et al. 2003). ZntR forms a homodimer and two cysteines in the zinc-binding loop region interact with zinc ions. Therefore, the metal-binding loop of ZntR was replaced by peptides with different lengths and sequences by two-step PCR, while the DNA-binding domain of ZntR recognizing the zntA promoter region was not altered. However, none of the ZntR mutants responded to metal(loid)s in BL21-zntR. Therefore, we moved on to the next strategy, disruption of metal homeostasis, by deleting the metal-exporting genes zntA and copA (Franke et al. 2001; Rensing et al. 1997; Wang et al. 2012). In the case of zntA/zntR deletion, the selectivity of WCBs was abolished because the responses toward metal(loid)s were too high (data not shown). However, ZntR-loop1 and ZntR-loop2 showed modulated metal-sensing properties in BL21-copA/zntR, which provided evidence that the properties of WCB could be modulated by disrupting metal homeostasis; thus, BL21-copA/zntR was used as a host strain for WCB in further investigations. Moreover, ZntR-loop1 and -loop2 possessing more than two cysteines showed modulated properties from the WT ZntR, whereas those with one or no cysteine showed no response to any of the tested metal(loid)s. Although it is not clear why the loop replacement changed the metal-binding properties, it is clear that more than two cysteines are necessary to interact with the metal(loid) ions. Importantly, loop1 with three cysteines specifically responded to Cd while loop2 with two cysteines favorably responded to Hg. Therefore, the coordination number of target metal(loid)s and number of cysteines in the metal-binding loop region of regulatory proteins are important considerations for engineering WCBs.
The new WCBs with ZntR-loop1 and ZntR-loop2 demonstrated modulated metal-sensing properties compared to WT WCB, which responded to both Cd and Hg and are thus insufficient to apply to contaminated environmental samples. Although the Cd-specific WCB responded only to Cd, the Hg-specific WCB responded to both Cd and Hg, even though the responses increased in an Hg concentration-dependent manner. Moreover, since its Hg specificity was too high, it was not sufficient to quantify the amount of Hg in contaminated samples, and it also responded to Cd. Therefore, to improve the Hg specificity, the WCB was further engineered. Among the several different methods reported for improving the sensitivity of WCBs (Nivens et al. 2004; Yagur-Kroll and Belkin 2011), we first tried to change the reporter gene from egfp to mcherry since the sensing properties of WCBs have been shown to be changed upon a change in reporter genes (Yoon et al. 2016c). This new system was named Hg-specific WCB-Red, and since the maturation time of mCherry is longer than that of eGFP, the sensitivity toward Cd or Hg would be decreased. As expected, Hg-specific WCB-Red showed a much lower fluorescent signal compared to the Hg-specific WCB and the response to Cd was diminished. Although the response to Cd could still be observed when the exposure duration was increased, we concluded that Hg-specific WCB-Red is a genuine Hg-specific sensor under the controlled experimental condition. Thus, we finally obtained both Cd-specific and Hg-specific WCBs among broad metal(loid)s-specific WCBs based on the znt-operon.
Since the Cd-specific and Hg-specific WCBs contain different colored fluorescent proteins, the amount of both metal(loid)s could be quantified simultaneously from contaminated environmental samples. The standard curves for Cd and Hg quantification were obtained at the same time with a mixed Cd and Hg solution, and we obtained over 90% accuracy in Cd and Hg quantification. This result proved the capability of the novel WCBs for the simultaneous detection of both Cd and Hg in artificially contaminated samples, which suggests that it could be equally applied to environmental samples such as contaminated water and soils.
In conclusion, we demonstrated that the metal-sensing properties of WCBs can be modulated by genetic and biochemical engineering approaches. Although the bacterial cell-based biosensor, WCB, is generally regarded as a simple, cheap, and convenient alternative tool to quantify contaminants, it has not yet been actively applied to environmental systems because of the limited number of gene systems available. This limitation could be overcome by identifying new genetic systems for WCBs from diverse living organisms. However, this would still be an insufficient approach because of the continuously increasing number of contaminants and broad specificity of genetic systems. Alternatively, we have demonstrated the good potential of developing new WCBs from established WCBs through genetic and biochemical engineering.
References
Belkin S (2003) Microbial whole-cell sensing systems of environmental pollutants. Curr Opin Microbiol 6(3):206–212
Bjerketorp J, Håkansson S, Belkin S, Jansson JK (2006) Advances in preservation methods: keeping biosensor microorganisms alive and active. Curr Opin Biotechnol 17(1):43–49
Branco R, Cristóvão A, Morais PV (2013) Highly sensitive, highly specific whole-cell bioreporters for the detection of chromate in environmental samples. PLoS One 8(1):e54005
Brocklehurst KR, Hobman JL, Lawley B, Blank L, Marshall SJ, Brown NL, Morby AP (1999) ZntR is a Zn (II)-responsive MerR-like transcriptional regulator of zntA in Escherichia coli. Mol Microbiol 31(3):893–902
Changela A, Chen K, Xue Y, Holschen J, Outten CE, O'Halloran TV, Mondragón A (2003) Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 301(5638):1383–1387
Close DM, Ripp S, Sayler GS (2009) Reporter proteins in whole-cell optical bioreporter detection systems, biosensor integrations, and biosensing applications. Sensors 9(11):9147–9174
Daunert S, Barrett G, Feliciano JS, Shetty RS, Shrestha S, Smith-Spencer W (2000) Genetically engineered whole-cell sensing systems: coupling biological recognition with reporter genes. Chem Rev 100(7):2705–2738
DeLano WL (2002) PyMOL
Franke S, Grass G, Nies DH (2001) The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiology 147(4):965–972
Good L, Nazar RN (1992) An improved thermal cycle for two-step PCR-based targeted mutagenesis. Nucleic Acids Res 20(18):4934
Harms H, Wells MC, van der Meer JR (2006) Whole-cell living biosensors—are they ready for environmental application? Appl Microbiol Biotechnol 70(3):273–280
Hong H, Park W (2014) TetR repressor-based bioreporters for the detection of doxycycline using Escherichia coli and Acinetobacter oleivorans. Appl Microbiol Biotechnol 98(11):5039–5050
Hynninen A, Tõnismann K, Virta M (2010) Improving the sensitivity of bacterial bioreporters for heavy metals. Bioengineered bugs 1(2):132–138
Hynninen A, Virta M (2009) Whole-cell bioreporters for the detection of bioavailable metals. Whole Cell Sensing System II. Springer, pp 31–63
Ibáñez MM, Checa SK, Soncini FC (2015) A single serine residue determines selectivity to monovalent metal ions in metalloregulators of the MerR family. J Bacteriol 197(9):1606–1613
Juhasz AL, Smith E, Weber J, Rees M, Rofe A, Kuchel T, Sansom L, Naidu R (2006) In vivo assessment of arsenic bioavailability in rice and its significance for human health risk assessment. Environ Health Perspect 114(12):1826–1831
Kang Y, Lee W, Kim S, Jang G, Kim B-G, Yoon Y (2018) Enhancing the copper-sensing capability of Escherichia coli-based whole-cell bioreporters by genetic engineering. Appl Microbiol Biotechnol 102(3):1513–1521
Magrisso S, Erel Y, Belkin S (2008) Microbial reporters of metal bioavailability. Microb Biotechnol 1(4):320–330
Merulla D, Buffi N, Beggah S, Truffer F, Geiser M, Renaud P, van der Meer JR (2013) Bioreporters and biosensors for arsenic detection. Biotechnological solutions for a world-wide pollution problem. Curr Opin Biotechnol 24(3):534–541
Nivens DE, McKnight T, Moser S, Osbourn S, Simpson M, Sayler G (2004) Bioluminescent bioreporter integrated circuits: potentially small, rugged and inexpensive whole-cell biosensors for remote environmental monitoring. J Appl Microbiol 96(1):33–46
Rensing C, Mitra B, Rosen BP (1997) The zntA gene of Escherichia coli encodes a Zn (II)-translocating P-type ATPase. Proc Natl Acad Sci U S A 94(26):14326–14331
Walker DJ, Clemente R, Roig A, Bernal MP (2003) The effects of soil amendments on heavy metal bioavailability in two contaminated Mediterranean soils. Environ Pollut 122(2):303–312
Wang D, Hosteen O, Fierke CA (2012) ZntR-mediated transcription of zntA responds to nanomolar intracellular free zinc. J Inorg Biochem 111:173–181
Yagur-Kroll S, Belkin S (2011) Upgrading bioluminescent bacterial bioreporter performance by splitting the lux operon. Anal Bioanal Chem 400(4):1071–1082
Yoon Y, Kang Y, Chae Y, Kim S, Lee Y, Jeong S-W, An Y-J (2016a) Arsenic bioavailability in soils before and after soil washing: the use of Escherichia coli whole-cell bioreporters. Environ Sci Pollut Res 23(3):2353–2361
Yoon Y, Kang Y, Lee W, Kim S, Oh K, Kim B (2017) Modulating the properties of metal-sensing whole-cell bioreporters by interfering Escherichia coli metal homeostasis. J Microbiol Biotechnol
Yoon Y, Kim S, Chae Y, Jeong S-W, An Y-J (2016b) Evaluation of bioavailable arsenic and remediation performance using a whole-cell bioreporter. Sci Total Environ 547:125–131
Yoon Y, Kim S, Chae Y, Kang Y, Lee Y, Jeong S-W, An Y-J (2016c) Use of tunable whole-cell bioreporters to assess bioavailable cadmium and remediation performance in soils. PLoS One 11(5):e0154506
Yoon Y, Kim S, Chae Y, Kim SW, Kang Y, An G, Jeong S-W, An Y-J (2016d) Simultaneous detection of bioavailable arsenic and cadmium in contaminated soils using dual-sensing bioreporters. Appl Microbiol Biotechnol 100(8):3713–3722
Funding
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2015R1C1A1A02037275 and 2017R1E1A1A01073894 to Y.Y.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This article did not involve any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 124 kb)
Rights and permissions
About this article
Cite this article
Kang, Y., Lee, W., Jang, G. et al. Modulating the sensing properties of Escherichia coli-based bioreporters for cadmium and mercury. Appl Microbiol Biotechnol 102, 4863–4872 (2018). https://doi.org/10.1007/s00253-018-8960-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8960-2