Abstract
Quorum sensing (QS), a communication system involved in virulence of pathogenic bacteria like Pseudomonas aeruginosa is a promising target to combat multiple drug resistance. In vitro studies using clove bud oil (CBO) in P. aeruginosa revealed a concentration dependent attenuation of a variety of virulence factors including motility, extracellular DNA, exopolysaccharides and pigment production. Furthermore, treatment with CBO demonstrated a distinct dose-dependent reduction in biofilm formation as well as promoting dispersion of already formed biofilm, observations that were also supported by porcine skin ex vivo studies. Expression studies of genes involved in signalling systems of P. aeruginosa indicated a specific decrease in transcription of pqsA, but not in the lasI or rhlI levels. Additionally, the expression of vfr and gacA genes, involved in regulation, was also not affected by CBO treatment. CBO also influenced the PQS signalling pathway by decreasing the levels of kynurenine, an effect which was reversed by the addition of exogenous kynurenine. Though the synthesis of the signalling molecules of the Las and Rhl pathways was not affected by CBO, their activity was significantly affected, as observed by decrease in levels of their various effectors. Molecular modelling studies demonstrated that eugenol, the major component of CBO, favourably binds to the QS receptor by hydrophobic interactions as well as by hydrogen bonding with Arg61 and Tyr41 which are key amino acid residues of the LasR receptor. These results thus elucidate the molecular mechanism underlying the action of CBO and provide the basis for the identification of an attractive QS inhibitor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomonas aeruginosa is the foremost cause of secondary infections in immune-compromised patients (Meynard et al. 1999) and accounts for 57 % of total nosocomial infections (Oncul et al. 2009). P. aeruginosa can grow as a planktonic, free-living organism or as attached matrix-enclosed communities called biofilm, which are important for microbial endurance in hostile settings, representing a secluded mode of growth. In clinical settings, the major challenge presented by biofilm forming bacteria is the increased protection they enjoy against host immune responses (Cady et al. 2012). Additionally, this makes them significantly more tolerant to various anti-microbial treatments, resulting in multidrug-resistant organisms with major public health implications (Tamma et al. 2012 ). Furthermore, P. aeruginosa possesses an array of cell associated and extracellular virulence factors (Jimenez et al. 2012), which makes it a versatile pathogen responsible for life-threatening infections. Production of most of these virulence factors are regulated by quorum sensing (QS) (Rumbaugh et al. 2000). Since QS regulates factors contributing to the virulence of a pathogen, it is a promising target for attenuation of bacterial pathogenicity. Hence, inhibitors that regulate biofilm formation and other virulence factors without interfering with bacterial growth have been the focus of recent studies.
The three major QS systems which have been found to control the virulence and biofilm formation in P. aeruginosa are LasI-LasR, RhlI-RhlR and PQS-MvfR (Venturi 2006). LasI produces an extracellular diffusible acyl homoserine lactone (AHL) signal molecule, N-(3-oxododecanoyl)-L-homoserine lactone (OdDHL), which is recognized by the transcriptional regulator LasR, resulting in diverse gene expression in the RhlI-RhlR system. Similarly, RhlI produces the N-butyryl-L-homoserine lactone (BHL) signal molecule which binds to its cognate transcriptional regulator RhlR. LasR and RhlR transcriptional regulators are activated when adequate levels of OdDHL and BHL are attained, resulting from a high population density of P. aeruginosa (Pesci et al. 1997). In the PQS-MvfR system, 2-heptyl-3-hydroxy-4(1H) quinolone (PQS), and its precursors bind to the transcriptional regulator MvfR and control the transcription of downstream targets (Heeb et al. 2011). The Las system regulates a complex hierarchy of other QS pathways that are regulators of many cellular processes. The PQS pathway is one such pathway that is directly regulated by the Las system (Bacalso et al. 2011). LasR upregulates the expression of pqsA/B/C/D/H and R genes. Of these, pqsA/B/C and D are involved in the synthesis of the precursor of PQS. In view of the fact that QS regulates factors contributing to the virulence of P. aeruginosa, it is a promising target for attenuation of bacterial pathogenicity.
Plant-derived compounds have traditionally been used to treat microbial infections (Mahady, 2005). Essential oils (EOs) are aromatic liquids obtained from plants and are rich sources of biologically active compounds. Extracts of various natural products and EOs of several plants (e.g., lavender, eucalyptus, cinnamon and citrus) (Szabo et al. 2010) exhibit anti-QS effects. Clove bud oil (CBO) demonstrates many useful properties and has been used extensively in dental remedies (Bhowmik et al. 2012). Applications of CBO in aromatherapy to aid with digestive problems are also well established (Sanlin et al. 2011). Additionally, CBO has also been used to alleviate muscular aches and pains (Shaaban et al. 2012) and used in vapour therapy for relief from respiratory problems (Bhowmik et al. 2012). The present study provides convincing evidence that CBO significantly inhibits P. aeruginosa biofilm formation, as well as promotes dispersion of formed biofilm. This effect is mediated by attenuation of a variety of virulence factors including motility, extracellular DNA, exopolysaccharide and pigment formation. CBO also causes a specific decrease in pqsA gene transcription and reduction in kynurenine levels. The hydrogen bonding and hydrophobic interactions of eugenol, the major component of CBO with Arg61 and Tyr47, which are key amino acid residues of the Las R receptor, are also critical for its inhibitory effect. The anti-QS effect of CBO towards P. aeruginosa, thus provides a better insight into infections caused by this pathogen, and the basis for identification and characterization of an effective means of QS inhibition.
Materials and Methods
Bacterial strains and treatments
P. aeruginosa wild type strain, PAO1 (ATCC 15692), obtained from American Type Culture Collection, Manassas, VA, USA was grown in Luria Bertani (LB) broth at 37 °C and treated with commercially obtained CBO (Syzygium aromaticum) (Plant Lipids Pvt. Ltd., Cochin, Kerala, India) in LB broth for 24 h.
Assessment of biofilm formation and biofilm dispersal
Biofilm formation was determined as previously described (Adonizio et al. 2008). Briefly, the stationary phase culture of PAO1 was diluted in fresh medium, followed by overnight incubation with CBO in polystyrene microtitre plates (BD, USA) at 37 °C for 24 h. Surface-attached cells were quantified using 0.1 % (w/v) aqueous solution of crystal violet, and the absorbance was measured at 600 nm. For biofilm dispersal assays, the biofilm was first allowed to develop in polystyrene microtitre plates for 24 h in the absence of CBO treatment. Following incubation, the plates were washed, and fresh media containing CBO was added and incubated for an additional 24 h (Quave et al. 2008). The staining techniques were the same as those described above.
Microscopic observation of biofilm
To observe the dispersal of mature biofilm, glass slides were used to develop the biofilm from cultures grown at 37 °C at 100 rpm for 24 h. The mature biofilm was incubated with CBO for an additional 24 h, and the unbound bacteria were removed by rinsing the slides with sterile saline. The samples were air dried and processed for atomic force microscopy (AFM) (Oh et al. 2009).
Growth curve
The effect of CBO on cell proliferation of P. aeruginosa PAO1 was determined as per Thenmozhi et al. (2009). CBO was added to 50 ml LB, to which a 1 % inoculum from the overnight culture was added. This was incubated at 37 °C. Growth medium, without the addition of P. aeruginosa PAO1, served as a negative control, as compared to medium with the addition of bacteria, which served as a positive control. The growth rate was monitored by taking the OD at 600 nm (OD600) for 24 h at hourly intervals.
Swarming motility
The bacteria was cultured in media containing 0.5 % agar, 0.5 % peptone, 0.2 % yeast extract and 1 % glucose in distilled water with or without CBO. Two microlitres of late log phase cultures of PAO1 diluted to 0.1 OD600 was inoculated at the centre of the agar, following which the plate was incubated for 16 h at 37 °C. The extent of swarming motility was determined by measuring the diameter of the swarming migration from the point of inoculation (Rashid and Kornberg 2000).
Swimming and twitching motility
Determination of swimming and twitching motility was carried out, according to the method described by Rashid and Kornberg 2000. The bacterial culture was inoculated in media containing 1 % tryptone, 0.5 % NaCl and 0.3 % agar with or without 1 % CBO and incubated for 16 h at 37 °C. Motility was assessed by the circular turbid zone formed by bacteria migrating away from the point of inoculation. To study twitching motility, cells were stab inoculated through 1 % LB agar layer, to the bottom of the petri dish. After incubation for 24 h at 37 °C, the extent of motility was determined by measuring the hazy zone of growth at the interface between the agar and the polystyrene surface.
Isolation and quantification of exopolysaccharides
Isolation and quantification of exopolysaccharides (EPS) was done as described previously (Myska and Czaczk, 2009). Bacteria treated with or without 1 % CBO was harvested by centrifugation at 8000g for 20 min at room temperature. The cells were resuspended in 1.5 ml of 30 % (w/v) NaOH, boiled for 15 min and centrifuged at 15,000g for 15 min, and the supernatant was added drop wise to 60 % (v/v) ethanol. The total EPS was determined using the acid hydrolysis method (Myska and Czaczk, 2009). The precipitated EPS was collected by centrifugation at 15,000g for 20 min. The pellet obtained was resuspended in 1 ml sterile water, mixed with 7 ml of 77 % (v/v) H2SO4 and cooled for 10 min in an ice bath. Subsequently, 1 ml of 1 % (w/v) of cold tryptophan was added, followed by heating in a boiling water bath for 20 min. The acid hydrolysis of EPS produced a furan, which condenses the tryptophan to form a coloured product that was read at OD500 after cooling. The calibration curve was prepared using standard dextran solution.
Measurements of extracellular DNA
Overnight cultures of PAO1 were grown in LB broth and were subsequently diluted to an OD600 of 0.1 in LB medium containing 0.05 mM propidium iodide. The diluted cultures were transferred to wells of polystyrene microtitre plates (100 μl/well) and incubated for 24 h at 37 °C. After incubation, the absorbance of propidium iodide was measured at OD480, and the cell density was measured at OD 600(Yang et al. 2007).
Quantification of pigment production
Pyocyanin was extracted from culture supernatants and measured using previously reported methods (Liang et al. 2011). Briefly, 3 ml of chloroform was added to 5 ml culture supernatant. Following extraction, the chloroform layer was transferred to a fresh tube and mixed with 1 ml of 0.2 M HCl. After centrifugation, the top layer (0.2 M HCl) was removed, and the absorbance at 520 nm was measured. The pyocyanin concentration was determined by multiplying the A520 by 17.072. To estimate Pyoverdin, 1 ml of culture supernatant was diluted in 50 mM Tris-HCl (pH 7.4), and the resulting fluorescence was measured at 465 nm by exciting the sample at 405 nm (Adonizio et al. 2008).
Kynurenine production assay
P. aeruginosa PAO1 was cultured in LB broth at 37 °C with a starting OD600 of 0.2. At the indicated incubation time, the supernatant recovered after centrifugation at 10,000g for 10 min was mixed with an equal volume of Kovac’s reagent. The amount of kynurenine produced was measured at 490 nm and computed from the standard curves (Genestet et al. 2014).
RNA isolation and RT PCR
To examine the effect of CBO on QS genes, the bacterial cultures were treated with different concentrations of CBO. Aliquots (1.5 ml) of bacterial cultures to be used for RNA extraction were transferred to a pre-chilled vial and the cells were harvested by centrifugation at 6000 g for 10 min to remove extracellular membranes, polysaccharides, and DNA. The RNA was extracted by TRIZOL (Life technologies) reagent, which was added to the pellet and incubated for 3 min at room temperature. Ice-cold chloroform (0.1ml) was added, mixed for 15 s and incubated for 15 min followed by centrifugation (8000g) at 4 °C for 15 min. The upper chloroform layer was transferred to a fresh tube to which 0.25 ml of 100 % isopropyl alcohol was added and incubated at −20 °C for 30 min. The mixture was then centrifuged at 14,000 rpm for 10 min at 4 °C to form pellets. The pellets were washed with 1 ml of 70 % ethanol and centrifuged at 14,000 rpm for 5 min at 4 °C. Washed pellets were dissolved in nuclease free water, and the quantity of total RNA was measured using Nanodrop spectrophotometer (Thermo Scientific). One microgram of template RNA was mixed with 1 pM/μl of specific primer (Table S1) and the PCR master mix, as described by the manufacturer (Origin, India). Amplification and detection of the product was performed by employing the one step reverse transcription PCR using Eppendorf Mastercycler (Eppendorf, Hamburg, Germany). Primers were designed as reported by Kruczek et al. 2014.
Comparative infrared analysis of CBO and eugenol
Infrared spectra were recorded (cm−1) on FT-IR spectrophotometer (Shimadzu model no. 01080). Sample spectrums were recorded on film. CBO was compared with the standard compound, eugenol.
In silico docking study of Las R and eugenol
In order to identify the binding mode of eugenol, Auto Dock Tools (http://mgltools.scripps.edu) was used (Nanjan et al. 2015). A PDB coordinate file of LasR (PDB ID: 2UVO) was downloaded from the RCSB PDB data bank and then utilized for the docking studies. 2UVO is the X-ray structure of the N-terminal OdDHL binding domains of LasR complexed with OdDHL. The best docking pose of eugenol was selected and analysed.
Ex vivo porcine skin model
Hides from carcasses of freshly killed adult pigs were obtained from a local slaughterhouse. Prior to tests, the hides were thoroughly washed with sterile water and de-haired. The excess fat deposits were removed, and the skin was cut into sections of 1.5 cm × 1.5 cm. The cut sections were soaked in 70 % ethanol for 5 min and washed thoroughly with sterile water. The disinfected sections were then placed on sterile petri plates and allowed to dry. The log phase culture (OD600 between 0.2 and 0.4, 10 μl) was added to the skin explants. The explants were then placed in culture plates and incubated (37 °C for 24 h, 5 % CO2) for the development of the biofilm. Following incubation with CBO for 24 h, the skin explants were processed for SEM analysis. Subsequently, the explants were processed by sonication in phosphate-buffered saline (PBS) with 5 μl/L Tween-80 to ensure the complete dispersal of biofilm. The accurate colony-forming unit (CFU) counts in the resulting bacterial suspensions were determined by plating serial dilutions on tryptic soy agar (TSA) in triplicates, and the data was reported as CFU/millilitre.
The skin explants were washed with PBS, fixed in Trump’s solution for 1 h and washed three times with PBS at 10 min intervals followed by a single 10-min wash with distilled water. Each fixed and washed explant was dehydrated in a graded ethanol series of 25, 50, 75, 95 and 100 % at 10-min intervals. The explants were then air dried overnight and processed for SEM analysis (Nataraj et al. 2013, Yang et al. 2013).
Statistical analysis
Statistical analyses were performed with the GraphPad Prism 5 software. Values were expressed as mean ± SD. Dunnett-ANOVA test was employed to compare the tests and control. Significance levels were at *p < 0.05 vs control, **p < 0.01 vs control and ***p < 0.001 vs control.
Results
Effect of CBO on biofilm formation and dispersion of pre-established biofilm of P. aeruginosa
A static biofilm assay was employed to evaluate the biofilm formation of P. aeruginosa in the presence of CBO. As illustrated in Fig. 1a, cultures treated with CBO concentrations ranging from 0.01 to 1.0 % demonstrated a dose-dependent reduction of biofilm formation by 5 to 85.3 %, respectively, when compared with the untreated control, indicating that this effect was mediated by CBO. In order to determine if CBO could also affect previously formed biofilm, cultures with pre-established PAO1 biofilm was treated with CBO, at concentrations ranging from 0.01 to 1.0 %. Figure 1a shows a dose-dependent increase in biofilm dispersal ranging from 2.7 to 50.4 %, as compared to the untreated control, demonstrating that CBO also abolished pre-established biofilm, in addition to inhibiting biofilm formation. Atomic force microscopy (AFM) was also utilized to visualize the effect of CBO on dispersion of pre-established biofilm on glass slides. The AFM images revealed distinct differences in surface morphology and topography. The topography of the biofilm treated with CBO showed fewer adherent cells on the surface as compared with the untreated control. Additionally, the thickness and architecture of biofilm in the presence of CBO indicated that the bacterial cells were scattered on the substratum in a thin layer, while in the untreated control, the biofilm appeared mature and stacked densely in multiple layers (Fig. 1b and Fig. S1).
a Effect of CBO on biofilm formation and biofilm dispersion. Pseudomonas aeruginosa (PAO1) was grown in medium containing 0.01–1.0 % CBO to study the effect on biofilm formation and biofilm dispersion. b AFM images of P. aeruginosa PAO1 mature biofilm grown on glass slide treated with or without CBO (c). To study the effect of CBO on bacterial growth, PAO1 was treated with 1.0 % CBO and growth was monitored by taking the OD600. Mean values of triplicate independent experiments and SD are shown. Dunnett’s test demonstrates significant difference between the tests and the control. Significance at *p < 0.05 vs control, **p < 0.01 vs control, ***p < 0.001 vs control
Growth curve analysis
The growth of PAO1 was monitored for 24 h at 37 °C to determine the effect of CBO on bacterial growth. As observed in Fig. 1c, there was no significant difference in the end point cell density of PAO1 in the presence of 1 % CBO as compared to the untreated control, indicating that CBO did not affect the growth of P. aeruginosa. Based on these observations, we concluded that the inhibitory effect exhibited by CBO, on biofilm formation and increased dispersal of already formed biofilm, is not due to alteration in growth patterns.
Effect of CBO on factors that influence biofilm formation
Effect on swarming, swimming and twitching motilities of PAO1
The effect of CBO on swarming, swimming, and twitching motilities of PAO1, all of which play a crucial role in biofilm formation, was tested (Fig. 2a). The swarming motility was substantially decreased by 59.5 % in the presence of 1 % CBO (Fig. 2b). When compared with the swarming motility, the diameter of the swimming zone was decreased by 35 % in the presence of 1 % CBO, while the twitching motility was reduced by 24 % as compared to the untreated control. These results indicated that CBO differentially regulated all three types of motility exhibited by P. aeruginosa.
a Impact of CBO on swarming, swimming and twitching motility exhibited by PAO1. b Motility was measured by taking the diameter of movement of bacteria. Mean values of triplicate independent experiments and SD are shown. Dunnett’s test demonstrates significant difference between the tests and the control. Significance at *p < 0.05 vs control, **p < 0.01 vs control, ***p < 0.001 vs control
Effect on exopolysaccharide level
Quantitative determination of EPS was carried out to determine the potential of CBO on EPS production. As indicated in Fig. 3, the amount of EPS produced by PAO1 was significantly reduced in a dose-dependent manner, i.e. 48.6, 85.9 and 91.9 % reduction in the presence of 0.1, 0.5 and 1 % of CBO, respectively, as compared to the untreated control.
Effect of CBO on exopolysaccharide and extracellular DNA content. P. aeruginosa (PAO1) was treated with 0.01–1.0 % CBO for 24 h and exopolysaccharide (EPS) and extra cellular DNA (e DNA) content was determined. Mean values of triplicate independent experiments and SD are shown. Dunnett’s test demonstrates significant difference between the tests and the control. Significance at *p < 0.05 vs control, **p < 0.01 vs control, ***p < 0.001 vs control
Effect on extracellular DNA
In order to quantify the extracellular DNA (e DNA), the level of fluorescence in the cultures, which was indicative of e DNA, was measured. The amount of e DNA of PAO1 was found to be decreased in a dose-dependent manner with increasing concentration of CBO (Fig. 3). Treatment with 0.5 and 1 % CBO resulted in 56.2 and 65.5 % reduction in the amount of e DNA respectively.
Effect on pigments of PAO1
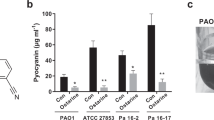
The levels of the pyocyanin and pyoverdine in PAO1 cultures treated with CBO were measured. As indicated in Fig. 4, both pyocyanin and pyoverdine concentrations were decreased by 49.9 and 54.3 %, respectively, in the presence of 1 % CBO as compared to the untreated control.
Effect of CBO on pyocyanin and pyoverdin. P. aeruginosa (PAO1) was treated with 0.01–1.0 % CBO and the amount of pyocyanin and pyoverdin were determined. Mean values of triplicate independent experiments and SD are shown. Dunnett’s test demonstrates significant difference between the tests and the control. Significance at **p < 0.01 vs control, ***p < 0.001 vs control
Effect of CBO on expression of QS genes
To determine whether the inhibition of biofilm formation was due to the decreased expression of QS genes, reverse transcription polymerase chain reaction of RNA from cultures grown in the presence of different concentrations of CBO was performed. P. aeruginosa has three QS systems—namely Las, Rhl and PQS. As shown in Fig. 5a and b our studies demonstrated that CBO did not affect the transcriptional activation of lasI or rhlI genes, indicating that the synthesis of the signalling molecules, OdDHL and BHL, was not affected by CBO. Additionally, the expression levels of gacA and vfr, the positive regulators of QS, were also not significantly affected by CBO. However, the transcriptional activation of pqsA, which is a part of pqsA-E operon, was specifically down regulated by CBO when compared to the untreated control (0.3 fold decrease in the expression compared to the control).
RT PCR analyses of genes involved in QS system of P. aeruginosa PAO1. a Gene expression was evaluated in control and cultures treated with 0.01, 0.1 and 1.0 % CBO. b Fold change in the expression of QS genes of P. aeruginosa treated with CBO. Mean values of triplicate independent experiments and SD are shown. Dunnett’s test demonstrates significant difference between the tests and the control. Significance at ***p < 0.001 vs control
Effect of CBO on kynurenine production
To evaluate the impact of CBO on the kynurenine pathway of P. aeruginosa, with a specific focus on kynurenine production, PAO1 (OD600=0.2) was treated with different concentrations of CBO. As shown in Fig. 6, there was a concomitant decrease in kynurenine levels observed with increasing concentrations of CBO. Kynurenine production was decreased by 61 % in the presence of 1 % CBO as compared to the untreated control. This data suggests that CBO affects the kynurenine pathway, which is essential for the production of PQS signalling molecules.
Effect of CBO on the production of kynurenine. P. aeruginosa PAO1 was grown in LB at 37 °C with different concentrations of CBO. The kynurenine production in the supernatant was measured after 8h. Mean values of triplicate independent experiments and SD are shown. Dunnett’s test demonstrates significant difference between the tests and the control. Significance at *p < 0.05 vs control, **p < 0.01 vs control
P. aeruginosa pyocyanin production in the presence of externally added kynurenine
Since pyocyanin production was regulated by PQS signalling mediated through the kynurenine pathway, the effect of externally added kynurenine on the levels of pyocyanin was determined in the presence of CBO. The addition of 12.5, 25 and 50 μM kynurenine to P. aeruginosa PAO1 cultures containing 1 % CBO led to a corresponding 1.39, 1.46 and 2.16 fold increase in pyocyanin production, relative to cultures treated with 1 % CBO alone (Fig. 7). These results clearly demonstrate that CBO interferes with the kynurenine pathway of P. aeruginosa, by decreasing the production of kynurenine, which in turn leads to a diminished level of PQS signalling, resulting in lowered levels of pyocyanin.
Change in pyocyanin production by P. aeruginosa PAO1 grown in the presence of different concentrations of kynurenine (Kyn) with 1 % CBO. The values are presented as fold change relative to P. aeruginosa treated with 1 % CBO. Mean values of triplicate independent experiments and SD are shown. Dunnett’s test demonstrates significant difference between the tests and the control. Significance at **p < 0.01 vs control
In silico molecular docking of Las R with eugenol
Eugenol is known to be the major component, constituting 88.5 % of CBO (Chaieb et al. 2007). As shown in Fig. S2, IR spectra studies confirmed that the CBO used in the present study also contained eugenol as its major component. Hence, eugenol was utilized for in silico analysis with Las R receptor to understand the binding pattern as compared to its ligand. Molecular modelling of eugenol with Las R receptor indicated that both the methoxy and hydroxyl groups of eugenol hydrogen bond with the amino acids Arg61 and Tyr47, which are key amino acid residues in the active site of Las R. Additionally, eugenol also forms hydrophobic interactions with Ile52, Gly126, Gly127, Lys79, Val76, Ala70, Tyr64 and Leu36. The aromatic hydroxyl and methoxy groups are involved in the inhibition, while the hydrophobic interactions stabilize the overall orientation of eugenol (Fig. 8a and b).
Ex vivo porcine skin explants study
SEM analysis of skin explants was prepared as described earlier (Nataraj et al. 2013, Yang et al. 2013). Explants treated with CBO were nearly devoid of the exopolysaccaride (EPS) matrix when compared with the untreated explants. A substantial number of attached bacterial cells were present on the surface of untreated explants, whereas there was a significant decrease in the bacteria attached on the surface after treatment with 1 % CBO (Fig. 9a, Fig S3). These observations were further substantiated by lower levels of CFU in treated explants, as compared with the untreated control (the mean bacterial load decreasing from 17 × 106 log total CFU to 6 × 105 log) (Fig. 9b). Collectively, both the SEM analyses and CFU levels indicated that CBO significantly reduced the extent of P. aeruginosa, in mature biofilm communities.
CBO inhibits biofilm formation in ex vivo porcine skin model. a Scanning electron micrographs of PAO1 biofilm attached to porcine skin explants. b Difference in colony-forming units in CBO treated and untreated porcine skin explants. Mean values of triplicate independent experiments and SD are shown. t test demonstrates significant difference between the test and the control. Significance at **p < 0.01 vs control
Discussion
The occurrence of multidrug resistance in pathogens like P. aeruginosa is an issue of great clinical concern. Since the antibiotic resistant variants of Pseudomonas were established to be more adherent and virulent in most cases (Aliaga et al. 2002; Wei et al. 2011), the identification of substances which attenuate the virulence, without killing the bacteria, is an attractive alternative. Quorum sensing (QS) is a perfect target for this approach, since most of the virulence factors in P. aeruginosa are under the control of QS. Traditional medicines, such as essential oils, have over the years, generated a great deal of interest as repositories for the development of new drugs and therapeutics (Kim et al. 2015). The present study clearly demonstrates that clove bud oil (CBO) inhibits biofilm formation as well as promotes dispersion of the formed mature biofilm in P. aeruginosa, in a concentration-dependent manner. Since concentrations greater than 1 % resulted in bactericidal effects, further studies were carried out at a concentration of 0.01–1 % clove bud oil.
The AFM images of biofilm treated with CBO revealed distinct differences in surface morphology as well as topography when compared to the untreated control, which showed a dense biofilm with multiple layers of EPS embedded with bacteria. In contrast, the topography of the CBO treated biofilm was thin, less intense and exhibited very little adherence of cells on the surface biofilm. These findings clearly demonstrate that not only does CBO affect the adherence of bacteria from forming biofilms but also causes the eventual dispersion of the biofilm matrix, leading to its structural loss. The CBO thereby makes the organism more vulnerable and provides a possible line of attack, by reducing the resistance of sessile cells to antibiotics.
The growth curve of P. aeruginosa was not altered in the presence of CBO, demonstrating that this compound did not exhibit any bactericidal or bacteriostatic effect. This indicates that the inhibition of biofilm formation was not due to any lethal effect on bacteria and might, therefore, be expected to reduce the resistance as well as restore sensitivity to antibiotics, in addition to allowing for successful clearance by the host immune system (Johnansson et al. 2008). Since QS signalling controls motility, EPS and e DNA production in Gram-negative bacteria like P. aeruginosa (Marketon et al. 2003), all of which play a crucial role in biofilm formation, the effect of CBO was also assessed against these properties. Bacterial motility has a pivotal role in both surface colonization and biofilm formation and is proven to be instrumental in spreading to new environments (Rashid and Kornberg 2000). P. aeruginosa exhibits different types of motility like swimming, swarming and twitching that help in adherence, formation and expansion of biofilm. The swimming motility, which is mediated by the flagella, is used to approach a chosen surface, while attachment and further spreading of the colony is mediated by swarming and twitching motilities, which are regulated by multiple flagella and type IV pili, respectively (Klausen et al. 2003). Earlier reports have indicated that swarming of P. aeruginosa is controlled mainly by the Rhl QS system and partly by the Las system, while the swimming and twitching motilities are under the control of the PQS system (Köhler et al. 2000). Since the adherence and subsequent establishment of biofilm by P. aeruginosa is mediated to a great extent by the different types of motility, the effect of CBO in inhibiting the different types of motility provides evidence that CBO impedes biofilm formation, at the beginning of the attachment stage itself.
Another key aspect of biofilm formation by P. aeruginosa is the production of the extracellular matrix, which is comprised of EPS, e DNA and protein, and it has typically been credited with structuring the mature biofilm (Wei and Ma 2013). It has been reported that overproduction of EPS leads to variation in biofilm architecture that correlates with an augmented resistance of the cells to osmotic and oxidative stress, as well as susceptibility to killing by biocides (Yildiz and Schoolnik, 1999). Reduction of EPS production and swarming motility in addition to inhibition of biofilm formation suggests that CBO not only affects the initial stages of biofilm formation but also affects structural design of the biofilm. In addition to EPS, e DNA is by far the most abundant polymer and comprises an important component of the P. aeruginosa biofilm matrix (Matsukawa and Greenberg, 2004). Production of e DNA, which is regulated by PQS signalling, facilitates expansion of biofilm by mediating twitching motility and facilitates cell aggregation by influencing physio-chemical interactions (Gloag et al. 2013; Das T and Manefield, 2012). The inhibition of e DNA observed in the presence of CBO clearly demonstrates that the expansion of the biofilm is also affected along with the cell aggregation.
Additionally, CBO inhibited the formation of both pyocyanin and pyoverdine, which are also controlled by PQS and Rhl signalling systems, respectively. Synthesis of the iron siderophore, pyoverdine, occurs in the stalk portion of the mushroom-shaped structures of the biofilm and is necessary for development of the cap-forming sub-population (Yang et al. 2009). Pyoverdin can also compete with mammalian transferrin for iron as well as promote pathogenicity by stimulating bacterial growth (Adonizio et al. 2008). Earlier reports have indicated that the production of pyocyanin contributes to virulence in chronic lung infections, enhanced e DNA production, microcolony formation and increased biomass, all of which are necessary for development of biofilm stability in P. aeruginosa (Das et al. 2013). Our observation of decreased e DNA and inhibition of biofilm formation is consistent with lowered levels of pyocyanin, which is seen in the presence of CBO. Taken together, these results clearly indicated that since CBO inhibited biofilm formation in addition to motility, pigment production as well as levels of EPS and e DNA, it could potentially be a very good candidate for attenuating a wide array of virulence factors exhibited by Pseudomonas.
To study the mechanism underlying these observations, we analysed the effect of CBO on the expression of genes in the QS circuitry. Our results indicated that CBO specifically decreased the expression of pqsA, implicated in the conversion of anthranilic acid to 2-heptyl-4-quinolone (HHQ), the precursor of PQS (Zhang et al. 2008; Farrow and Pesci 2007). These results support and corroborate our earlier observations (inhibition of e DNA and pyocyanin) that the presence of CBO results in weak biofilm formation. Additionally, CBO also inhibited the levels of kynurenine, the precursor to anthranilate. The downregulation of pqsA gene could be due to the lowered concentration of kynurenine. Anthranilate is also known to induce genes for pyocyanin biosynthesis (Calfee et al. 2001; Mavrodi et al. 2001). The reduced amount of pyocyanin and e DNA levels could possibly be an effect of CBO interfering with the PQS signalling pathway, an observation which was corroborated by the fact that the amount of pyocyanin was increased significantly in response to the external addition of kynurenine. Taken together, these results clearly establish the mechanism by means of which CBO affects tryptophan metabolism, particularly the kynurenine pathway of P. aeruginosa, by decreasing the production of kynurenine, which in turn leads to a diminished level of PQS signalling, resulting in lowered levels of pyocyanin.
The other two QS systems in P. aeruginosa are Las and Rhl. Our data demonstrated that even though CBO did not affect the transcriptional activation of lasI gene which encodes AHL synthase, it reduced QS controlled factors like pyoverdin levels and swimming motility regulated mainly by the Las system (Stintzi et al. 1998). Similarly, the transcriptional activation of the rhlI gene was also not affected in response to CBO, whereas swarming and twitching motilities, which are regulated by rhl system, were reduced. Furthermore, CBO did not significantly affect the expression of vfr and gac A genes. In P. aeruginosa, the Gac/Rsm system positively regulates the expression of the quorum-sensing signal N-butanoyl-homoserine lactone (C4-HSL) and the extracellular virulence factors, such as hydrogen cyanide (HCN), pyocyanin, and elastase production (Kay et al. 2006). The virulence factor regulator (vfr) is a central player in a transcriptional cascade that enhances the pathogenicity of P. aeruginosa through its role as a global transcriptional regulator (Serate et al. 2011). vfr positively regulates quorum sensing by promoting the transcription of lasR and upregulates transcription of the type III secretion system. These results indicated that CBO does not have any effect on the synthesis of the AHL, N-(3-oxododecanoyl)-L-homoserine lactone (OdDHL) and N-butyryl-L-homoserine lactone (BHL). Since the synthesis of these auto-inducers were not affected, whereas the virulence factors associated with these signalling systems were affected by CBO, we were interested in determining if the binding of these molecules to their receptors was affected by CBO. To address this, we have analysed the binding of eugenol, the major component of CBO, with the Las R receptor using in silico docking analysis. These studies suggest that the binding pattern of eugenol to the Las R receptor was comparable to that of its natural ligand OdDHL. Hence, eugenol could compete with OdDHL for binding to its cognate receptor, Las R, resulting in inhibition of QS.
Presences of appropriate substrates for attachment and adherence, as well as nutritional availability, are factors that are known to contribute to the development of bacterial biofilm. In this study, we used an ex vivo porcine skin explant biofilm model, which more closely replicates conditions in skin wounds as compared to the other models (Yang et al. 2013). The SEM images clearly demonstrate that CBO was effective in inhibiting the bacterial biofilm attached to the porcine skin with significant differences in the depth of biofilm, as well as the bacterial density.
In conclusion, the present study provides convincing evidence that CBO successfully attenuates a wide array of virulence factors of P. aeruginosa and proposes a mechanism by which CBO circumvents QS in PAO1 (Fig. 10). The study further shows that in addition to exhibiting a potent inhibitory effect against the AHL-QS systems, CBO also inhibits the species-specific PQS system in P. aeruginosa. The use of CBO to combat P. aeruginosa infections should not raise safety concerns since clove traditionally has been used for medicinal and culinary purposes. Furthermore, the present study can also be used as a basis for utilizing eugenol as a suitable template in the design of new drug candidates focused on QS while reducing the possibility of the development of drug resistance in microorganisms.
References
Adonizio AL, Kong KF, Mathee K (2008) Inhibition of quorum sensing controlled virulence factor production in P. aeruginosa by South Florida plant extract. Antimicrob Agents Chemother 52:198–203. doi:10.1128/AAC.00612-0
Aliaga L, Mediavilla JD, Cobo F (2002) A clinical index predicting mortality with P.aeruginosa bacteraemia. J Med Microbiol 51:615–661. doi:10.1099/0022-1317-51-7-615
Bacalso M, Xu T, Yeung K, Zheng D (2011) Biofilm formation of P. aeruginosa PA14 required lasI and was stimulated by the Pseudomonas quinolone signal although salicylic acid inhibition is independent of the PQS pathway. J Exp Microbiol Immun 15:84–89
Bhowmik D, Sampathkumar KP, Yadav A, Srivastava S, Paswan S, Dutta A (2012) Recent trends in Indian traditional herb Syzygium aromaticum and its health benefits. J Pharmacogn Phytochem 1(1):13–22
Cady NC, McKean KA, Behnke J, Kubec R, Mosier AP, Stephen H, Kasper SH, Burz DS, Musah RA (2012) Inhibition of biofilm formation, quorum sensing and infection in Pseudomonas aeruginosa by natural products-inspired organosulfur compounds. PLoS One 7(6):e38492. doi:10.1371/journal.pone.0038492
Calfee MW, Coleman JP, Pesci EC (2001) Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by P. aeruginosa. Proc Natl Acad Sci U S A 98:11633–11637. doi:10.1073/pnas.201328498
Chaieb K, Hajlaoui H, Zmantar T, Kahla-Nakbi AB, Rouabhia M, Mahdouani K, Bakhrouf A (2007) The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phyother Res 21:501–506. doi:10.1002/ptr 2124
Das T, Manefield M (2012) Pyocyanin promotes extracellular DNA release in P. aeruginosa. PLoS ONE 7(10):e46718. doi:10.1371/journal.pone.0046718
Das T, Kutty SK, Kumar N, Manefield M (2013) Pyocyanin facilitates extracellular DNA binding to P. aeruginosa influencing cell surface properties and aggregation. PLoS ONE 8(3):e58299. doi:10.1371/journal.pone.0058299
Farrow JM III, Pesci EC (2007) Two distinct pathways supply anthranilate as a precursor of the Pseudomonas quinolone signal. J Bacteriol 189(9):3425–3433. doi:10.1128/JB.00209-07
Genestet C, Gouellec AL, Chaker H, Polack B, Guery B, Toussaint B, Stasia MJ (2014) Scavenging of reactive oxygen species by tryptophan metabolites help P. aeruginosa escape neutrophil killing. Free Radic Biol Med 73:400–410. doi:10.1016/j.freeradbiomed.2014.06.003
Gloag ES, TurnbullL HA, Vallotton P, Wang H, Nolan M, Mililli L, Hunt C, Lu J, Osvath SR, Monahan LG, Cavaliere R, Charles GI, Wand MP, Gee ML, Prabhakar R, Whitchurch CB (2013) Self-organization of bacterial biofilms is facilitated by extracellular DNA. Proc Natl Acad Sci U S A 110(28):11541–11546. doi:10.1073/pnas.1218898110
Heeb S, Fletcher MP, Chhabra SR, Diggle SP, Williams P, Cámara M (2011) Quinolones from antibiotics to autoinducers. FEMS Microbiol Rev 35:247–274. doi:10.1111/j.1574-6976.2010.00247
Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH (2012) The multiple signalling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev 76:46–65. doi:10.1128/MMBR.05007-11
Johnansson EM, Cruz SA, Kolomiets E, Buts L, Kadam RU, Cacciarini M, Bartels KM, Diggle SP, Camara M, Williams P, Loris R, Nativi C, Rosenau F, Jaeger KE, Darbre T, Reymond JL (2008) Inhibition and dispersion of P.aeruginosa biofilm by glycopeptide dendrimers targeting the fucose—specific lectin LecB. Chem Biol 15:1249–1257. doi:10.1016/j.chembiol.2008.10.009
Kay E, Humair B, De’nervaud V, Riedel K, Spahr S, Eberl L, Valverde C, Haas D (2006) Two GacA-dependent small RNAs modulate the quorum sensing response in P. aeruginosa. J Bacteriol 188:6026–6033. doi:10.1128/JB.00409-06
Kim HS, Lee SH, Byun Y, Park HD (2015) 6-Gingerol reduces P. aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci Rep 5:8656. doi:10.1038/srep08656/srep08656
Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jørgensen A, Molin S, Tolker-Nielsen T (2003) Biofilm formation by P. aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol 48:1511–1524. doi:10.1046/j.1365-2958.2003.03525
Kruczek C, Qaisar U, Colmer-Hamood JA, Hamood AN (2014) Serum influences the expression of P.aeruginosa quorum sensing genes and QS-controlled virulence genes during early and late stages of growth. MicrobiologyOpen 3(1):64–79. doi:10.1002/mbo3.147
Köhler T, Curty LK, Barja F, van Delden C, Pechère J-C (2000) Swarming of P. aeruginosa is dependent on cell-to-cell signalling and requires flagella and pili. J Bacteriol 182(21):5990–5996. doi:10.1128/JB.182.21.5990-5996.2000
Liang H, Duan J, Sibley CD, Surette MG, Duan K (2011) Identification of mutants with altered phenazine production in P. aeruginosa. J Med Microbiol 60(1):22–34. doi:10.1099/jmm.0.022350-0
Mahady GB (2005) Medicinal plants for the prevention and treatment of bacterial infections. Curr Pharm Des 11:2405–2427
Marketon MM, Glenn SA, Eberhard A, González JE (2003) Quorum sensing controls exopolysaccharide production in Sinorhizobium mliloti. J Bacteriol 185(1):325–331. doi:10.1128/JB
Matsukawa M, Greenberg EP (2004) Putative exopolysaccharide synthesis genes influence P. aeruginosa biofilm development. J Bacteriol 186(14):4449–4456. doi:10.1128/JB.186.14.4449-4456.2004
Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS (2001) Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from P. aeruginosa PAO1. J Bacteriol 183:6454–6465. doi:10.1128/JB.183.21.6454-6465.2001
Meynard JL, Barbut F, Guiguet M, Batisse D, Lalande V, Lesage D, Guiard-Schmid JB, Petit JC, Frottier J, Meyohas MC (1999) Pseudomonas aeruginosa infection in human immunodeficiency virus infected patients. J Infect 38:176–181
Myska K, Czaczk K (2009) Characterization of adhesive exopolysacchride (EPS) produced by P. aeruginosa under starvation conditions. Current Microbiol 58(6):541–546. doi:10.1007/s00284-009-9365-3
Nanjan P, Nambiar J, Nair BG, Banerji (2015) Synthesis and discovery of (I-3, II-3) -biacacetin as a novel non-zinc binding inhibitor of MMP-2 and MMP-9. Bioorg Med Chem 23:3781–3787. doi:10.1016/j.bmc.2015.03.084
Nataraj N, Anjusree GS, Madhavan AA, Priyanka P, Sankar D, Nisha N, Lakshmi SV, Jayakumar R, Balakrishnan A, Biswas R (2013) Synthesis and anti-staphylococcal activity of TiO2 nanoparticles and nanowires in ex vivo porcine skin model. J Biomed Nanotechnol 9:1–7. doi:10.1166/jbn.2013.1756
Oh J, Lee NR, Jo W, Jung WK, Lim JS (2009) Effects of substrates on biofilm formation observed by atomic force microscopy. Ultramicroscopy 109:874–880. doi:10.1016/j.ultramic.2009.03.042
Oncul O, Ulkur E, Acar A (2009) Prospective analysis of nosocomial infections in a burn care unit, Turkey. Indian J Med Res 130:758–764
Pesci EC, Pearson JP, Seed PC (1997) Regulation of las and rhl quorum sensing in P. aeruginosa. J Bacteriol 179:3127–3132
Quave CL, Planto LR, Pantuso T, Bennet BL (2008) Effect of Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin resistant S. aureus. J Ethanopharmacol 118(3):418–428. doi:10.1016/j.jep.2008.05.005
Rashid MH, Kornberg A (2000) Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of P. aeruginosa. Proc Natl Acad Sci USA 97:4885–4890. doi:10.1073/pnas.060030097
Rumbaugh KP, Griswold JA, Hamood AN (2000) The role of quorum sensing in in vivo virulence of Pseudomonas aeruginosa. Microbes Infect 2(14):1721–1731. doi:10.1016/S1286-4579(00)01327-7
Sanlin JR, Lemos M, Klein-Junior LC, Machado ID, Costa P, de Oliveira AP, Tilia C, de Souza JP, Bastos JK, de Andrade SF (2011) Gatroprotective activity of essential oil of Syzygium aromaticum and its major component eugenol in different animal models. Naunyn Schmiedeberg’s Arch Pharmacol 383(2):149–159. doi:10.1007/ 5002-010-2582
Serate J, Roberts GP, Berg O, Youn H (2011) Ligand responses of Vfr, the virulence factor regulator from P. aeruginosa. J Bacteriol 193:4859–4868. doi:10.1128/JB.00352-11
Shaaban HAE, Ei-Ghorab AH, Shibamoto T (2012) Bioactivity of essential oils and their volatile aroma compounds: review. J Essent Oil Res 24(2):203–212
Stintzi A, Evans K, Meyer JM, Poole K (1998) Quorum-sensing and siderophore biosynthesis in P. aeruginosa lasR/lasI mutants exhibit reduced pyoverdine biosynthesis. FEMS Microbiol Lett 166:341–345
Szabó MA, Varga GZ, Hohmann J, Schelz Z, Szegedi E, Amaral L, Molnár J (2010) Inhibition of quorum-sensing signals by essential oils. Phytother Res 24:782–786. doi:10.1002/ptr.3010
Tamma PD, Cosgrove SE, Maragakis LL (2012) Combination therapy for treatment of infections with Gram-negative bacteria. Clin Microbiol Rev 25(3):450–470. doi:10.1128/CMR.05041-11
Thenmozhi R, Nithyanand P, Rathna J, Pandian SK (2009) Antibiofilm activity of coral associated bacteria against different clinical M serotypes of Streptococcus pyogenes. FEMS Immunol Med Microbiol 57:284–294. doi:10.1111/j.1574-695X.2009.00613.x
Venturi V (2006) Regulation of quorum sensing in Pseudomonas. FEMS Microbiol Rev 30:274–291
Wei Q, Tarighi S, Dötsch A, Häussler S, Müsken M, Wright VJ, Cámara M, Williams P, Haenen S, Boerjan B, Bogaerts A, Vierstraete E, Verleyen P, Schoofs L, Willaert R, De Groote VN, Michiels J, Vercammen K, Crabbé A, Cornelis P (2011) Phenotypic and genome-wide analysis of an antibiotic-resistant small colony variant (SCV) of P. aeruginosa. PLoS One 6(12):e29276. doi:10.1371/journal.pone.0029276
Wei Q, Ma LZ (2013) Biofilm matrix and its regulation in P. aeruginosa. Int J Mol Sci 14(10):20983–21005. doi:10.3390/ijms141020983
Yang L, Barken KB, Skindersoe ME, Christensen AB, Givskov M, Tolker-Nielsen (2007) Effect of iron on DNA release and biofilm development in P. aeruginosa. Microbiology 153:1318–1328. doi:10.1099/mic.0.2006/004911-0
Yang L, Nilsson M, Gjermansen M, Givskov M, Tolker-Nielsen T (2009) Pyoverdine and PQS mediated subpopulation interactions involved in P. aeruginosa biofilm. Mol Microbiol 74(6):1380–1392. doi:10.1111/j.1365-2958.2009.06934.x
Yang Q, Phillips PL, Sampson EM, Progulske-Fox A, Jin S, Antonelli P, Schultz GS (2013) Development of a novel ex vivo porcine skin explant model for the assessment of mature bacterial biofilms. Wound Repair Regen 21:704–714. doi:10.1111/wrr.12074
Yildis HF, Schoolnik GK (1999) Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc Natl Acad Sci U S A 96:4028–4033
Zhang YM, Frank MW, Zhu K, Mayasundari A, Rock CO (2008) PqsD is responsible for the synthesis of 2, 4-dihydroxyquinoline, an extracellular metabolite produced by P. aeruginosa. J Biol Chem 283:28788–28794. doi:10.1074/jbc.M804555200
Acknowledgments
The authors graciously acknowledge Sri Mata Amritanandamayi, Chancellor, Amrita University, for her constant support and guidance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants performed by any of the authors. Approval for experiments in this study to be conducted with porcine skin was obtained from the Institutional Ethics Committee.
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(PDF 288 kb)
Rights and permissions
About this article
Cite this article
H., J., Omanakuttan, A., Pandurangan, N. et al. Clove bud oil reduces kynurenine and inhibits pqs A gene expression in P. aeruginosa . Appl Microbiol Biotechnol 100, 3681–3692 (2016). https://doi.org/10.1007/s00253-016-7313-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7313-2