Abstract
Pseudomonas aeruginosa synthesizes large quantities of exopolysaccharide (EPS), making it an excellent model organism for the study of EPS-mediated adhesion. The purpose of this investigation was to evaluate the influence of limited nutrients availability in the culture medium on the composition of EPS produced by P. aeruginosa. The relationship between the EPS production and the adhesion process of the P. aeruginosa cells to stainless steel surface (type 316 L) under starvation conditions were also examined. In all experimental variants P. aeruginosa produced more EPS with an increase of incubation period upon starvation conditions. Under limited nutrients condition, glucose dominated in the EPS materials. After 6 days of the process, only glucosyl units were detected in the extracellular matrix produced by nutrient-deprived P. aeruginosa cells. These extracellular molecules promoted more advanced stages of P. aeruginosa biofilm formation on the surface of stainless steel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A better understanding of bacterial adhesion process is needed for the production of microbiologically safe and good quality products in the food industry. Bacteria colonizing the processing equipment are a potential source of food contamination, which may lead to food spoilage, or transmission of diseases [2, 11]. From a medical perspective, the attached bacteria on catheters, drains, implants, or lenses can cause serious infections [7]. Despite rigorous cleaning and disinfection procedures, pathogenic and spoilage microorganisms may be isolated from many types of surfaces [11].
Gram et al. [11] suggested that solid materials are often colonized by Gram-negative bacteria that originally exist in aquatic environment (with limited nutrients availability). Among marine bacteria species, Pseudomonas aeruginosa is one of the most important food spoilage and human opportunistic bacteria [17]. P. aeruginosa may cause off-flavor or the textural changes resulting in sensory rejection of vacuum-packed meats, vegetables, and fish [2]. P. aeruginosa cells have also been described as etiological agents in urinary and pulmonary tract infections, as well as in gastroenteritis resulting from the consumption of contaminated food [6, 11].
The ability of P. aeruginosa to adhere on surfaces is associated with the extensive production of exopolysaccharide (EPS) [6, 7]. Chen and Stewart [4] concluded that EPS is responsible for both adhesion and cohesion interactions and play a crucial role in maintaining structural integrity of P. aeruginosa biofilms. It is also considered that EPS can promote a preconditioning of surface, making the adhesion process more favorable. In some cases, EPS matrix also enables the marine bacteria to capture nutrients [7].
The problem of P. aeruginosa attachment process to solid materials has not been solved yet. Most researches have focused so far on the mechanism of EPS secretion and its role in biofilm formation process under optimal nutrients availability in the medium. These cultivation conditions do not correspond to natural environment, where Pseudomonas spp. is widely distributed [12]. Reduced nutrient availability in the medium changes the manner of attachment process of cells to solid materials [19]. Moreover, upon conditions of starvation the properties of the cell envelope are being modified because of the changes in the monosaccharide composition of EPS [3]. Analysis of the extracellular mechanisms determining the biofilm phenotype on abiotic surfaces will help in the eradication of the attached bacteria [2].
The aim of this study was to define the influence of limited nutrient availability on the monosaccharide composition of EPS produced by P. aeruginosa cells. The relationship between the EPS production and the adhesion process of the P. aeruginosa cells to stainless steel surface (type 316 L) under starvation condition was also examined. This type of steel is well-suited for making food processing equipments and surgical instruments [18].
Materials and Methods
Bacterial Strains and Growth Conditions
P. aeruginosa strain ATCC 10145 was obtained from the American Type Culture Collection (Rockville, MD, USA). The strains of P. aeruginosa ATCC 10145 commonly thrive in water environment. During the investigations, the microorganisms were passaged three times after every 48 h on the ABPG medium according to Schubert [20]. The ABPG medium contained: 17.0 g/l of peptone from casein, 3.0 g/l of peptone from soymeal, 0.5 g/l of glucose, 5.0 g/l of sodium chloride, 10.0 g/l of l-arginine-monohydrochloride, 0.015 g/l of bromothymol blue, 0.02 g/l of cresol red and 0.0038 g/l of brilliant green. From each passage 10 ml of inoculum of P. aeruginosa was added to 100 ml of the fresh medium. The cultures were incubated at 35°C under shaking conditions (100 rpm/min) on the ABPG medium with optimal (as described above) and reduced by 90% (w/v) of optimal nutrients availability. The pH value of the culture medium at the beginning of incubation was 7. The incubation lasted in total 144 h.
Isolation of Bacterial Exopolysaccharides
Isolation of EPS was based on the procedure employed by Forde and Fitzgerald [8]. The bacteria were harvested by centrifugation at 3000g for 20 min at the room temperature after 24, 48, 72, 96, 120 and 144 h of each experiment. The cells were resuspended in 1.5 ml of 30% (w/v) NaOH. Samples were boiled for 15 min, centrifuged at 15000g for 15 min and the supernatant fluids were added dropwise to 60% (v/v) ethanol.
Quantification of EPS
The total EPS (expressed as μg/CFU) was determined using the acid hydrolysis method of Parkar et al. [16]. The precipitated EPS was collected by centrifugation (15000g, 20 min) and was resuspended in 1 ml of sterile water. The samples were mixed with 7 ml of 77% (v/v) H2SO4 and cooled for 10 min in an ice-bath. 1 ml of 1% (w/v) of cold tryptophan was added and the samples were heated in a boiling bath for 20 min to effect hydrolysis. The acid hydrolysis of EPS produced a furan which condenses with the tryptophan and forms a colored product. This was evaluated after cooling the samples by measuring O.D.500. Calibration curves were prepared against standard dextran (Mp. 40000) solutions (Sigma, USA).
Determination of the EPS Monomer Composition
The method described by Forde and Fitzgerald [8] was used for the preparation of EPS for chromatographic analysis. One milli liter of sample was hydrolysed with an equivalent volume of 10% (v/v) H2SO4 for 60 min at 100°C. After cooling, trichlorocetic acid (TCA) was added to a final concentration of 5% (w/v) to precipitate proteins, followed by centrifugation at 15,000g for 20 min at 4°C. The samples were passed through 0.22 μm Millex-GS filters (Milipore, USA). Determination of EPS components (glucose, fructose, galactose, arabinose) was carried out using HPLC technique, on MERCK-HITACHI system with autosampler (model L-7250), pump (model L-7100) and refractive index detector (model L-7490). Analysis were performed isocratically at a flow rate of 0.6 ml/min (0.005 M H2SO4), at 35°C, on column Aminex HPX-87H, 300 × 7.8 mm (Bio-Rad, USA). Standards were used to identify peaks in chromatograms. Peak areas were used to determine the samples concentration. It was carried out by computer integration (Chromatography Data Station Software, MERCK-HITACHI) operated in the mode of external standard. Standard calibration solutions for examined sugars were prepared within the range 0.1–0.01 mg/ml.
Stainless Steel Plates Preparation
Stainless steel plates (type 316 L) sized 1 cm × 6.5 cm × 1 mm were treated with 50% (v/v) solution of HNO3 for 10 min at 70°C. After soaking under distilled water, the plates were put into glass containers and sterilized at 121°C for 15 min [16].
Bacterial Adhesion Analysis
Bacterial adhesion analysis was started after 144 h of experiments. The stainless steel surface was put into P. aeruginosa cultures resulting from the final passage in ABGP medium. At 145 h the plates were removed from glass containers and washed with PBS solutions (pH 7.2) in order to remove unattached cells from the surfaces. The plates were stained with 0.01% (w/v) solution of acridine orange (2 min). Stained adherent bacteria were observed under oil immersion, using a fluorescence microscope (CARL-ZEISS, Axiovert 200, Germany). To determine the level of P. aeruginosa adhesion to the surface of stainless steel the method described by Le Thi et al. [15] was carried out. This method is based on the direct classification by the operator of randomly selected 50 visual fields according to a 9-degrees scale:
-
1st degree: from 0 to 5 bacteria cells in visual field.
-
2nd degree: from 5 to 50 bacteria cells in visual field.
-
3rd degree: only single bacterial cells (above 50 bacterial cells in visual field; no microcolonies.
-
4th degree: single bacterial cell + small microcolonies.
-
5th degree: large but not confluent microcolonies + single bacterial cell.
-
6th degree: confluent microcolonies + single bacterial cell.
-
7th degree: 1/4 visual field covered by the biofilm.
-
8th degree: 1/2 visual field covered by the biofilm.
-
9th degree: visual field totally covered by the biofilm.
The classification procedure excluded bacteria situated on the edges of each visual field.
Statistical Analysis
Presented results are the average of three independent experiments. Student’s t-test was used to determine the significant difference (P < 0.05) between the carbohydrate compositions of EPS produced by cells under different nutrients availability in the medium.
Results
Quantitative Determination of EPS
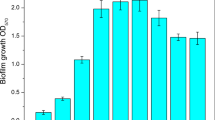
To characterize the physiological potential of P. aeruginosa cells for colonization of the abiotic surfaces under starvation conditions, the quantitative determination of EPS was carried out. The EPS production capacity of P. aeruginosa at different nutrients availability in the medium is shown in Fig. 1. P. aeruginosa produced more EPS with an increased incubation period upon starvation conditions. In the first 96 h of the process, the EPS production by examined bacteria was not higher than 5 μg/109 CFU. At 120 h of cultivation the EPS synthesis was significantly increased to the level of 18 μg/109 CFU. From 120 h of the experiment the EPS secretion by P. aeruginosa remained relatively constant. In contrast, upon favorable conditions the maximum EPS production by P. aeruginosa (20 μg/109 CFU) was observed in 48 h of the process. After 72 h the EPS synthesis decreased to the level of 3 μg/109 CFU.
Composition of EPS
Determination of the EPS monomer composition was carried out after acid hydrolysis method. The results of the experiments were expressed as the percentage of examined components in EPS material measured by the spectrophotometric method. Table 1 presents the influence of nutrients availability in the medium on the monosaccharide compositions of EPS produced by P. aeruginosa cells. Under conditions of starvation, glucose dominated in the EPS materials. From 120 h of experiments, the content of glucose in the EPS materials increased to the level of 41%. Interestingly, at 144 h of the process, only glucosyl units were detected in the extracellular matrix. In all experimental variants, the percentage of fructose, galactose, and arabinose in extracellular materials was not higher than 4%. Upon nutrient-rich conditions, P. aeruginosa synthesized the EPS composed of glucose, fructose, galactose, and arabinose. However, the contents of sugars in the extracellular matrix equaled between 3 and 7%. At 144 h of cultivation under optimal nutrients availability, the content of glucose in the EPS materials significantly increased to the level of 26%.
Adhesion
The bacterial colonization of solid material is a multi-step process. In this work to define the rate of biofilm development process, the 9-degree scale according to Le Thi et al. [15] was used.
The results of the influence of nutrients availability on the attachment of P. aeruginosa cells to stainless steel (type 316 L) are presented in Table 2. In this work, the adhesion analysis started when P. aeruginosa produced the high amount of EPS composed of glucose under starvation conditions. Approximately 107–108 CFU/ml P. aeruginosa cells were present in the culture medium during the experiments.
In this work when a particular degree of adhesion occurred with a minimum amount of 20% that degree became the dominant one. Stainless steel type 316 L was efficiently colonized by P. aeruginosa under starvation conditions. Limited nutrients availability induced more developed stages of P. aeruginosa biofilm formation process on the surface of stainless steel (6 and 7th adhesions degree). P. aeruginosa grown upon nutritionally favorable conditions colonized the studied surface at the 4th degree of adhesion and no higher level of adhesion (6–9th degrees) was observed in the work.
Discussion
In this work, we aimed to characterize the EPS synthesis by P. aeruginosa cells during growth on low nutrients availability in the medium. The work of Wai et al. [21] concluded that bacterial EPS production is favored by nutrients deprivation. However, in the literature there are not clear evidences about the mechanisms of that process. Nutrient limited conditions represent the natural aquatic habitat of P. aeruginosa cells [12].
In the food industry and hospital conditions, EPS-rich strains are difficult to overcome [2]. The EPS surrounding the microorganisms restrict disinfectant and antibiotic penetration [10]. The extensive production of EPS by microorganisms may seriously affect the quality and safety of the processed food and pose a potential risk to patients [7]. In our study, long-term starvation influenced higher productivity of EPS matrix by P. aeruginosa cells. The highest yield of EPS production by examined bacteria was observed after 120 h of cultivation. Kiliς and Dönmez [14] noticed similar effects when monitoring EPS production by P. aeruginosa cells. According to this study, EPS synthesis by P. aeruginosa was effected by the increasing incubation period. The highest production of extracellular matrix by examined cells was noticed after incubation for 96 h. Dunne [7] reported that extensive production of EPS by nutrient-deprived bacteria is a logical strategy of cells. The EPS matrix is an efficient system for trapping nutrients from surrounding environments [7].
In this work, monosaccharide compositions of EPS matrix produced by P. aeruginosa upon low nutrients availability in the medium were also investigated. This knowledge may improve the elimination the particular pathogenic and spoilage promoting compounds [11]. However, in the literature the qualitative data on the composition of EPS produced by marine bacteria upon starvation are lacking. Our results indicated that glucose dominated in the EPS material produced by P. aeruginosa under starvation conditions. In contrast, Hung et al. [13] concluded that galactose, mannose, and arabinose were the main compositions of EPS produced by Pseudomonas fluorescens Biovar II. According to this data the content of glucose in EPS material synthesized by examined bacteria was not higher than 5%.
In our study, at 144 h under starvation conditions only glucosyl units were detected in the EPS matrix. Our results underscore the genetic analysis of extracellular material produced by the strains of P. aeruginosa [9]. These investigations demonstrated that the synthesis of a glucose-rich polymer is the feature of P. aeruginosa strains. These results differ from those published by Grobe et al. [12] where they reported that P. aeruginosa synthesized large quantities of extracellular alginate.
In this work, the relationship between the EPS production and the adhesion process of P. aeruginosa cells to stainless steel type 316 L upon starvation were also investigated. Microbial attachment process to abiotic surfaces is determined by EPS production [7]. In our study, the attachment analysis of P. aeruginosa cells started when examined bacteria producing EPS matrix composed of glucose upon starvation conditions. According to the results published by Costerton et al. [5], we assumed that this extracellular polysaccharide may determine the biofilm phenotype upon starvation conditions. The researchers prepared antibodies against a planktonically synthesized polymer and used them to reveal interaction with material in the biofilm matrix. This indicated that some components of the biofilm EPS had the same compositions as the planktonic product. In this work, starvation conditions increased the adhesion of microorganisms to abiotic surfaces. Our studies also indicated that high quantities of EPS are not required for the first step in biofilm formation but are needed to develop a true biofilm matrix. Similar results were observed for a wild type of P. fluorescens and a nonpolysaccharide-producing mutant [1]. Both strains adhered to a glass surface, but over time, the wild bacteria formed three-dimensional structure while the mutant remained as single adherent cells on the surface.
Our data support the notion that the composition of adhesive EPS produced by P. aeruginosa cells should be examined under conditions in which marine bacteria are widely distributed. The production of EPS molecules by examined microorganisms is more extensive upon long-term starvation. In response to nutrient limitations in the medium P. aeruginosa synthesized EPS composed of glucose. These extracellular molecules promoted more advanced stages of P. aeruginosa biofilm formation on the surface of stainless steel upon starvation conditions.
References
Allison DA, Sutherland IW (1987) The role of exopolysaccharides in adhesion of freshwater bacteria. J Gen Microbiol 133:1319–1327
Bower CK, McGuire J, Daeschel MA (1996) The adhesion and detachment of bacteria and spores on food-contact surfaces. Trends Food Sci Tech 7:152–157
Celik GY, Aslim B, Beyatli Y (2008) Characterization and production of the exopolysaccharide (EPS) from Pseudomonas aeruginosa G1 and Pseudomonas putida G12 strains. Carbohydr Polym 73:178–182
Chen X, Stewart PS (2002) Role of electrostatic interactions in cohesion of bacterial biofilms. Appl Microbiol Biotechnol 59:718–722
Costerton JW, Cheng K-I, Geesey G (1981) Bacterial biofilms in nature and disease. Annu Rev Microbiol 35:399–424
Drenkard E (2003) Antimicrobial resistance of Pserudomonas aeruginosa biofilms. Microbes Infect 5:1213–1219
Dunne WM (2002) Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev 15:155–166
Forde A, Fitzgerald GF (1999) Analysis of exopolysaccharide (EPS) production mediated by the bacteriphage adsorption blocking plasmid, pCI658, isolated from Lactococcus lactis ssp. cremoris HO2. Int Dairy J 9:465–472
Friedman L, Kolter R (2004) Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol Microbiol 51:675–690
Fuster-Valls N, Hernandez-Herrero M, Marin-de-Mateo M et al (2008) Effect of different environmental conditions on the bacteria survival on stainless steel surfaces. Food Control 19:308–314
Gram L, Ravn L, Rasch M et al (2002) Food spoilage––interactions between food spoilage bacteria. Int J Food Microbiol 78:79–97
Grobe S, Wingender J, Flemming H-C (2001) Capability of mucoid Pseudomonas aeruginosa to survive in chlorinated water. Int J Hyg Environ Health 204:139–142
Hung C-C, Santschi PH, Gillow JB (2005) Isolation and characterization of extracellular polysaccharides produced by Pseudomonas fluorescens Biovar II. Carbohydr Polym 61:141–147
Kiliς NK, Dönmez G (2008) Environmental conditions affecting exopolysaccharide production by Pseudomonas aeruginosa, Micrococcus sp., and Ochrobactrum sp. J Hazard Mater 154:1019–1024
Le Thi TT, Prigent-Combaret C, Dorel C et al (2001) First stages of biofilm formation: characterization and quantification of bacterial functions involved in colonization process. Methods Enzymol 336:152–159
Parkar SG, Flint SH, Palmer JS et al (2001) Factors influencing attachment of thermophilic bacilli to stainless steel. J Appl Microbiol 11:675–685
Pontefract RD (1991) Bacterial adherence: its consequences in food processing. Can I Food Sci Tech J 24:113–117
Precival SL, Knapp JS, Edyvean RGJ et al (1998) Biofilms, mains water and stainless steel. Water Res 32:2187–2201
Sanin SL, Sanin SD, Bryers JD (2003) Effect of starvation on the adhesive properties of xenobiotic degrading bacteria. Process Biochem 38:909–914
Schubert R (1989) The use of arginine briliantgreen glucose peptone broth (ABGP medium) as a primary culture medium for Pseudomonas aeruginosa. Zbl Bakt Mik Hyg 187:166–268
Wai SN, Mizunoe Y, Yoshida S (1999) How Vibrio cholerae survive during starvation. FEMS Microbiol Lett 180:123–131
Acknowledgment
This study was supported by the Ministry of Science and Higher Education, Poland, project no NN 312326533.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Myszka, K., Czaczyk, K. Characterization of Adhesive Exopolysaccharide (EPS) Produced by Pseudomonas aeruginosa Under Starvation Conditions. Curr Microbiol 58, 541–546 (2009). https://doi.org/10.1007/s00284-009-9365-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9365-3