Abstract
A Pseudomonas sp. strain WL2 that is able to efficiently metabolize ethyl mercaptan (EM) into diethyl disulfide (DEDS) through enzymatic oxidation was isolated from the activated sludge of a pharmaceutical wastewater plant. One hundred percent removal of 113.5 mg L−1 EM and 110.3 mg L−1 DEDS were obtained within 14 and 32 h, respectively. A putative EM degradation pathway that involved the catabolism via DEDS was proposed, which indicated DEDS were further mineralized into carbon dioxide (CO2), bacterial cells, and sulfate (SO4 2−) through the transformation of element sulfur and ethyl aldehyde. Degradation kinetics for EM and DEDS with different initial concentrations by strain WL2 were evaluated using Haldane-Andrews model with maximum specific degradation rates of 3.13 and 1.33 g g−1 h−1, respectively, and maximum degradation rate constants of 0.522 and 0.175 h−1 using pseudo-first-order kinetic model were obtained. Results obtained that aerobic degradation of EM by strain WL2 was more efficient than those from previous studies. Substrate range studies of strain WL2 demonstrated its ability to degrade several mercaptans, disulfides, aldehydes, and methanol. All the results obtained highlight the potential of strain WL2 for the use in the biodegradation of volatile organic sulfur compounds (VOSCs).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Foul odors are always serious environmental issues, which have also been identified as atmospheric contamination due to a variety of adverse health effects on human beings (An et al. 2010; Charlson et al. 1987; Zhang et al. 2013). Odor emissions are usually related to hydrogen sulfide (H2S), mercaptans (R-SH), organic sulfides (R-S-R), and disulfides (R-S-S-R) (Barbosa et al. 2002; Hernandez et al. 2012; Zhang et al. 2013). As far as mercaptan is concerned, ethyl mercaptan (EM) is always used as an intermediate and starting material in the manufacture of plastics, insecticides, and antioxidants and as an odorant to serve as a warning property for natural gas (An et al. 2010; NRC US 2013). The low detection threshold, high volatility, and potential toxicity of EM make it inevitable to be treated as a main organic sulfur pollutant in waste gas streams (NRC US 2013; Wan et al. 2011).
To date, common studies have focused on the removal of EM using a continuous bioreactor (An et al. 2010; Arellano-Garcia et al. 2010; Hernandez et al. 2012; Wan et al. 2011). However, these studies showed that 100 % removal of EM could only be achieved when low loading rate was applied under higher empty bed residence times (EBRTs) of 110 ~ 322 s. Therefore, isolation and identification of a highly efficient strain for EM biodegradation is of great importance to ensure its complete removal in an effective and economical bioreactor system. Also, it is crucial to have a thorough understanding of biodegradation characterization of EM (Rappert and Müller 2005). For now, only two different kinds of pure strains were isolated to evaluate the biodegradation characteristics of EM using batch reactors (Sedighi et al. 2013; Wan et al. 2010). Nevertheless, the degradation rates of EM obtained from using these two pure strains are not satisfactory, due to their longer incubation period at lower substrate concentrations. And little has been known regarding the formation of the final product, such as carbon dioxide (CO2), biomass, and sulfate (SO4 2−), during EM biodegradation by pure cultures. Sedighi et al. (2013) have proposed a putative pathway for EM degradation by Ralstonia eutropha without indentifying organic intermediates or final products. Hence, a comprehensive study on EM biodegradation pathway needs to be further carried out.
In this study, a newly isolated Pseudomonas sp. WL2 that is able to efficiently transform EM into diethyl disulfide (DEDS) through the enzymatic oxidation was identified. Characteristics of both EM and DEDS biodegradation were discussed along with the increase of biomass concentration. A complete pathway for EM biodegradation by Pseudomonas sp. WL2 was presented. Besides, kinetic analysis for substrate degradation and bacterial growth were fitted using Haldane-Andrews model and pseudo-first-order model, respectively. All the results would enhance the understanding of this novel strain WL2 on the removal of volatile organic sulfur compounds (VOSCs).
Material and methods
Chemicals and cultivation medium
Ethyl mercaptan, diethyl sulfide, diethyl disulfide, propyl mercaptan, dipropyl sulfide, dipropyl disulfide, n-butyl mercaptan, dibutyl sulfide, dimethyl sulfide, dimethyl disulfide, methanol, methyl aldehyde, and ethyl aldehyde were obtained from Aladdin Industrial Inc. (Shanghai, China) and Sigma-Aldrich (Mainland, China). All other chemicals were of analytical grade, commercially available and used without further purification.
Mineral salts medium (MSM) was applied for cultivation and enrichment of bacterial strains. It contained the following compositions per liter of deionized water: 1.5 g of NH4Cl, 0.2 g of MgCl2, 4.5 g of Na2HPO4, 1.0 g of KH2PO4, 0.01 g of FeCl2 · 4H2O, 0.00002 g of CuCl2, 0.000014 g of H3BO3, 0.0001 g of MnCl2 · 4H2O, 0.0001 g of ZnCl2, 0.00002 g of Na2MoO4 · 2H2O, and 0.00002 g of CoCl2 · 6H2O. The initial pH value of the medium was about 7.13.
Isolation and identification of strain WL2
The bacterial strain used in this study was isolated from the activated sludge of a pharmaceutical wastewater plant (Hangzhou, China) and cultured in 250-mL conical flasks containing 100 mL liquid medium at 30 °C and 180 r min−1 on a rotary shaker with 100 mg L−1 of EM as the sole source of carbon and energy. The spread-plate method using R2A agar plates was applied for isolation of pure strains. Single colonies were transferred to additional new plates until homogenous colonies were observed. Finally, the organisms were inoculated into the mineral medium with EM as the only carbon source to prove their degrading ability.
The isolate was identified according to its phenotypic and genotypic characteristics. The colony morphology of the isolated strain was observed on agar plates after 36 h of culturing at 30 °C. The cell morphology was observed using an H-7650 transmission electron microscope (Hitachi, Japan). Further identification was completed by 16S ribosomal RNA (rRNA) gene sequencing, as described in Su et al. (2013).
Substrate degradation by strain WL2
One hundred milliliters of the MSM were added to a 630-mL serum bottle sealed with Teflon-faced silicone rubber septa and parafilm, and a certain amount of liquid-phase substrate was added to the sterilized bottles using a 50-μL gas-tight microsyringe. The bottles were then sealed and shaken (30 °C, 180 r min−1) for 2 h to ensure equilibrium of the substrate between the headspace and the solution. The initial substrate concentrations in the liquid phase of the batch reactors were controlled at 22.7, 41.8, 71.7, and 113.5 mg L−1 for EM and 24.6, 44.8, 86.2, and 110.3 mg L−1 for DEDS, respectively. The batch reactors were incubated using 10 mL of the microbial suspension from the mid-log phase of EM degradation, with the initial cell concentration of 2.73 mg cell dry weight (CDW) L−1. A sterile control experiment was carried out to evaluate the abiotic substrate loss when the biomass endogenous decay rate was negligible.
EM degradation intermediates and final products
Strain WL2 was grown to the log phase in 630-mL serum bottle containing 100 mL MSM with 20 μL of initial EM. Solid phase extraction (SPE) was used to extract the intermediates with the microbial suspension for EM degradation. The SPE cartridges used, 3 mL/60 mg N-methacrylate polymer-divinylbenzene copolymer, were obtained from Shimadzu-GL (InertSep Pharma, Shanghai, China). The cartridges were conditioned as recommended by the manufacturer. The intermediates formed during the EM transformation by strain WL2 was qualitatively analyzed using gas chromatography/mass spectrometry (6890 N/5975B Inert XL MSD, Agilent, USA) equipped with a HP-5MS capillary column (30 m × 0.25 mm × 0.25 μm, J&W Scientific, USA) and a flame ionization detector. Helium (purity 99.999 %) was used as carrier gas with a constant column flow of 1.0 mL min−1. The GC oven temperature was programmed from 50 °C (held 5 min) to 180 °C at 10 °C min−1 (held 10 min). The injector temperature was set at 180 °C. The mass spectrometry (MS) interface temperature was set at 280 and 250 °C for the ionization source. The mass spectra were obtained at 70 eV. All metabolites were identified by GC/MS by matching retention times and ion spectra with authentic standards and NIST library data.
The mineralization for EM degradation by Pseudomonas sp. WL2 was confirmed by determining the final products, including CO2 production, bacterial yield, and sulfate production. For determination of CO2 production and bacterial yield, each batch reactor was carried out in a sterilized 630-mL air-sealed serum bottle (as described above) with initial added mass of 4.2, 8.4, 16.8, and 25.2 mg for EM, respectively.
Accumulation of the sulfate concentration for different initial EM consumed was analyzed to evaluate the sulfur balance for EM transformation. The batch reactors were carried out with initial added EM of 6.7, 15.1, and 23.5 mg, respectively. After the substrates were fully consumed, 20 mL of the microbial suspension was centrifuged, and 10 mL of the supernatant was diluted to 100 times before determining the sulfate concentration (mg L−1). A control test was established by incubating 5 mL microbial suspension into 100 mL of sulfate-free MSM without EM.
Substrate range of strain WL2
Substrate range of Pseudomonas sp. WL2 was also examined through the degradation of several other VOSCs with similar chemical structures, including propyl mercaptan, dipropyl sulfide, dipropyl disulfide, n-butyl mercaptan, dibutyl sulfide, dimethyl sulfide and dimethyl disulfide, and methanol, methyl aldehyde, and ethyl aldehyde were also examined. The initial addition of each possible growth substrate in the batch reactors was controlled at 10 μL. Furthermore, accumulation of the biomass concentration was calculated at the inoculation time of 12 h, which was used to prove whether an organic pollutant could be degraded by strain WL2 as growth substrate.
Analytical methods
The gas chromatograph response to the gas-phase sample was calculated to give the liquid-phase concentration. Assuming the headspace and the liquid phase were in equilibrium, the corresponding liquid-phase concentration could be calculated using the following equation:
where S L and S G are the liquid-phase and the gas-phase concentrations (mg L−1), respectively, and H c is the dimensionless Henry’s constant at 30 °C. Gas-phase sampling was withdrawn at regular time intervals to determine the liquid-phase concentration with 0.167 and 0.145 of the dimensionless Henry’s constants (Staudinger and Roberts 2001) for EM and DEDS, respectively. Gas-phase VOSC samples in this study were quantitatively analyzed using a gas chromatograph (Model 9790, Fuli, Zhejiang, China) with a DB-624 capillary column (30 m × 0.32 mm × 0.33 μm, J&W Scientific, USA) and a flame ionization detector. The temperatures of injection port, oven, and detection port were set at 180, 180, and 200 °C, respectively.
CO2 contents was analyzed by injecting 200 μL gas-phase samples into a 7890A gas chromatograph (Agilent Technologies) with a HP-Plot-Q column (30 m × 0.32 mm × 20 μm), and a thermal conductivity detector was used. The column and detector temperatures were set at 40 and 200 °C, respectively.
Bacterial growth in the batch reactor was monitored with a spectrophotometer (722s, Leng-Guang Technology, China) by measuring the optical density (OD) at 600-nm wavelength. The cell concentration in milligram CDW per liter correlated with different OD values were determined after 50 mL of cell suspension was filtered and dried (24 h, 105 °C) using a 0.22-μm microporous membrane filter (Xingya, Shanghai, China) and then weighted.
The sulfate concentration was determined using an ion chromatography system (ICS-2000, Dionex, USA), which is equipped with suppressor and conductivity detector and an AS11-HC column (4 × 250 mm, Dionex, USA). The temperature of column was controlled at 30 °C.
Kinetic model
Specific growth rates (μ, h−1) of strain WL2 with different initial substrate concentrations were calculated as the following relationship:
where X is the biomass concentration (mg L−1) at the time of t (h).
Furthermore, specific degradation rate (q, gS gX −1 h−1) was calculated according to the following equation:
where S is the substrate liquid-phase concentration (mg L−1) at time (t).
The bacterial specific growth rate is often described using Monod kinetics (Monod 1949). However, higher substrate concentrations may become inhibitory to bacterial growth (Hao et al. 2002). Therefore, a modified Monod type (Haldane-Andrews model) was widely used to estimate the kinetic parameters, as described by the following equations (Andrews 1968; Saravanan et al. 2009):
where μ max is the maximum specific growth rate (h−1), K s is the Monod half-saturation constant (mg L−1) which indicates the substrate concentration when half of the μ max was obtained, and K i is an inhibition constant (mg L−1) which was used to express the inhibitory effect of substrate concentration on bacterial growth.
For estimating the maximum specific degradation rate (q max, gS gX −1 h−1) using the Haldane-Andrews model, μ and μ max in the above equation were replaced with q and q max, respectively, as in Eq. (4):
Furthermore, various models, including zero-order model, first-order model, and pseudo-first-order model, have also been widely used for modeling the kinetics of VOC or VOSC degradation (Saravanan et al. 2009; Silambrasan and Abraham 2013; Wan et al. 2010). In this study, pseudo-first-order model was also used to evaluate the degradation rate constant for EM and DEDS biodegradation, as in Eq. (6).
where S t and S 0 are the liquid-phase concentrations at the time of 0 and t. K 1p is the degradation rate constants using pseudo-first-order model. The model Eqs. (2)–(6) were solved using the nonlinear least squares regression analysis to estimate the kinetic constants.
GenBank accession number
The 16S rRNA sequence of strain WL2 has been deposited in the GenBank database under the accession number of KF233595.
Results
Isolation and identification of strain WL2
In this work, a bacterial strain isolated from the activated sludge of a pharmaceutical wastewater treatment plant, designated as WL2, was able to efficiently degrade EM as the sole carbon and energy source. Strain WL2 has been deposited in the China General Microbiological Culture Collection Center (CGMCC, Beijing, China) with accession no. 7898. It is an aerobic, Gram-negative, nonspore, motile, and short rod-shaped bacterium (Fig. 1a).
A 1334-bp 16S rRNA fragment amplified from the total DNA of strain WL2 was partially sequenced. After alignment with other 16S rRNA sequences in GenBank, a phylogenetic tree was constructed (Fig. 1b), showing a high degree of similarity (99 %) to Pseudomonas putida (GenBank accession no. AM921634). Therefore, strain WL2 was identified as a strain of Pseudomonas species (named Pseudomonas sp. WL2).
Substrate degradation by Pseudomonas sp. WL2
Degradation of EM and DEDS with different concentrations
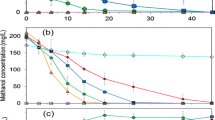
Degradation characteristics and bacterial growth for EM as the sole substrate by strain WL2 were investigated. As shown in Fig. 2a, 100 % removal of 23.3 mg L−1 EM was observed at 5 h, while the removal efficiencies for initial concentrations of 41.8, 71.7, and 113.5 mg L−1 at 6 h were 91.1, 56.1, and 29.9 %, respectively. Within 5 ~ 14 h, 23.3 ~ 113.5 mg L−1 of initial liquid-phase EM were completely metabolized. Different concentrations of the major intermediate (DEDS) in the liquid phase were accumulated with the detectable maximum values of 13.6, 21.0, 40.6, and 66.4 mg L−1, respectively. Complete degradation of DEDS was observed within 11 ~ 23 h (Fig. 2a). The increase of biomass concentrations from 2.7 ~ 3.5 mg CDW L−1 to 12.7 ~ 41.1 mg CDW L−1 was obtained within 0 ~ 23 h, as shown in Fig. 2b. Although EM could be spontaneously converted to DEDS during the incubation period, only 3 % total loss of initial 23.3 mg L−1 EM in the control experiment was found within 11 h (data not shown). Therefore, EM was quickly transformed into DEDS through enzymatic oxidation by strain WL2, and chemical oxidation of EM into DEDS was negligible.
EM and its major intermediate (DEDS) degradation (a) by Pseudomonas sp. WL2 along with bacterial growth (b) for initial EM concentrations of 23.3 ~ 113.5 mg L−1. Solid line, EM concentration; dotted line, DEDS concentration. Empty square, 23.3 mg L−1; filled circle, 41.8 mg L−1; empty triangle, 71.7 mg L−1; filled inverted triangle, 113.5 mg L−1; empty star, trend of CO2 accumulation for 113.5 mg L−1. DEDS degradation (c) by Pseudomonas sp. WL2 along with bacterial growth (d) for initial DEDS concentrations of 24.6 ~ 110.3 mg L−1. Empty star, 24.6 mg L−1; filled star, 44.8 mg L−1; empty diamond, 86.3 mg L−1; filled diamond, 110.3 mg L−1
In the present study, strain WL2 was further examined for its DEDS degradation characteristics accompanied by the bacterial growth. As shown in Fig. 2c, 24.6 ~ 110.3 mg L−1 of initial DEDS were degraded by strain WL2 within 14 ~ 32 h. At 14 h, 97.2 % removal for the DEDS with initial concentration of 24.6 mg L−1 was obtained, while 34.8 ~ 90.8 % of removal efficiency were obtained for 44.8 ~ 110.3 mg L−1. After 32 h of the inoculation, almost complete degradation was observed for 110.3 mg L−1 of initial DEDS. As far as bacterial growth is concerned, 12.4 , 18.6, 30.3, and 33.8 mg CDW L−1 of the maximum biomass concentration for 24.6 ~ 110.3 mg L−1 of initial DEDS were obtained at the time of 14, 16, 23, and 32 h (Fig. 2d), respectively.
Degradation intermediates and final products for EM mineralization
According to GC/MS chromatogram of the SPE sample and mass spectra of the major intermediate (Fig. S1 and Fig. S2 in supplementary materials), DEDS (7.906 min, m/z = 122.0) was the identified intermediate during EM degradation. It was concluded that strain WL2 could degrade EM as a carbon source into DEDS through the enzymatic oxidation.
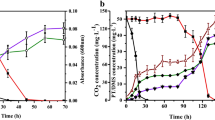
CO2 production (mg) and bacterial yield (mg CDW) as a function of different initial EM (mg) consumed was presented in Fig. 3. It is obvious that higher CO2 production and bacterial yield in the batch reactors were achieved when more substrate were consumed. For 4.2 ~ 25.2 mg of initial EM degradation, there were 8.9 ~ 29.0 mg CO2 produced and 1.23 ~ 3.48 mg CDW accumulated in the batch reactors after the substrate was completely consumed within 24 h.
To examine the sulfate production from oxidation of EM, water soluble metabolite in the liquid medium was determined using ion chromatography after 2, 7, and 20 days of incubation, respectively. For this experiment, a control reactor was used to measure the sulfate production originating from the sulfur oxidation of 5 mL inoculum (the equivalent of 0.84 mg EM added). The sulfate production was evaluated for different initial VOSCs consumed, as shown in Table 1.
After different initial EM of 6.7, 15.1, and 23.5 mg were fully consumed within 24 h, the sulfur in EM was transformed into other inorganic sulfur compounds via DEDS. However, inorganic sulfur compounds such as hydrogen sulfide (H2S), sulfite, and thiosulfate were not detected in the gas phase/liquid phase of the batch reactors. There was no sulfate immediately formed after the EM was completely degraded within 2 days. Also, after removing the bacterial cells by centrifugation within the first 2 days, the supernatant was cultured for 1 month and the sulfate concentration was not detectable (data not shown). The presence of sulfate concentration was found after 7 days of incubation. For 6.7 mg of initial EM added, the sulfate concentration accumulated from 0 mg L−1 (2 days) to 15.6 mg L−1 (7 days) and then to 100.3 mg L−1 for 20 days of incubation (Table 1). For 15.1 and 23.5 mg of initial EM added, it would take longer incubation period to be completely oxidized into the sulfate.
Degradation pathway
The major objective of this study was to establish the degradation pathway that involved the complete mineralization of EM by strain WL2. A complete pathway was proposed for EM degradation by Pseudomonas sp. WL2 (Fig. 4). The EM degradation was initiated by the formation of DEDS resulted from the enzymatic oxidation by strain WL2. As other inorganic sulfur compounds were not detected in the liquid, it was speculated that formation of the elemental sulfur (S0) occurred inside of the bacterial cells via the transformation of DEDS before being further oxidized to sulfate. Ethyl aldehyde could be a possible intermediate for EM degradation by strain WL2. Combining the production of CO2, bacterial cells, and sulfate with other possible intermediates, a putative degradation pathway of EM by strain WL2 was presented.
Substrate range of Pseudomonas sp. WL2
Substrate range studies of Pseudomonas sp. WL2 were performed to test its ability to degrade other sulfur compounds, aldehydes, and methanol. The utilization of several other organic compounds by strain WL2 is listed and compared in Table 2. It can be seen that strain WL2 could efficiently degrade EM, propyl mercaptan, and n-butyl mercaptan as growth substrate with biomass concentration increasing to 16.3, 16.4, and 13.7 mg CDW L−1, respectively, within 12 h. Based on the increasing biomass for other organic sulfur compounds, it is concluded that strain WL2 could also use several organic disulfides as growth substrate, and organic sulfides could not be metabolized by strain WL2. For example, strain WL2 isolated with sole EM could not degrade dimethyl sulfide and dimethyl disulfide, indicating these compounds with methyl groups could not be degraded by Pseudomonas sp. WL2, except to the utilization of methanol (11.0 mg CDW L−1) and methyl aldehyde (1.8 mg CDW L−1) for bacterial growth. In addition, degradation of ethyl aldehyde with a growth value of 10.2 mg CDW L−1 was also obtained.
Kinetic analysis
The relationships between specific growth (degradation) rates and liquid-phase concentrations were shown with the experimental data and the model fitting data (Fig. S3 in supplementary materials). Moreover, the corresponding kinetic constants were obtained. For EM degradation, the experimental values of specific growth rates and specific degradation rates for initial concentrations of 22.7 ~ 113.5 mg L−1 were estimated at 0.120 ~ 0.166 h−1 and 1.193 ~ 1.465 gS gX −1 h−1, respectively.
The kinetic constants of model fitting corresponding with the specific degradation rate and specific growth rate of EM were also shown (Fig. S3a in supplementary materials). The estimated maximum specific growth rate (μ max = 0.379 h−1) of strain WL2 was obtained with K s = 23.01 mg L−1 and K i = 60.98 mg L−1. In terms of the specific degradation rate, q max, K s, and K i of strain WL2 for EM degradation were estimated at 3.13 gS gX −1 h−1, 31.91 mg L−1, and 103.19 mg L−1, respectively. Higher K i value indicates that this strain could grow well at a higher substrate concentration.
According to the kinetic analysis for DEDS degradation by strain WL2 using the Haldane-Andrews model (Fig. S3b in supplementary materials), the maximum specific degradation rate (1.33 gS gX −1 h−1) and maximum growth rate (0.263 h−1) were both lower than the estimated kinetic parameters for EM degradation. Comparatively, Pseudomonas sp. WL2 degraded EM at a maximum specific degradation rate that is 2.35 times higher than that for DEDS degradation. The corresponding values of K s and K i were 52.98 and 81.77 mg L−1 for bacterial growth while 22.57 and 65.68 mg L−1 for DEDS degradation, respectively.
Table 3 presents the values of degradation rate constants using pseudo-first-order models for both EM and DEDS degradation by Pseudomonas sp. WL2. In terms of EM degradation, the R 2 values for the pseudo-first-order model at initial concentrations of 23.3 ~ 113.5 mg L−1 were greater than 0.99, indicating a best fit using the pseudo-first-order model with the degradation rate constants K 1p of 0.232 ~ 0.522 h−1. With the increase of initial substrate concentration, the degradation rate constant decreased because of the effects of substrate inhibition. Similarly, the values of degradation rate constant for DEDS degradation using the pseudo-first-order model reached a maximum value 0.175 h−1 at a lower concentration of 24.6 mg L−1. It is indicated that Pseudomonas sp. WL2 could degrade EM at a maximum degradation rate constant that is 2.98 times higher than that for DEDS degradation.
Discussion
In this study, strain Pseudomonas sp. WL2 was firstly reported to efficiently mineralize EM via DEDS using a putative degradation pathway. Besides, DEDS is always used as an intermediate for bactericide and organic synthesis, and its degradation by pure cultures was firstly studied. The ability of strain Pseudomonas species to degrade EM was firstly reported by Barreiros et al. (2008) for the mineralization of the thiocarbamate herbicide molinate, indicating the transformation of EM via the possible intermediate (ethyl methyl sulfide). In fact, several other studies have been conducted to screen the pure cultures that are capable of utilizing EM as the sole carbon and energy source using the batch reactors. Sedighi et al. (2013) found that 115 ~ 320 mg m−3 of gaseous EM was completely degraded by R. eutropha within 120 ~ 168 h. Moreover, Wan et al. (2010) noted that complete biodegradation of 2 ~ 4 mg L−1 EM in an aqueous medium was achieved by a new Lysinibacillus sphaericus strain RG-1 within 96 h. Besides, EM could be oxidized by methyl mercaptan oxidase, which was purified from Thiobacillus thioparus TK-m grown on dimethyl sulfide (Gould and Kanagawa 1992).
Degradation characteristics of EM and DEDS with different initial concentrations by Pseudomonas sp. WL2 were evaluated in terms of the growth rate and the degradation rate, respectively. The bacterial cells in the liquid-phase were not saturated at lower initial substrate concentration and the slight increase of substrate concentration would be beneficial to the substrate degradation and the bacterial growth of strain WL2. Comparatively, both the degradation rates and bacterial growth rates for EM were much higher than those for DEDS by strain WL2. This was further confirmed by the kinetic analysis using the Haldane-Andrews model and the pseudo-first-order model, respectively. All of the results showed that the degradation rates for EM by strain WL2 achieved in this study were much higher (2.35 ~ 2.98 times) than those for DEDS degradation. This probably indicated that transformation of DEDS is the limitation step for EM degradation by strain WL2.
In the previous studies, Sedighi et al. (2013) have fitted the kinetic parameters using the Monod model and obtained the maximum specific degradation rate of 0.23 mgS gX −1 h−1 and K s of 1.379 mg L−1 at the initial EM concentrations of 1 ~ 4 mg L−1 by R. eutropha. These values were much lower than those of strain WL2. Obviously, EM degradation rate of strain WL2 is 13.6 × 103 times higher than that of R. eutropha. Furthermore, the maximum degradation rate constant of 0.0308 h−1 for EM degradation by L. sphaericus (Wan et al. 2010) was obtained using the pseudo-first-order model, which was 0.059 times lower than that by strain WL2 (0.522 h−1). Results obtained that aerobic degradation of EM by strain WL2 was more efficient than those from previous studies.
In the process of aerobic biodegradation by microorganisms, organic compounds are always aerobically degraded into water and carbon dioxide and are used as the essential carbon source for bacterial growth (Kennes et al. 2009). In addition to the production of CO2 and biomass, the sulfur element in organic sulfur compounds would be transformed into the final sulfate. Hence, CO2 production, bacterial yield, and sulfate production are important parameters to provide valuable information on VOSC mineralization. Theoretically, the stoichiometric ratio should be 1.416 mg in the case of complete mineralization to CO2 for 1 mg EM according to Eq. (7). The coefficient of CO2 production was estimated at 1.23 g g−1 for EM degradation by strain WL2. In addition, a bacterial yield coefficient (0.158 g g−1) of Pseudomonas sp. WL2 was obtained (Fig. 3).
In the present study, 86.86 % of the carbon element in EM was transformed into CO2, and the other carbon atoms were used as the essential carbon source for bacterial growth when the abiotic substrate loss was not taken into account. Both the CO2 production and bacterial yield coefficient proved that strain WL2 could efficiently mineralize EM into harmless products.
As reported previously, the elemental sulfur was always formed and transformed into the final sulfate when metabolizing the sulfur element in VOSCs (Li et al. 2010; Sipma et al. 2004; Zhang et al. 2013). According to Eq. (7), the stoichiometric ratio should be 15.5 mg L−1 of the sulfate formed under complete mineralization of 1 mg EM. Based on the sulfur balance analysis (Table 1), 96.5 % of the sulfur element was oxidized to sulfate for 6.7 mg of EM by strain WL2, within 20 days. Only 13.9 and 4.2 % of the sulfur element were converted into sulfate with the initial EM of 15.1 and 23.5 mg, respectively, within 20 days. Similarly, previous research on the biological oxidation of 2.5 mM methanethiol (MM) to sulfate at neutral pH conditions within 350 h was also obtained via DMDS as intermediate (Sipma et al. 2004). Barreiros et al. (2003, 2008) have reported that EM originating from the initial breakdown of molinate could be mineralized to CO2 and sulfate by Pseudomonas chlororaphis ON1 and Stenotrophomonas maltophilia ON2 through the further conversion to ethyl methyl sulfide. Moreover, Li et al. (2010) has also reported the oxidation of elemental sulfur to sulfate via thiosulfate by strain Fusarium solani THIF01 isolated from deteriorated sandstones of Angkor monuments.
DEDS was identified as the first and major intermediate for EM degradation by strain WL2. Similarly, Sipma et al. (2004) found that biological oxidation of methyl mercaptan (MM) was firstly oxidized into dimethyl disulfide (DMDS). Furthermore, formaldehyde was reported as a main intermediate for methyl mercaptan degradation (Gould and Kanagawa 1992; Zhang et al. 2013). Also, ethyl aldehyde could be degraded by strain WL2 as a growth substrate (Table 2). Therefore, it was speculated that ethyl aldehyde was a possible intermediate along with the desulfuration of DEDS by strain WL2. Based on the identified intermediates or final products for EM degradation by Pseudomonas sp. WL2, a putative degradation pathway was presented. Degradation pathway of EM was previously proposed by Sedighi et al. (2013). And sulfate production and the EM degradation pathway by R. eutropha were discussed based on the putative pathway for the mineralization of the thiocarbamate herbicide molinate (Barreiros et al. 2008). However, there was no direct evidence for observing the accumulation of the possible intermediates (ethyl methyl sulfide or sulfide) in the above studies.
Besides, investigation on substrate range of Pseudomonas sp. WL2 indicated strain WL2 is the most metabolically VOSC-degrading organism. The ability of strain WL2 to degrade several mercaptans, disulfides, aldehydes, and methanol makes it a promising aspirant for the removal of industrial VOSC emissions.
In conclusion, strain Pseudomonas sp. WL2 isolated from the activated sludge of a pharmaceutical wastewater treatment plant was able to efficiently degrade EM as the sole carbon and energy source. Kinetic analysis for EM and DEDS degradation with different initial concentrations was proved to be more efficient than other pure cultures reported previously. Also, it was shown that strain WL2 could oxidize EM to CO2, bacterial cells and sulfate through enzymatic oxidation into DEDS, indicating a novel pathway for EM degradation.
References
An TC, Wan SG, Li GY, Sun L, Guo B (2010) Comparison of the removal of ethanethiol in twin-biotrickling filters inoculated with strain RG-1 and B350 mixed microorganisms. J Hazard Mater 183:372–380
Andrews JF (1968) A mathematical model for the continuous culture of microorganisms utilizing inhibitory substrates. Biotechnol Bioeng X:707–723
Arellano-Garcia L, Gonzalez-Sanchez A, Baquerizo G, Hernandez-Jimenez S, Revah S (2010) Treatment of carbon disulfide and ethanethiol vapors in alkaline biotrickling filters using an alkaliphilic sulfo-oxidizing bacterial consortium. J Chem Technol Biotechnol 85:328–335
Barbosa VL, Burgess JE, Darke K, Stuetz RM (2002) Activated sludge biotreatment of sulphurous waste emissions. Rev Environ Sci Biotechnol 1:345–362
Barreiros L, Nogales B, Manaia CM, Ferreira ACS, Pieper DH, Reis MA, Nunes OC (2003) A novel pathway for mineralization of the thiocarbamate herbicide molinate by a defined bacterial mixed culture. Environ Microbiol 5:944–953
Barreiros L, Fernandes A, Silva Ferreira AC, Helena P, Bastos MMSM, Manaia CM, Nunes OC (2008) New insights into a bacterial metabolic and detoxifying association responsible for the mineralization of the thiocarbamate herbicide molinate. Microbiology 154:1038–1046
Charlson RJ, Lovelock JE, Andreae MO, Warren SG (1987) Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 326:655–661
Gould WD, Kanagawa T (1992) Purification and properties of methyl mercaptan oxidase from Thiobacillus thioparus TK-m. J Gen Microbiol 138:217–221
Hao OJ, Kim MH, Seagren EA, Kim H (2002) Kinetics of phenol and chlorophenol utilization by Acinetobacter species. Chemosphere 46:797–807
Hernandez J, Lafuente J, Prado OJ, Gabriel D (2012) Simultaneous removal of H2S, NH3, and ethyl mercaptan in biotrickling filters packed with poplar wood and polyurethane foam: impact of pH during startup and crossed effects evaluation. Water Air Soil Pollut 223:3485–3497
Kennes C, Rene ER, Veiga MC (2009) Bioprocesses for air pollution control. J Chem Technol Biotechnol 84:1419–1436
Li XS, Sato T, Ooiwa Y, Kusumi A, Gu JD, Katayama Y (2010) Oxidation of elemental sulfur by Fusarium solani strain THIF01 harboring endobacterium Bradyrhizobium sp. Microb Ecol 60:96–104
Monod J (1949) The growth of bacterial cultures. Annu Rev Microbiol 3:371–394
National Research Council of United States (N.R.C. U.S.) (2013) Acute exposure guideline levels for selected airborne chemicals: volume 15. The National Academies Press, Washington, DC, http://www.nap.edu/catalog.php?record_id=18449
Rappert S, Müller R (2005) Microbial degradation of selected odorous substances. Waste Manag 25:940–954
Saravanan P, Pakshirajan K, Saha P (2009) Batch growth kinetics of an indigenous mixed microbial culture utilizing m-cresol as the sole carbon source. J Hazard Mater 162:476–481
Sedighi M, Vahabzadeh F, Zamir SM, Naderifar A (2013) Ethanethiol degradation by Ralstonia eutropha. Biotechnol Bioproc Eng 18:827–833
Silambrasan S, Abraham J (2013) Kinetic studies on enhancement of degradation of chlorpyrifos and its hydrolyzing metabolite TCP by a newly isolated Alcaligenes sp. JAS1. J Taiwan Inst Chem Eng 44:438–445
Sipma J, Svitelskays A, Van Der Mark B, Hulshoff Pol LW, Lettinga G, Buisman CJN, Janssen AJH (2004) Potentials of biological oxidation processes for the treatment of spent sulfidic caustics containing thiols. Water Res 38:4331–4340
Staudinger J, Roberts PV (2001) A critical compilation of Henry’s law constant temperature dependence relations for organic compounds in dilute aqueous solutions. Chemosphere 44:561–576
Su Y, Xia FF, Tian BH, Li W, He R (2013) Microbial community and function of enrichment cultures with methane and toluene. Appl Microbiol Biotechnol 98:3121–3131
Wan SG, Li GY, An TC, Guo B, Sun L, Zu L, Ren AL (2010) Biodegradation of ethanethiol in aqueous medium by a new Lysinibacillus sphaericus strain RG-1 isolated from activated sludge. Biodegradation 21:1057–1066
Wan SG, Li GY, An TC, Guo B (2011) Co-treatment of single, binary and ternary mixture gas of ethanethiol, dimethyl disulfide and thioanisole in a biotrickling filter seeded with Lysinibacillus Sphaericus RG-1. J Hazard Mater 186:1050–1057
Zhang CZ, Zhang WJ, Xu J (2013) Isolation and identification of methanethiol-utilizing bacterium CZ05 and its application in bio-trickling filter of biogas. Bioresour Technol 150:338–343
Acknowledgments
The work was sponsored by the National High Technology Research and Development Program of China (No. 2012AA063102).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 191 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Wu, C., Liu, N. et al. Degradation of ethyl mercaptan and its major intermediate diethyl disulfide by Pseudomonas sp. strain WL2. Appl Microbiol Biotechnol 99, 3211–3220 (2015). https://doi.org/10.1007/s00253-014-6208-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6208-3