Abstract
The present work discusses the startup and operation of different biotrickling filters during the simultaneous removal of NH3, H2S, and ethyl mercaptan (EM) for odor control, focusing on (a) the impact of pH control in the stability of the nitrification processes during reactor startup and (b) the crossed effects among selected pollutants and their by-products. Two biotrickling filters were packed with poplar wood chips (R1 and R2A), while a third reactor was packed with polyurethane foam (R2B). R2A and R2B presented a pH control system, whereas R1 did not. Loads of 2–10 g N–NH3 m−3 h−1, 5–16 g S–H2S m−3 h−1, and 1–6 g EM m−3 h−1 were supplied to the bioreactors. The presence of a pH control loop in R2A and R2B proved to be crucial to avoid long startup periods and bioreactors malfunctioning due to biological activity inhibition. In addition, the impact of the presence of different concentrations of a series of N species (NH +4 , NO −2 , and NO −3 ) and S species (SO 2−4 and S2−) on the performance of the two biotrickling filters was studied by increasing their load to the reactors. Sulfide oxidation proved to be the most resilient process, since it was not affected in any of the experiments, while nitrification and EM removal were severely affected. In particular, the latter was affected by SO 2−4 and NO −2 , while nitrification was significantly affected by NH +4 . The biotrickling filter packed with polyurethane foam was more sensitive to crossed effects than the biotrickling filter packed with poplar wood chips.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
As of today, the abatement of foul-smelling mixtures remains the main market for gas-phase bioreactors all over the world. A vast majority of industrial gaseous emissions comprise perceptible concentrations of two or more odorous pollutants, commonly belonging to different chemical groups and, accordingly, with a distinct behavior in the bioreactor. This is especially notorious in wastewater treatment plants (WWTP), where gaseous emissions from different plant locations may contain dozens of odorous compounds. However, only very recently, research has focused on the simultaneous treatment of mixtures of odorous volatile inorganic compounds such as H2S and NH3 and volatile organic compounds (VOCs) (Hort et al. 2009; Hernández et al. 2010; Ho et al. 2008). Although biofilters have been generally considered as the most adequate bioreactor configuration for the treatment of VOC-containing emissions (Devinny et al. 1999; Kennes and Thalasso 1998), biotrickling filters are expanding their range of application to the treatment of complex odorant mixtures containing VOCs (Hassan and Sorial 2011; Sempere et al. 2010; Paca et al. 2007). However, the need for cheap, durable packing materials remains a challenge for real-scale bioreactors (Prado et al. 2009). In this sense, it has been hypothesized that certain organic materials, as some types of wood, can compete with inert materials in terms of durability in biotrickling filters at a significantly lower price.
When treating complex mixtures of pollutants, the effects that the presence of a certain pollutant in the off-gas or that of metabolites resulting from the oxidation of a given compound might have on the biodegradation of others remain relatively unknown. Crossed effects have been studied in depth in the field of water treatment, mostly focused on the influence of free ammonia (NH3) and free nitrous acid (HNO2) on the nitrification process (Anthonisen et al. 1976; Jubany et al. 2008). However, analogous studies in gas-phase bioreactors considering different metabolites (as sulfur compounds) and processes (as sulfur oxidation or heterotrophic VOC consumption) are scarce. Crossed effects can be either due to a direct inhibitory effect on the metabolism of sensitive microbial species or to an eventual modification of the conditions of the bioreactor environment such as pH changes. The latter is particularly important, taking the main oxidation mechanisms of S and N species into account. H2S biodegradation, undertaken by sulfide-oxidizing bacteria (SOB), produces 2 mol of H+ per mol of H2S oxidized. Similarly, mercaptan oxidation under aerobic conditions produces sulfate (Wan et al. 2010), which indicates that ethyl mercaptan (EM) oxidation takes place following an oxidative pathway via H2S (Lomans et al. 2002) once the S–C bonds have been broken. Heterotrophic biomass is responsible for the oxidation of the organic fraction of mercaptans. In addition, the nitrification process, which comprises the two-step oxidation of NH3 via NO −2 to NO −3 catalyzed by autotrophic ammonium-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB), respectively, is a well-known alkalinity-consumer process.
The aim of the present study was to assess the performance of several biotrickling filters packed with an organic packing material (poplar wood chips, seldom used in biotrickling filters) and an inert one (namely, polyurethane foam) and to determine the crossed effects taking place in them by increasing the concentrations of N species (NO −3 , NO −2 , and NH +4 ) and S species (S2− and SO 2−4 ) in the makeup water. These reactors were used to treat a complex mixture composed of NH3, H2S, and EM. EM was employed as a model VOC due to its low odor detection threshold value and to being a commonly produced pollutant in many industrial activities. Moreover, the impact of the presence of a pH control system on the startup was studied.
2 Materials and Methods
2.1 Experimental Setup
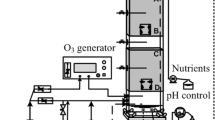
The experimental setup (Fig. 1) consisted of several PVC columns with a height of 70 cm and an internal diameter of 8.6 cm. Reactors were packed up to a height of 50 cm, resulting in a packed bed volume of 2.9 L per biofilter. Compressed, humidified air was fed with pure NH3 and H2S using digital mass flow controllers (Bronkhorst, The Netherlands). EM was fed by means of a double-head peristaltic pump (Mod. 403 U/VM2; Watson Marlow, Wilmington, MA, USA) which pumped air into glass bubblers containing liquid EM (97 %; Sigma-Aldrich, St. Louis, MO, USA). The resulting gas mixture was fed in upflow mode. The liquid phase was continuously renewed by the automated supply of mineral medium containing (in grams per liter): NH4Cl, 0.01; KH2PO4, 0.05; K2HPO4, 0.05; CaCl2, 0.02; MgSO4·7H2O, 0.2; trace elements, 1 mL L−1 (Pfenning et al. 1981). The recirculation flow rate was set to 130 mL min−1. All reactors were inoculated with a mixture containing enriched ammonia-oxidizing biomass, aerobic sludge from a municipal WWTP (Manresa, Spain), and mineral medium. Biotrickling filters were operated at an empty bed retention time of 32 s at room temperature. A homemade Visual Basic™ application was used for automating and monitoring the biofiltration setup.
Schematic representation of the experimental setup: 1 air inlet, 2 humidification tower, 3 humidification tower level control pump, 4 water level control reservoir, 5 NH3 and H2S gas cylinders, 6 flow meters, 7 EM supply pump, 8 gas bubbler with pure EM, 9 mixing chamber, 10 rotameter, 11 biotrickling filter, 12 adsorption chamber, 13 air outlet, 14 pH control system, 15 recirculation pump, 16 purge pump, 17 purge reservoir, 18 makeup water pump, 19 makeup reservoir
2.2 Analytical Methods
Inlet and outlet H2S concentrations were measured using an electrochemical H2S sensor (Sure-cell; Euro-Gas Management Services Ltd., Plymouth, UK). NH3 concentration was determined after bubbling the gas stream in acidified water and later analyzed in an ammonium continuous flow analyzer (CFA) (Baeza et al. 1999). For EM determination, a calibrated GC 6890N (Agilent Tech. S.A., Barcelona, Spain) was employed. Chloride, N-nitrite, N-nitrate, S-sulfate, and P-phosphate concentrations in the leachate samples were determined by means of an ICS-1000 Ion Chromatograph (Dionex Corp., Sunnyvale, CA, USA), equipped with an IonPac AS9-HC column. Sulfide concentration was determined by means of a CFA (Montebello et al. 2010). Leachate pH and conductivity were measured by means of a pH 28 sensor and a MicroCM 2100 sensor (Crison Instr. S.A., Barcelona, Spain), respectively. According to the specifications of the sensors, the following standard deviations were considered: 10 % for EM removal efficiency (RE), 1.5 % for H2S RE, 7 % for nitritation and nitratation percentages (defined in the next section), and 1 % for the ammonium concentration. The detection limit of the methodologies employed were 1 ppmv for H2S and EM, 1 mg N–NH +4 L−1 for ammonium, 0.1 mg L−1 for all ions in the ion chromatograph, and 0.6 mg S L−1 for sulfide.
2.3 Experimental Conditions During Startup
Several runs were performed to assess biotrickling filters operation. In a first run, a biotrickling filter packed with poplar wood chips (thereafter R1) was operated without any automated pH control system. R1 was fed with 49 ± 9 ppmv NH3, 40 ± 3 ppmv H2S, and 12 ± 4 ppmv EM corresponding to loads of 3.5 ± 0.9 g N–NH3 m−3 h−1, 6.5 ± 1.1 g S–H2S m−3 h−1, and 3.6 ± 1.2 g EM m−3 h−1, respectively. In a second run, two biotrickling filters (R2A and R2B) were operated in parallel with an automated pH control system. Biotrickling filter R2A was packed with poplar wood chips, while R2B was packed with polyurethane foam. The pH regulation (HCl or NaOH 0.5 M addition) of R2A and R2B consisted of an on/off controller with a pH set point value of 7. During the startup, R2A and R2B were fed with 135 ± 14 ppmv NH3, 39 ± 3 ppmv H2S, and 10 ± 4 ppmv EM corresponding to loads of 8.8 ± 1.0 g N–NH3 m−3 h−1, 5.9 ± 0.6 g S–H2S m−3 h−1, and 2.9 ± 1.4 g EM m−3 h−1, respectively.
2.4 Crossed Effects Tests Conditions
After R2A and R2B startup, the following inlet concentrations were kept in the gas phase throughout the study: 90 ± 7 ppmv of NH3, 103 ± 2 ppmv of H2S, and 14 ± 3 ppmv of EM, corresponding to inlet loads of 5.9 ± 0.5 g N–NH3 m−3 h−1, 15.4 ± 0.5 g S–H2S m−3 h−1, and 3.9 ± 1.4 g EM m−3 h−1. Crossed effects tests were performed in reactors R2A and R2B between days 155 and 280 of operation by stepwise increase of the makeup water concentration to have a better control of the liquid-phase concentrations. In experiments A to C, the SO 2−4 concentration in the makeup water was increased stepwise in three steps of 367, 1,011, and 1,696 mg S L−1. In experiments D and E, due to experimental limitations, a concentration ramp experiment was performed instead of a concentration step, consisting in supplying the sulfide concentration desired just in the makeup water by means of an Na2S solution. The two concentrations tested were 468 and 841 mg S L−1. All makeup water concentration increases lead to the S species concentrations in the liquid phase at the end of each step provided in Table 1.
Similarly, three series of experiments were carried out by increasing the concentrations of NO −3 , NO −2 , and NH +4 in the makeup water. Two NO −3 concentration steps of 610 and 2,490 mg N L−1 were performed in experiments F and G, while four NO −2 concentration steps of 25, 75, 160, and 310 mg N L−1 were performed in experiments H to K. Finally, two concentration steps of 100 and 300 mg N L−1 for NH +4 were enough to evaluate the impact on pollutants abatement in experiments L and M. Table 1 shows the N species concentrations in the liquid phase at the end of each step. A period of at least three hydraulic residence times was ensured prior to performing the next concentration step, except for experiment F, in which experimental constraints allowed only for two hydraulic residence times.
2.5 Performance Parameters
Nitritation and nitratation percentages were calculated according to Eqs. 1 and 2, respectively, where m(NO −2 )l, m(NO −3 )l, and m(N-drain)l are the mass of nitrite, nitrate and the sum of all nitrogen species, respectively, measured in the drain of the reactor under pseudo-steady-state conditions. On the other hand, the crossed effects impact on H2S and EM were evaluated in terms of RE, since elemental sulfur was not measured and SO 2−4 production could be attributed to the biodegradation of both H2S and EM.
3 Results and Discussion
3.1 Effect of pH Control on the Startup of Biotrickling Filters
Although conditions in a biological reactor are generally highly selective, microbial selected species are, at some point, always grown. However, long startup phases are undesirable from an industrial perspective because of the impact of potential emissions derived from low bioreactors performance. In the case of simultaneous removal of NH3, H2S, and organic compounds at low concentrations, as those found in odor treatment facilities, 100 % removal efficiencies of NH3 are usually reached right after reactor startup with or without pH control due to the high solubility of NH3. Complete mercaptans and H2S removal may generally take longer to be reached (Webster et al. 1997; Gabriel and Deshusses 2003).
In the two biotrickling filters packed with poplar wood chips studied herein (R1 and R2A), a significantly worse performance was encountered for a pH-uncontrolled biotrickling filter (Fig. 2a) compared with a pH-controlled biotrickling filter (Fig. 3a) in terms of gas-phase removal. In the case of H2S and EM, 7 and 10 days, respectively, were needed in the pH-controlled biotrickling filter (R2A) to reach RE above 95 %. This was significantly lower than the time needed in the uncontrolled biotrickling filter (R1), which lasted 14 and 26 days, respectively (Figs. 2a and 3a). However, 100 % removal in the gas phase may correspond as well to a reactor operation governed by absorption with nil biological activity. The distribution of S and N species in the liquid phase in both biotrickling filters tested was different (Figs. 2b and 3b). In all cases, no EM was detected in the liquid phase along the period under study.
Profiles of nitrogen and sulfur species in a biotrickling filter packed with poplar wood without pH control. a Profiles of inlet and outlet H2S concentration and H2S, NH3, and EM removal efficiencies and b liquid-phase species. Arrows indicate when manual corrective actions were applied to adjust reactor pH
Between days 5 and 20 of operation, the biotrickling filter without pH control (R1) exhibited a progressive decrease of pH from 7 to 5, mainly due to H2S and EM oxidation (Fig. 2b). It is worth mentioning that the sulfate production and pH decrease were mostly due to H2S removal, since the S load due to EM corresponded to 23 % of the overall S loaded to the reactor. According to the optimum pH range for nitrifying bacteria reported in the literature, which ranges between 6 and 8 (Jiang et al. 2009b), as soon as the pH dropped below 6, a washout of the remaining NO −2 and NO −3 was observed, thus indicating that the nitrifying activity ceased. At this point, the biotrickling filter performed as an absorption column in terms of N removal. On day 14, the makeup water flow rate in R1 was doubled to minimize the pH drop. Such actuation increased the H2S and EM absorption capacities. Still, the makeup water flow rate was doubled again on day 18, which led to a progressive stabilization of the concentration of NH +4 and SO 2−4 and a recovery of pH. On day 28, NaOH was added to the makeup water to reach a constant pH of 9–10 at the entrance of the biotrickling filter. Such manual, expert-based actuations led to a progressive increase in the nitrification capacity from day 35 onwards. On day 50, the biotrickling filter reached the desired operating conditions, i.e., complete nitrification to nitrate. However, EM RE suffered a decrease of around 30 %.
Instead, the biotrickling filter with pH control (R2A) exhibited a much more stable operation during startup (Fig. 3b), with a probably unavoidable initial accumulation of NH +4 together with a sustained nitrification activity that led to a much shorter startup. It is worth noticing that such improved startup of reactor R2A took place by feeding an NH3 load almost three times higher than that of R1 without pH control. On day 20, complete nitrification and sulfide oxidation were reached. In addition, on day 27, the ammonia load was increased to around 10 g N–NH3 m−3 h−1 without a significant impact in the performance of the biotrickling filter. Even though R2A achieved complete nitrification and sulfur oxidation during the pseudo-steady state between days 45 and 55 of operation, it is important to remark that not all the nitrogen and sulfur fed to R2A were recovered. Average deviations of 10 ± 5 and 17 ± 4 % were measured in the nitrogen and sulfur mass balances performed in the biofilter, respectively. Such deviations are in the range of those previously reported (Sakuma et al. 2008). Nitrogen and sulfur mass balances in R1 showed average deviations of 23 ± 8 and 13 ± 4 %, respectively. Such differences with respect to R2A were attributed to the lack of an automated pH control loop in R1.
The results clearly show the intrinsic relationship between pH and biological sulfide oxidation and nitrification processes and the benefits of a pH-controlled startup to avoid nitrification inactivation. A better understanding of the role of pH may help in improving system design and operation of numerous full-scale biotrickling filters currently operated without pH control (Juhler et al. 2009; Lafita et al. 2011). Also, an additional benefit would be found in the case of treatment of higher, changing loads of NH3. As an example, Juhler et al. (2009) found that several biotrickling filters for NH3 removal from pig farms in Denmark are generally operated in such a way that the sump water drainage and renewal are performed discontinuously based on a preset conductivity threshold without pH control in the reactor. Under these conditions, large ammonium and nitrite concentrations accumulate in the liquid, leading to partial inhibition of nitrification processes. Since free ammonia (FA) or free nitrous acid (FNA) concentrations as well as inhibition thresholds are pH-dependent, then pH control would be a strategy to minimize the impact of potential inhibitions in the system during reactor startup.
It is worth mentioning that simultaneous treatment of NH3 and H2S in a conventional biofilter, in which control of parameters in the liquid phase is hardly feasible, is likely to lead to reactor failure more easily. As an example, Malhautier et al. (2003), treating a mixture of NH3 and H2S in a conventional biofilter, observed a poor nitrification activity probably caused by the acidification of the biofilter due to the oxidation of high amounts of H2S. Results found herein reinforce the crucial importance that pH control has on biotrickling filters performance and the impact that pH may have in not well-controlled systems. Process control may help tip the balance in favor of successful reactor startup and operation, particularly in biotrickling filters.
3.2 Crossed Effects Analysis
After reactor startup and prior to the crossed effects tests started on day 155, both biotrickling filters exhibited a markedly different performance (Table 2). Systematically, lower REs for S pollutants, especially for EM, were obtained in R2B (polyurethane foam). One hypothesis which may explain these differences would be the influence of biomass density (not measured) on the bioreactor’s performance. In this sense, considering the H2S biodegradation stoichiometry proposed by Rittman and McCarty (2003), which includes SOB growth, and the EC for H2S obtained during the first 155 operations, a slight difference of around 5 % in the biomass production coupled to H2S biodegradation was estimated between reactors. Analogously, a stoichiometry including biomass growth on EM was derived, taking into account that EM biodegradation is carried out by a heterotrophic–autotrophic consortium. Biomass yields of 0.62 g biomass g−1 EM and 0.19 g biomass g−1 H2S led to a roughly 25 % higher biomass production in R2A in comparison with R2B which was a consequence of such systematic lower removal capacities in R2B. Even though the inaccuracy of these estimations is due to several non-evaluated factors such as biomass detachment, this assumption is consistent with experiments aimed at evaluating the effect of biomass concentration and its adaptation on the performance of bioreactors, which prove that a higher biomass concentration and adapted inoculum improved the bioreactor’s performance (Prado et al. 2005). However, other factors such as the water holdup or the water distribution throughout the bed, which have been shown to impact the performance of biotrickling filters, cannot be discarded as factors playing a role in the present study.
3.2.1 S Species Crossed Effects
The set of experiments aimed at evaluating the effect of supplying different concentrations of SO 2−4 and S2− led to the results summarized in Table 3, in which REs and nitritation and nitratation percentages based on the concentrations encountered before and at the end of each step under pseudo-steady-state conditions are shown. During 16 days, the concentration of SO 2−4 supplied to the bioreactors was stepwisely increased in three steps of 367, 1,011, and 1,696 mg S L−1. Subsequently, the concentration of SO 2−4 supplied was lowered to its former value of 40 mg S L−1 and the concentration of S2− was increased to 468 and 841 mg S L−1, respectively.
S–SO 2−4 Experiments
No significant effect of the presence of S–SO 2−4 on nitrification and sulfur oxidation was found neither on R2A nor on R2B. The decay in the nitritation percentage found in R2A during the last S–SO 2−4 concentration step (Table 3), experiment C, was due to a pipe clog in the NaOH supply line, which led to a pH decrease from 7 to 4 and to a subsequent nitrification decay (Lee et al. 2002; Galera et al. 2008). Once the pH control was fixed, R2A recovered its initial nitrification rate and EM RE. Moreover, NH3 RE remained over 99 % during the entire experiment.
Also, H2S oxidation remained unaffected (Table 3) even though maximum S–SO 2−4 concentrations in R2A and R2B up to 2.3 and 2.1 g S L−1, respectively, were found in the drain (Table 1). This is consistent with results obtained by Sercu et al. (2005) in a study treating a mixture of dimethyl sulfide and H2S, which found that H2S RE did not significantly vary even when S–SO 2−4 concentrations of 4 g S L−1 were found in the drain. Interestingly, Ramírez et al. (2009) found that increasing S–SO 2−4 concentrations from 1 to 3.3 g S L−1 led to a decrease in the H2S RE from 78 to 54 %. As aforementioned, acclimation of microbial populations and biomass density might have played an important role in order to determine the inhibition thresholds and effects of potentially inhibiting compounds.
Regarding EM abatement, the increase of the S–SO 2−4 concentration led to an EM RE decrease from 69 to 59 % in R2B already during experiment A, where maximum S–SO 2−4 concentrations of 1.0 g S L−1 were measured. An et al. (2010) did not observe any negative effect on EM RE either at S–SO 2−4 concentrations around 1.0 g S L−1. However, the RE of EM in R2A was affected in experiment C at an S–SO 2−4 concentration of 2.3 g S L−1. Again, it is very likely that the instability of pH caused the RE decrease in this bioreactor during experiment C. R2A, packed with poplar wood, showed a higher stability in comparison to R2B, packed with polyurethane foam, to sudden concentration changes. This behavior was related to a probable lower microbial density onto the packing material, even if the sorption capacity due to different water contents cannot be discarded.
S–S2− Experiments
Once the same concentration of the species analyzed in the liquid phase was achieved, an experiment aimed at assessing the effect of an increase in the S–S2− inlet concentration was carried out. Due to experimental constrains, a concentration ramp experiment was performed instead of a concentration step experiment. No changes in the nitrification or sulfur oxidation capacity were observed in R2A, in which an S2− concentration of 5.8 mg S L−1 was found in the liquid phase at the end of the experiment. On the other hand, nitrification was partially inhibited in R2B in experiment D, in which an S2− concentration of 1.7 mg S L−1 was measured in the drain. Considering that a sulfide concentration of 0.5 mg L−1 can cause considerable negative effects on the nitrification activity (Esøy et al. 1998), it is interesting to emphasize the stability of the reactor packed with the organic material (R2A) to hold the same nitrification rates at higher sulfide concentrations. Probably, the inexistent adsorption capacity of polyurethane foam (Dorado et al. 2010) could favor the instability observed in comparison with poplar wood. To avoid further damage of R2B performance, experiment E was only performed in reactor R2A. Later on, R2B recovered its nitrification capacity in <10 days.
Regarding EM abatement, no negative effect was observed by feeding S2−. It is interesting to notice that, during experiment D, R2B achieved S–SO 2−4 concentrations of 1.1 g S L−1 without any effect on EM RE, while the same S–SO 2−4 concentrations reached in experiment A significantly affected the EM RE in R2B. Based on biomass growth estimations, a 7.5 % increase in autotrophic biomass was estimated during the S2− experiments, which supports the idea that such an SOB increase during S2− experiments could have helped in overcoming the inhibition caused by the same S–SO 2−4 concentrations without affecting the EM RE. An analogous behavior was observed in R2A during experiment E.
Overall, further investigation is warranted since several hypotheses can be proposed in order to explain the different effects of S2− in the performance of both reactors.
3.2.2 N Species Crossed Effects
Three series of experiments were carried out with NO −3 , NO −2 , and NH +4 . The REs of all three pollutants and nitritation and nitratation percentages based on concentrations encountered before and at the end of each step under pseudo-steady-state conditions are summarized in Table 4.
N–NO −3 Experiments
N–NO −3 concentrations as high as 2.4 g N L−1 were measured in both reactors. Nitrification and H2S RE remained unaffected (Table 4). EM RE in R2A remained unaffected, while in R2B in experiment G, it suffered a decrease from 79 to 59 %. It is worth noticing that the SO 2−4 concentration decreased around 23 % in both reactors during experiments F and G (Table 1) with no S2− accumulation, which suggests that the presence of NO −3 led to an incomplete S2− biodegradation to elemental sulfur. As it has been already discussed herein, experiments confirmed that EM-degrading strains were the most sensitive population to changes taking place in the reactors and that the reactor packed with polyurethane foam was more sensitive than that packed with poplar wood chips.
N–NO −2 Experiments
No negative effect on R2A was noticed neither on nitrification nor on sulfide oxidation (Table 4). However, EM RE in R2A was reduced from 94 to 66 % during experiment K when N–NO −2 concentrations up to 4 mg N L−1 were found. EM RE in R2B was more severely affected than in R2A, decreasing from values of 78 to 48 % in experiment J (with N–NO −2 concentrations of 1.4 mg N L−1) to 23 % in experiment K (with N–NO −2 concentrations of 3.6 mg N L−1). In both cases, a slight N–NH +4 accumulation in experiment K (Table 1) was discarded to affect the EM RE according to the results of the N–NH +4 steps L and M in both reactors (see the next section).
During experiment K, average N–FA concentration values of 1.6 mg N–FA L−1 were measured in R2B (with maximum FA concentrations up to 13 mg N–FA L−1), while N–FNA concentration was always below 0.008 mg N L−1. Considering inhibition threshold values of 5.8 mg N–FA L−1 for AOB, 0.16 mg N–FNA L−1 for AOB, and 0.02 mg N–FNA L−1 for NOB reported by Jubany et al. (2008), the nitritation process was probably affected by FA accumulation.
Finally, sulfide oxidation was slightly affected in R2B, which resulted in an RE decrease from 96 to 92 %. White deposits on the surface of both packing materials were observed, which probably indicated the existence of elemental sulfur. This correlates well with the SO 2−4 production decrease in both reactors (Table 1). So, besides a possible FA accumulation, sulfur oxidation RE decay could contribute to the nitrification rate decrease since it has been suggested that incomplete H2S biodegradation products cause a negative effect on nitrification processes (Jiang et al. 2009a).
N–NH +4 experiments
Only the nitritation process was negatively affected by increasing the makeup water NH +4 concentration in two steps of 100 and 300 mg N–NH +4 L−1. Overall, N–NH +4 concentrations supplied during experiments L and M would be equivalent to 135 and 203 ppmv of NH3, respectively (9.0 and 13.5 g N–NH3 m−3 h−1, respectively), if fed in the gas phase, while the inlet concentration H2S in the gas phase was kept constant at 96 ppmv (15.1 g S–H2S m−3 h−1). Chung et al. (2001) observed a reduction of H2S RE from 96 to 90 % when H2S and NH3 were mixed in a ratio of 1:2, suggesting that the high NH3 concentration (120 ppmv corresponding to 3.5 g N–NH3 m−3 h−1) inhibited H2S metabolism of Pseudomonas putida. Jones et al. (2004), using an organic packing material (wood chips and compost), observed a decrease in the RE of H2S from 90 to 85 % when loads of H2S and NH3 of 11.9 g S–H2S m−3 h−1 and 4.2 g N–NH3 m−3 h−1, respectively, were simultaneously supplied. According to these studies, the present work has shown a good capacity to avoid toxicity of high loadings of H2S and NH3 with the coexistence of a problematic VOC such as EM. Nevertheless, Jiang et al. (2009c) observed that H2S RE was not influenced significantly when the inlet NH3 concentration was increased up to 200 ppmv (56 g N–NH3 m−3 h−1), even though not 100 % of the NH3 elimination was biological.
As aforementioned, nitrification rates in both reactors were affected. During experiment L in R2B, where inlet concentration values in the gas phase would correspond to 135 ppmv (9.0 g N–NH3 m−3 h−1), average N–FA concentration values of 1.5 mg N–FA L−1 were measured (with several maximum FA concentrations of up to 12.9 mg N–FA L−1). R2B presented again a higher sensitivity and nitritation was reduced from 95 to 78 %, even though NH3 RE remained constant at 100 %. During experiment M, an average N–FA concentration of 2.7 mg N–FA L−1 was measured in R2A (with maximum FA concentrations of up to 23.1 mg N–FA L−1). According to Jubany et al. (2008), it is likely that nitritation was somehow inhibited by FA accumulation in both reactors. R2B showed again a higher sensitivity than R2A, probably related mainly to a lower biomass density. Galera et al. (2008), treating a mixture of NH3, H2S, and toluene at loads of 20.3 g N–NH3 m−3 h−1, 22.2 g S–H2S m−3 h−1, and 53.2 g toluene m−3 h−1, observed an NH3 RE decrease, attributed as well to the high NH3 inlet load itself. Kim et al. (2007) observed inhibition by FA at the same inlet NH3 loading rate as in this study, decreasing its RE from 100 to 60 %. In the present study, both reactors were able to oxidize all the NH3 to nitrate treating NH3 loads of 6.2 g N–NH3 m−3 h−1.
4 Conclusions
The results presented herein demonstrate that proper pH control dramatically improves the startup and operation of biotrickling filters for odor treatment. A shorter, more stable startup was found under pH-controlled conditions even under significantly higher pollutant loads. A study on the impact of the presence of NH +4 , NO −2 , NO −3 , SO 2−4 , and S2− on NH3, H2S, and EM abatement helped in elucidating the crossed effects in these bioreactors. Also, the biotrickling filter packed with poplar wood showed a lower sensitivity than the one packed with polyurethane foam, probably due to a larger adsorption capacity and higher microbial density. Overall, the biotrickling filter packed with poplar wood only suffered a clear impact on the nitritation percentage and the EM RE by the presence of ammonium and nitrite, respectively. Oppositely, in the biotrickling filter packed with polyurethane foam, sulfate, nitrate, and nitrite significantly affected the EM RE. Sulfide, nitrite, and ammonium affected the nitritation process, while nitrite was the sole compound that affected the H2S RE. Further research is warranted in order to clarify the interactions between these pollutants and the active microbial populations present in the bioreactors.
References
An, T., Wan, S., Li, G., Sun, L., & Guo, B. (2010). Comparison of the removal of ethanethiol in twin-biotrickling filters inoculated with strain RG-1 and B350 mixed microorganisms. Journal of Hazardous Materials, 183, 372–380.
Anthonisen, A. C., Loehr, R. C., Prakasan, T. B. S., & Srinath, E. G. (1976). Inhibition of nitrification by ammonia and nitrous acid. J. Water Pollut. Control Fed., 48, 835–852.
Baeza, J. A., Gabriel, D., & Lafuente, J. (1999). An expert supervisory system for a pilot WWTP. Environ. Modell. Softw., 14, 383–390.
Chung, Y. C., Huang, C., & Tseng, C. P. (2001). Biological elimination of H2S and NH3 from waste gases by biofilter packed with immobilized heterotrophic bacteria. Chemosphere, 43, 1043–1050.
Devinny, J. S., Deshusses, M. A., & Webster, T. (1999). Biofiltration for air pollution control. Boca Raton: CRC/Lewis.
Dorado, A. D., Lafuente, J., Gabriel, D., & Gamisans, X. (2010). A comparative study based on physical characteristics of suitable packing materials in biofiltration. Environmental Technology, 31, 193–204.
Esøy, A., Ødegaard, H., & Bentzen, G. (1998). The effect of sulphide and organic matter on the nitrification activity in a biofilm process. Water Science and Technology, 37, 115–122.
Gabriel, D., & Deshusses, M. A. (2003). Retrofitting existing chemical scrubbers to biotrickling filters for H2S emission control. Proc. Natl. Acad. Sci. USA, 100, 6308–6312.
Galera, M. M., Cho, E., Tuuguu, E., Park, S. J., Lee, C., & Chung, W. J. (2008). Effects of pollutant concentration ratio on the simultaneous removal of NH3, H2S and toluene gases using rock wool-compost biofilter. Journal of Hazardous Materials, 152, 624–631.
Hassan, A. A., & Sorial, G. A. (2011). Treatment of dynamic mixture of hexane and benzene vapors in a trickle bed air biofilter integrated with cyclic adsorption/desorption beds. Chemosphere, 82, 521–528.
Hernández, J., Prado, Ó. J., Almarcha, M., Lafuente, J., & Gabriel, D. (2010). Development and application of a hybrid inert/organic packing material for the biofiltration of composting off-gases mimics. Journal of Hazardous Materials, 178, 665–672.
Ho, K. L., Chung, Y. C., Lin, Y. H., & Tseng, C. P. (2008). Microbial populations analysis and field application of biofilter for the removal of volatile-sulfur compounds from swine wastewater treatment system. Journal of Hazardous Materials, 152, 580–588.
Hort, C., Gracy, S., Platel, V., & Moynault, L. (2009). Evaluation of sewage sludge and yard waste compost as a biofilter media for the removal of ammonia and volatile organic sulfur compounds (VOSCs). Chemical Engineering Journal, 152, 44–53.
Jiang, X., Luo, Y., Yan, R., & Tay, J. H. (2009a). Impact of substrates acclimation strategy on simultaneous biodegradation of hydrogen sulfide and ammonia. Bioresource Technol., 100, 5707–5713.
Jiang, X., Yan, R., & Tay, J. H. (2009b). Transient-state biodegradation behavior of a horizontal biotrickling filter in co-treating gaseous H2S and NH3. Applied Microbiology and Biotechnology, 81, 969–975.
Jiang, X., Yan, R., & Tay, J. H. (2009c). Simultaneous autotrophic biodegradation of H2S and NH3 in a biotrickling filter. Chemosphere, 75, 1350–1355.
Jones, K. D., Martinez, A., Maroo, K., Deshpande, S., & Boswell, J. (2004). Kinetic evaluation of H2S and NH3 biofiltration for two media used for wastewater lift station emissions. J. Air Waste Manage., 54, 24–35.
Jubany, I., Carrera, J., Lafuente, J., & Baeza, J. A. (2008). Start-up of a nitrification system with automatic control to treat highly concentrated ammonium wastewater: experimental results and modelling. Chemical Engineering Journal, 144, 407–419.
Juhler, S., Revsbech, N. P., Schramm, A., Herrmann, M., Ottosen, L. D. M., & Nielsen, L. P. (2009). Distribution and rate of microbial processes in an ammonia-loaded air filter biofilm. Applied and Environmental Microbiology, 75, 3705–3713.
Kennes, C., & Thalasso, F. (1998). Waste gas biotreatment technology. J. Chem. Technol. Biot., 72, 303–319.
Kim, J. H., Rene, E. R., & Park, H. S. (2007). Performance of an immobilized cell biofilter for ammonia removal from contaminated air stream. Chemosphere, 68, 274–280.
Lafita, C., Penya-roja, J.-M., Sempere, F., Waalkens, A., Gabaldón, C. (2011). Two case studies of hydrogen sulfide and odor removal in municipal wastewater treatment plants. In: Proceedings of the 4th International Conference on Biotechniques for Air Pollution Control, A Coruña, pp. 467–474.
Lee, E. Y., Cho, K. S., Han, H. D., & Ryu, H. W. (2002). Hydrogen sulfide effects on ammonia removal by a biofilter seeded with earthworm casts. Journal of Environmental Quality, 31, 1782–1788.
Lomans, B. P., van der Drift, C., Pol, A., & Op den Camp, H. J. M. (2002). Microbial cycling of volatile organic sulfur compounds. Cellular and Molecular Life Sciences, 59, 575–588.
Malhautier, L., Gracian, C., Roux, J. C., Fanlo, J. L., & Le Cloirec, P. (2003). Biological treatment process of air loaded with an ammonia and hydrogen sulfide mixture. Chemosphere, 50, 145–153.
Montebello, A., Baeza, M., Lafuente, J., & Gabriel, D. (2010). Monitoring and performance of a desulphurizing biotrickling filter with an integrated continuous gas/liquid flow analyser. Chemical Engineering Journal, 165, 500–507.
Paca, J., Klapkova, E., Halecky, M., Jones, K., & Soccol, C. R. (2007). Performance evaluation of a biotrickling filter degrading mixtures of hydrophobic and hydrophilic compounds. Clean Technol. Envir., 9, 69–74.
Pfenning, N., Widdel, F., & Trüper, H. G. (1981). In M. P. Starr, H. Stolp, H. G. Trüper, A. Balows, & H. G. Schlegel (Eds.), The prokaryotes (pp. 926–940). New York: Springer.
Prado, Ó. J., Veiga, M. C., & Kennes, C. (2005). Treatment of gas-phase methanol in conventional biofilters packed with lava rock. Water Research, 39, 2385–2393.
Prado, Ó. J., Gabriel, D., & Lafuente, J. (2009). Economical assessment of the design, construction and operation of open-bed biofilters for waste gas treatment. Journal of Environmental Management, 90, 2515–2523.
Ramírez, M., Gómez, J. M., Aroca, G., & Cantero, D. (2009). Removal of hydrogen sulfide by immobilized Thiobacillus thioparus in a biotrickling filter packed with polyurethane foam. Bioresource Technol., 100, 4989–4995.
Rittman, B. E., & McCarty, P. L. (2003). Environmental biotechnology: principles and applications. In B. E. Rittman & P. L. McCarty (Eds.), Stoichiometry and bacterial energetics (pp. 287–314). New York: McGraw-Hill.
Sakuma, T., Jinsiriwanit, S., Hattori, T., & Deshusses, M. A. (2008). Removal of ammonia from contaminated air in biotrickling filter-denitrifying bioreactor combination system. Water Research, 42, 4507–4513.
Sempere, F., Martinez-Soria, V., Penya-roja, J.-M., Izquierdo, M., Palau, J., & Gabaldón, C. (2010). Comparison between laboratory and pilot biotrickling filtration of air emissions from painting and wood finishing. J. Chem. Technol. Biot., 85, 364–370.
Sercu, B., Van Langenhove, H., Nuñez, D., Aroca, G., & Verstraete, W. (2005). Operational and microbiological aspects of a bioaugmented two-stage biotrickling filter removing hydrogen sulfide and dimethyl sulfide. Biotechnology and Bioengineering, 90, 259–269.
Wan, S., Li, G., An, T., Guo, B., Sun, L., Zu, L., & Ren, A. (2010). Biodegradation of ethanethiol in aqueous medium by a new Lysinibacillus sphaericus strain RG-1 isolated from activated sludge. Biodegradation, 21, 1057–1066.
Webster, T. S., Devinny, J. S., Torres, E. M., & Basrai, S. S. (1997). Microbial ecosystems in compost and granular activated carbon biofilters. Biotechnology and Bioengineering, 53, 296–303.
Acknowledgments
The Spanish government (MEC) provided financial support through the project CICYT CTM2009-14338-C03-01. This study was partially funded by the company Ros Roca S.A. (Barcelona, Spain). The Department of Chemical Engineering at UAB (Universitat Autònoma de Barcelona) is a unit of Biochemical Engineering of the Xarxa de Referència en Biotecnologia de Catalunya (XRB), Generalitat de Catalunya.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hernández, J., Lafuente, J., Prado, Ó.J. et al. Simultaneous Removal of H2S, NH3, and Ethyl Mercaptan in Biotrickling Filters Packed with Poplar Wood and Polyurethane Foam: Impact of pH During Startup and Crossed Effects Evaluation. Water Air Soil Pollut 223, 3485–3497 (2012). https://doi.org/10.1007/s11270-012-1126-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-012-1126-4