Abstract
Polyhydroxyalkanoates (PHAs), a promising family of bio-based polymers, are considered to be alternatives to traditional petroleum-based plastics. Copolymers like poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (P(HB-co-HHx)) have been shown to exhibit favorable physical and mechanical properties, due to decreased crystallinity resulting from the presence of medium-chain-length 3-hydroxyhexanoate (3HHx) monomers. In this study, we produced P(HB-co-HHx) using engineered Ralstonia eutropha strains containing deletions of the acetoacetyl-CoA reductase (phaB) genes and replacing the native PHA synthase with phaC2 from Rhodococcus aetherivorans I24 and by using butyrate, a short-chain organic acid, as the carbon source. Although the wild-type R. eutropha did not produce P(HB-co-HHx) when grown on mixed acids or on butyrate as the sole carbon source, we are able to produce polymer containing up to 40 wt% 3HHx monomer with the aforementioned engineered R. eutropha strains using various concentrations of just butyrate as the sole carbon source. This is the first report for the production of P(HB-co-HHx) copolymer in R. eutropha using butyrate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The polyhydroxyalkanoate (PHA) family of bio-based, biodegradable polymers is a promising next-generation product that can potentially substitute for petroleum-based plastics and is synthesized by many living microorganisms as part of their natural metabolism (Madkour et al. 2013; Steinbuchel and Fuchtenbusch 1998). PHA has many advantages, such as biodegradability and biocompatibility, compared to petroleum-based plastics while exhibiting thermal and mechanical properties similar to existing plastics (Sudesh et al. 2000). As a result, PHA can be used as a raw material to manufacture many different plastic products including biomedical devices, drink bottles, food packaging, etc. (Poirier et al. 1995). Many companies, such as Imperial Chemical Industries, Dupont, ADM, Biomer, DSM, Jiangsu TianAn, Meridian, Metabolix, PHB Industrial, and Tianjin Green Bio-Science, have established pilot or industrial-scale processes to produce PHAs, expecting to achieve global-scale commercialization in the near future (Chen 2009; Verlinden et al. 2007).
There are two major pathways by which organisms typically make specific PHA monomers. The first starts from acetyl-CoA, adding a carbon chain (C2 or C3) to the backbone and reducing it, thus resulting in a C4 (3-hydroxybutyryl-CoA) or a C5 (3-hydroxyvaleryl-CoA) precursor for polymerization. These are both short-chain-length (scl) precursors. PHA produced in this manner is synthesized by the enzymes PhaA or BktB (β-ketothiolases), PhaB (acetoacetyl-CoA reductase), and PhaC (PHA synthase) from various organisms, such as Vibrio, Caulobacter, Ralstonia, and engineered Escherichia (Fuchtenbusch et al. 2000; Lee et al. 1994). The second precursor generation pathway starts from fatty acid β-oxidation or fatty acid biosynthesis intermediates and results in PHA precursors, considered medium-chain-length (mcl), converted from enoyl-CoA or 3-hydroxyacyl-ACP, respectively. These mcl PHA precursors produced using the second supply pathway are synthesized by PhaJ ((R)-specific enoyl coenzyme-A hydratase) or PhaG (3-hydroxyacyl-ACP:CoA transferase), and PhaC (PHA synthase) through fatty acid metabolism in organisms like Pseudomonas (Dellomonaco et al. 2011; Fiedler et al. 2002; Lu et al. 2003; Park et al. 2012; Rehm et al. 1998). In most cases, polymers containing C4–C5 monomers, such as 3-hydroxybutyrate (3HB) and 3-hydroxyvalerate (3HV), are made by the first pathway using various sugars, propionate, or valerate as carbon sources. Polymers containing monomers greater than C6 are usually made by the second pathway (Aldor and Keasling 2003). As a result, production of C6 units typically requires hexanoate, octanoate, or longer-chain-length fatty acids (Bhubalan et al. 2008; Chen et al. 2001; Doi et al. 1995). When short-chain precursors or substrates such as acetate, propionate, and butyrate were used for PHA production in Ralstonia eutropha H16, it resulted in the biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (P(HB-co-HV)), and butyrate (C4) was not used in R. eutropha H16 for the production of poly(3-hydroxybutryrate-co-3-hydroxyhexanoate) (P(HB-co-HHx)) (Yang et al. 2010).
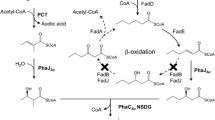
Among various PHAs, (P(HB-co-HHx)) is a promising candidate polymer for use in the biomedical field because of its superior biocompatibility, biodegradability, and mechanical properties (Misra et al. 2006; Williams et al. 1999; Wu et al. 2009). As a result, many studies have focused on exploiting microbial fatty acid degradation pathways in order to produce P(HB-co-HHx) (Budde et al. 2011a, b; Khanna and Srivastava 2005). Indeed, previous reports have shown P(HB-co-HHx), containing greater than 20 % content of 3-hydroxyhexanoate (3HHx) monomer, can be produced using plant oils as the sole carbon source (Budde et al. 2011b; Kahara et al. 2004; Riedel et al. 2012). Although its biosynthetic pathway from butyrate through butyryl-CoA, 3-ketohexanoyl-CoA, and 3-hydroxyhexanoyl CoA to PHA can be predicted in butyrate metabolism (Fig. 1), there is no report on anabolism of 3HHx-CoA and production of P(HB-co-HHx) using butyrate in R. eutropha.
Putative scheme of butyrate utilization for PHB production in R. eutropha H16. The pathway involving PhaA (or BktB) and PhaB is regarded as the main pathway for the biosynthesis of PHB homopolymer (bold arrows) (Dellomonaco et al. 2011)
In this study, we demonstrate that engineered R. eutropha can produce P(HB-co-HHx) from the volatile organic acid, butyrate, which is prepared as the end product of anaerobic fermentation of organic materials by obligate anaerobic bacteria, such as Clostridium and Fusobacterium (Lehmann et al. 2012; Potrykus et al. 2007). We had previously demonstrated that R. eutropha prefers butyrate to other volatile acids, such as acetate and propionate (Yang et al. 2010). This was made possible by a combination of a marked decrease in the major precursor supply pathway from acetoacetyl-CoA to 3-hydroxybutyryl-CoA by acetoacetyl-CoA reductase (PhaB) along with the expression of phaC2, a heterologous, broad-specificity PHA synthase gene originating from Rhodococcus aetherivorans I24 (Budde et al. 2010).
Materials and methods
Bacterial strains and media
Bacterial strains and plasmids used in this study are listed in Table 1. R. eutropha H16 (Wilde 1962) and its derivatives were precultured in 3 mL of tryptic soy broth (TSB) supplemented with 10 μg/mL gentamicin for 24 h at 30 °C. The cells were harvested and washed twice with sterilized water and then used to inoculate 5 mL of polyhydroxybutyrate (PHB) minimal media. The PHB minimal medium was prepared as previously described (York et al. 2003). Cell growth was monitored by measuring optical density at 600 nm (OD600), starting from an initial value of 0.05. For cell culture media containing high phosphate buffer (10×), we used 67 mM of Na2HPO4, 62.5 mM of NaH2PO4, 26 mM of K2SO4, 10 mM of NaOH with 0.3 % (w/v) of organic acids and 0.01 % (w/v) of NH4Cl. Cells used for growth experiments were grown initially in 5 mL PHB minimal media in a test tube with different concentrations of butyrate as the main carbon source for 48 h by a roller drum or shaker. For 50-mL volume experiments with high phosphate media containing 0.1–0.5 % butyrate (Yang et al. 2010), 1 mL of cells from an overnight culture grown in TSB medium, supplemented with 10 μg/mL gentamicin, was used to inoculate 50 mL of high-phosphate minimal media containing 0.1–0.5 % butyrate in 250-mL flasks and then grown for 48 h at 30 °C. The cells were harvested, washed twice with cold water, and lyophilized with a FreeZone 4.5 (Labconco Corp., MO, USA) for 48 h.
Analytical methods
PHA quantity and composition were determined by gas chromatography using a slight modification of a method described previously (Braunegg et al. 1978). Approximately 10 mg of freeze-dried cells from each experiment was weighed and placed in Teflon-stoppered glass vials, and methanolysis of PHA samples was performed as described previously (Yang et al. 2010). These samples were then injected into a gas chromatograph (Young-lin, Seoul, South Korea) equipped with a fused silica capillary column (Agilent HP-FFAP, 30 m × 0.32 mm, i.d. 0.25 μm film) with hydrogen as the carrier gas. A 1-μL portion of the organic phase was injected using an autosampler. The inlet was maintained at 250 °C. The oven was held at 80 °C for 5 min, heated to 220 °C at 20 °C/min, and then held at 220 °C for 5 min. Peak detection was performed by a flame ionization detector, which was maintained at 300 °C. Butyrate and other organic compounds present in the growth media were monitored by high-performance liquid chromatography (HPLC, Young-lin, Seoul, South Korea) isocratically using an Aminex HPX-87H column (Bio-Rad, CA, USA) at 60 °C with a diode array detector at a wavelength of 210 nm using 0.008 N sulfuric acid as the mobile phase with a flow rate of 0.6 mL/min, as described (York et al. 2003).
Mechanical properties
To obtain P(HB-co-HHx) film, Re2133/pCB81 cells grown in a 3-L volume of high-phosphate minimal media liquid cultures were collected by centrifugation (5,000 × g for 6 min) and washed twice with 100 % ethanol and distilled water, respectively. After lyophilization overnight, intracellular PHA polymers were extracted from dried cells using methyl isobutyl ketone (MIBK), at a cell dry weight (CDW)/MIBK ratio of 1 g/66 mL, at 60 °C for 4 h. After cooling to room temperature, the PHA was filtered through a Whatman no. 1 paper filter to remove any residual cell debris; then, the polymer dissolved in MIBK was precipitated with 10-fold volume of ice-cold ethanol. The polymer solution was then spread on the glass as a thin film, and the solvent was evaporated at room temperature for 3 h. Prepared samples were cut into dumbbell shapes with a width of 7 mm and a thickness of approximately 158 μm. Prior to mechanical property studies, the samples were maintained at the room temperature for 1 week to allow stable crystallization. Tensile mechanical properties of the polymer were studied using ASTM D638 at room temperature at a speed of 10 mm/min.
Results
Examination of R. eutropha strains producing 3HV and 3HHx from volatile organic acids
Initially, we examined growth and PHA production of different engineered R. eutropha strains using an organic acid mixture, similar to that obtained from treated palm oil mill effluent (POME), containing a ratio of acetate, propionate, and butyrate (Yang et al. 2010). In most cases, P(HB-co-HV) was produced, because propionate is a precursor of the 3HV monomer in P(HB-co-HV), although the percentage of 3HV in the resulting copolymers was different, depending on the strains used (Table 2). The 3HHx monomer was not detected in PHA produced from most strains grown with mixed organic acids as the main carbon source. Although butyrate has been regarded as a potential precursor of 3HHx monomer (Park et al. 2012), no production of P(HB-co-HHx) from butyrate was observed. This is an interesting observation, especially considering that Re2001, containing the PHA synthase gene from R. aetherivorans strain I24 (phaC2 Re ) (Budde et al. 2010), demonstrated the ability to polymerize 3-hydroxyhexanoyl-CoA precursors, resulting in the biosynthesis of P(HB-co-HHx) when cells were grown on palm oil as the sole carbon source (Budde et al. 2011b). R. eutropha strain Re2001 was expected to produce P(HB-co-HHx) or poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) terpolymer (P(HB-co-HV-co-HHx)) when grown in the presence of butyrate. However, no production of P(HB-co-HHx) or P(HB-co-HV-co-HHx) was detected using Re2001 cells when grown on mixed acids (Table 2).
We discovered that strains with deletions of the acetoacetyl-CoA reductase genes phaB1, phaB2, and phaB3, and also containing the phaC2 Re gene, did produce P(HB-co-HV-co-HHx) terpolymer. As shown in Table 2, both strain Re2135, containing phaC2 Re but with gene deletions in phaB1, phaB2, and phaB3, as well as strain Re2133/pCB81, containing phaJ from Pseudomonas and phaC2 Re but also containing deletions of phaB1, phaB2, and phaB3 genes, produced P(HB-co-HV-co-HHx). Strain Re2115, without phaB1, phaB2, and phaB3, could produce P(HB-co-HV), but notably not P(HB-co-HV-co-HHx), suggesting that phaC2 Re is important for incorporation of 3HHx monomer. These data suggest that the deletion of acetoacetyl-CoA reductase genes is important for de novo biosynthesis of 3HHx monomer from butyrate for incorporation into PHA by broad-substrate specificity PHA synthases like PhaC2 Re .
3HHx production is dependent on butyrate concentration
To confirm the production of P(HB-co-HHx) from butyrate and to compare the effect on P(HB-co-HHx) production using different amounts of butyrate in culture media, measurements of growth, PHA content, and 3HHx content of PHA in strains H16 and Re2133/pCB81 were taken in cultures with different concentrations of butyrate as the sole carbon source. Compared with wild-type H16, the strain Re2133/pCB81 showed less robust growth (Fig. 2a) and produced a lower amount of PHA (69.3 wt%, Fig. 2b). However, Re2133/pCB81 produced P(HB-co-HHx) copolymer containing a surprising 40 wt% of 3HHx, whereas H16 produced only PHB homopolymer, containing no 3HHx monomer (Fig. 2c). Use of butyrate in the PHA production media, in the absence of other volatile acids like acetate and propionate, resulted in the biosynthesis of P(HB-co-HHx) by Re2133/pCB81 and exhibited a higher 3HHx content, between 20 and 40 wt%, depending on culture time and volume. Deletion of acetoacetyl-CoA reductases and replacement of the native PHA synthase with a broad-specificity synthase resulted in lower amounts of final biomass and PHA content. However, these genetic modifications clearly established the incorporation of 3HHx, resulting in P(HB-co-HHx) copolymer production when butyrate was the sole carbon source present in the media.
Production of P(HB-co-HHx) over time from butyrate as the main carbon source
Production of P(HB-co-HHx) was monitored at different time points using 0.3 % (w/v) butyrate as the sole carbon source in 250-mL flasks. When 0.01 % (w/v) of NH4Cl was used, PHA production was detected at 24 h, with R. eutropha H16 accumulating up to 80 wt% PHA and Re2133/pCB81 accumulating 20 wt% PHA (Fig. 3a, b). When the cells were grown for 48 h, PHA content reached up to 90 % with H16 and 60 % with Re2133/pCB81. Strain H16 produced only PHB, whereas Re2133/pCB81 synthesized P(HB-co-HHx) containing about 20 wt% of 3HHx (Fig. 3c). The pattern of butyrate consumption showed slight differences between the two strains, although total butyrate consumption was observed to be similar (Fig. 3d).
H16 and Re2133/pCB81 both produced fumarate and an unidentified organic acid (retention time is 25 min) during cultivation, but only Re2133/pCB81 produced glycoxylic acid and acetate by-products, as monitored by HPLC (Fig. S1). When PHA production on fructose was compared with that on butyrate, Re2133/pCB81 could only produce PHB with fructose as the main carbon source (data not shown). This suggests that fatty acid metabolism is not involved in facilitating PHA production using fructose as the main carbon source, since P(HB-co-HHx) is not synthesized. Also, butyrate appears to be used as a direct precursor to produce P(HB-co-HHx) through an as-yet unidentified ketothiolase, an acetyl-CoA C acetyltransferase enzyme, or a reductase, as well as the heterologously expressed PHA synthase.
Mechanical properties of P(HB-co-HHx) produced from butyrate
When purified P(HB-co-HHx) polymer, containing 42 wt% 3HHx (see Fig. 2), was examined for its mechanical properties, the Young’s modulus, tensile strength, and elongation to break were measured to be 0.2 GPa, 72 MPa, and 690 %, respectively (Fig. S2). Considering that the Young’s modulus, tensile strength, and elongation to break low-density polyethylene are 0.2 GPa, 10 MPa, and 620 %, respectively, the mechanical properties of the P(HB-co-HHx) obtained here show similar results to polyethylene, except for higher tensile strength, as an elastomer (Brigham et al. 2011; Doi et al. 1995).
Discussion
P(HB-co-HHx) has superior physical and mechanical properties, such as lower melting point, enhanced flexibility, and better impact strength than PHB homopolymer, due to the presence of the longer-chain-length 3HHx monomer fraction (Feng et al. 2002; Qiu et al. 2005). P(HB-co-HHx) is mainly produced from related carbon sources such as fatty acids or plant oils in wild-type Aeromonas, Pseudomonas, as well as engineered Ralstonia, Escherichia, and others (Chen et al. 2001; Doi et al. 1995; Lee et al. 2000; Park et al. 2001; Qiu et al. 2005). However, long-chain fatty acids or plant oils, when used as carbon sources during fermentation, can cause some undesirable problems, such as severe foaming in the fermentation process and challenge in downstream processing of the removal of residual fatty acids (Qiu et al. 2005; Riedel et al. 2013). In addition, plant oils are more expensive in comparison to waste organic acids and are often considered controversial due to the conflicting production of biochemicals versus food, similar to the issue of corn being used for biofuel versus food (Flammini 2008). Therefore, using unrelated carbon sources such as glucose, fructose, and gluconate for P(HB-co-HHx) synthesis will be helpful to overcome those problems (Agnew et al. 2012; Wang et al. 2012). However, to achieve them, many pathways in wild-type strains must be modified to synthesize P(HB-co-HHx) from unrelated carbon sources and the resulting 3HHx incorporation is very low (Fukui et al. 2002; Qiu et al. 2005). Interestingly, although butyrate would be the most straightforward carbon substrate for P(HB-co-HHx) production based on the reaction of PHA biosynthetic enzymes such as PhaA and PhaB (Fig. 1), there is no prior report of the production of P(HB-co-HHx) from butyrate in R. eutropha. In neither R. eutropha H16 nor Re2001 containing phaC2 Re could P(HB-co-HHx) be produced (Fig. 1). However, when butyrate is fed to cultures of strains Re2135 and Re2133/pCB81, neither of which contains functional phaB1, phaB2, nor phaB3 genes, P(HB-co-HHx) copolymer was produced, likely as a result of a decrease in 3HB monomer production (Fig. 4). Considering butyrate is easily prepared as the end product of anaerobic fermentation of organic materials by obligate anaerobic bacteria such as Clostridium and Fusobacterium (Lehmann et al. 2012; Potrykus et al. 2007) and that R. eutropha also prefers butyrate to other volatile acids such as acetate and propionate (Yang et al. 2010), P(HB-co-HHx) production from butyrate alone could potentially be more straightforward and cost-effective than the use of oils or refined sugars.
Potential scheme of butyrate utilization for P(HB-co-HHx) production in engineered R. eutropha strains, such as Re2135 and Re2133/pCB81. The production of 3-hydroxyhexanoyl-CoA is regarded as a major pathway, along with the production of 3-hydroxybutyryl-CoA, without functional PhaB enzymes, in order to make P(HB-co-HHx) copolymer (bold arrows)
Although the exact identity of the gene encoding the enzyme, the 3-hydroxybutyryl-CoA dehydrogenase in R. eutropha (Dekishima et al. 2011), that utilizes 2-hexenoyl-CoA or 3-ketohexanoyl-CoA as a substrate to make 3-hydroxyhexanoyl-CoA in the absence of PhaB1, PhaB2, and PhaB3 has not yet been revealed, we have discovered that engineered R. eutropha strains, such as Re2135 and Re2133/pCB81 (Table 1), could produce P(HB-co-HHx) from butyrate. Further, we have shown that the resulting 42 wt% of the HHx monomer in the biopolymer produced on this sole carbon source confers highly favorable mechanical properties to the resulting PHA. This copolymer was not only possible by simply substituting the native PHA synthase from R. eutropha for phaC2 Re but also by deleting the reductase genes phaB1, phaB2, and phaB3. Our results suggest that copolymer biosynthesis was possible due to a marked decrease in the major precursor supply pathway from acetoacetyl-CoA to 3-hydroxybutyl-CoA by acetoacetyl-CoA reductase (PhaB). To our knowledge, this is the first report for the production of P(HB-co-HHx) using butyrate in R. eutropha.
References
Agnew DE, Stevermer AS, Youngquist JT, Pfleger BF (2012) Engineering Escherichia coli for production of C12–C14 polyhydroxyalkanoate from glucose. Metab Eng 14(6):705–713
Aldor IS, Keasling JD (2003) Process design for microbial plastic factories: metabolic engineering of polyhydroxyalkanoates. Curr Opin Biotechnol 14(5):475–483
Bhubalan K, Lee WH, Loo CY, Yamamoto T, Tsuge T, Doi Y, Sudesh K (2008) Controlled biosynthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) from mixtures of palm kernel oil and 3HV-precursors. Polym Degrad Stab 93(1):17–23
Braunegg G, Sonnleitner B, Lafferty RM (1978) Rapid gas-chromatographic method for determination of poly-beta-hydroxybutyric acid in microbial biomass. Eur J Appl Microbiol Biotechnol 6(1):29–37
Brigham CJ, Kurosawa K, Rha C, Sinskey AJ (2011) Bacterial carbon storage to value added products. J Microbial Biochem Technol 83(5):S3–S002
Budde CF, Mahan AE, Lu J, Rha C, Sinskey AJ (2010) Roles of multiple acetoacetyl coenzyme A reductases in polyhydroxybutyrate biosynthesis in Ralstonia eutropha H16. J Bacteriol 192(20):5319–5328
Budde CF, Riedel SL, Hubner F, Risch S, Popovic MK, Rha C, Sinskey AJ (2011a) Growth and polyhydroxybutyrate production by Ralstonia eutropha in emulsified plant oil medium. Appl Microbiol Biotechnol 89(5):1611–1619
Budde CF, Riedel SL, Willis LB, Rha C, Sinskey AJ (2011b) Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from plant oil by engineered Ralstonia eutropha strains. Appl Environ Microbiol 77(9):2847–2854
Chen GQ (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38(8):2434–2446
Chen GQ, Zhang G, Park SJ, Lee SY (2001) Industrial scale production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Appl Microbiol Biotechnol 57(1–2):50–55
Dekishima Y, Lan EI, Shen CR, Cho KM, Liao JC (2011) Extending carbon chain length of 1-butanol pathway for 1-hexanol synthesis from glucose by engineered Escherichia coli. J Am Chem Soc 133(30):11399–11401
Dellomonaco C, Clomburg JM, Miller EN, Gonzalez R (2011) Engineered reversal of the beta-oxidation cycle for the synthesis of fuels and chemicals. Nature 476(7360):355–359
Doi Y, Kitamura S, Abe H (1995) Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 28(14):4822–4828
Feng L, Watanabe T, Wang Y, Kichise T, Fukuchi T, Chen GQ, Doi Y, Inoue Y (2002) Studies on comonomer compositional distribution of bacterial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)s and thermal characteristics of their factions. Biomacromolecules 3(5):1071–1077
Fiedler S, Steinbuchel A, Rehm BH (2002) The role of the fatty acid beta-oxidation multienzyme complex from Pseudomonas oleovorans in polyhydroxyalkanoate biosynthesis: molecular characterization of the fadBA operon from P. oleovorans and of the enoyl-CoA hydratase genes phaJ from P. oleovorans and Pseudomonas putida. Arch Microbiol 178(2):149–160
Flammini A. (2008) Biofuels and the underlying causes of high food prices. Food and Agriculture Organization of the United Nations (FAO). Vol. 112 p 1–23
Fuchtenbusch B, Wullbrandt D, Steinbuchel A (2000) Production of polyhydroxyalkanoic acids by Ralstonia eutropha and Pseudomonas oleovorans from an oil remaining from biotechnological rhamnose production. Appl Microbiol Biotechnol 53(2):167–172
Fukui T, Abe H, Doi Y (2002) Engineering of Ralstonia eutropha for production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from fructose and solid-state properties of the copolymer. Biomacromolecules 3(3):618–624
Kahara P, Tsuge T, Taguchi K, Doi Y (2004) High yield production of polyhydroxyalkanoates from soybean oil by Ralstonia eutropha and its recombinant strain. Polym Degrad Stab 83(1):79–86
Khanna S, Srivastava AK (2005) Recent advances in microbial polyhydroxyalkanoates. Process Biochem 40(2):607–619
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM 2nd, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166(1):175–176
Lee SY, Lee KM, Chan HN, Steinbuchel A (1994) Comparison of recombinant Escherichia coli strains for synthesis and accumulation of poly-(3-hydroxybutyric acid) and morphological changes. Biotechnol Bioeng 44(11):1337–1347
Lee SH, Oh DH, Ahn WS, Lee Y, Choi J, Lee SY (2000) Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by high-cell-density cultivation of Aeromonas hydrophila. Biotechnol Bioeng 67(2):240–244
Lehmann D, Radomski N, Lutke-Eversloh T (2012) New insights into the butyric acid metabolism of Clostridium acetobutylicum. Appl Microbiol Biotechnol 96(5):1325–1339
Lu X, Zhang J, Wu Q, Chen GQ (2003) Enhanced production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) via manipulating the fatty acid beta-oxidation pathway in E. coli. FEMS Microbiol Lett 221(1):97–101
Madkour MH, Heinrich D, Alghamdi MA, Shabbaj II, Steinbuchel A (2013) PHA recovery from biomass. Biomacromolecules 14(9):2963–2972
Misra SK, Valappil SP, Roy I, Boccaccini AR (2006) Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules 7(8):2249–2258
Park SJ, Ahn WS, Green PR, Lee SY (2001) Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by metabolically engineered Escherichia coli strains. Biomacromolecules 2(1):248–254
Park SJ, Kim TW, Kim MK, Lee SY, Lim SC (2012) Advanced bacterial polyhydroxyalkanoates: towards a versatile and sustainable platform for unnatural tailor-made polyesters. Biotechnol Adv 30(6):1196–1206
Poirier Y, Nawrath C, Somerville C (1995) Production of polyhydroxyalkanoates, a family of biodegradable plastics and elastomers, in bacteria and plants. Biotechnology (N Y) 13(2):142–150
Potrykus J, Mahaney B, White RL, Bearne SL (2007) Proteomic investigation of glucose metabolism in the butyrate-producing gut anaerobe Fusobacterium varium. Proteomics 7(11):1839–1853
Qiu YZ, Han J, Guo JJ, Chen GQ (2005) Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from gluconate and glucose by recombinant Aeromonas hydrophila and Pseudomonas putida. Biotechnol Lett 27(18):1381–1386
Rehm BH, Kruger N, Steinbuchel A (1998) A new metabolic link between fatty acid de novo synthesis and polyhydroxyalkanoic acid synthesis. The PHAG gene from Pseudomonas putida KT2440 encodes a 3-hydroxyacyl-acyl carrier protein-coenzyme a transferase. J Biol Chem 273(37):24044–24051
Riedel SL, Bader J, Brigham CJ, Budde CF, Yusof ZA, Rha C, Sinskey AJ (2012) Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by Ralstonia eutropha in high cell density palm oil fermentations. Biotechnol Bioeng 109(1):74–83
Riedel SL, Brigham CJ, Budde CF, Bader J, Rha C, Stahl U, Sinskey AJ (2013) Recovery of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from Ralstonia eutropha cultures with non-halogenated solvents. Biotechnol Bioeng 110(2):461–470
Steinbuchel A, Fuchtenbusch B (1998) Bacterial and other biological systems for polyester production. Trends Biotechnol 16(10):419–427
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25(10):1503–1555
Verlinden RA, Hill DJ, Kenward MA, Williams CD, Radecka I (2007) Bacterial synthesis of biodegradable polyhydroxyalkanoates. J Appl Microbiol 102(6):1437–1449
Wang Q, Tappel RC, Zhu C, Nomura CT (2012) Development of a new strategy for production of medium-chain-length polyhydroxyalkanoates by recombinant Escherichia coli via inexpensive non-fatty acid feedstocks. Appl Environ Microbiol 78(2):519–527
Wilde E (1962) Untersuchungen Uber Wachstum Und Speicherstoffsynthese Von Hydrogenomonas. Arch Mikrobiol 43(2):109–137
Williams SF, Martin DP, Horowitz DM, Peoples OP (1999) PHA applications: addressing the price performance issue: I. Tissue engineering. Int J Biol Macromol 25(1–3):111–121
Wu Q, Wang Y, Chen GQ (2009) Medical application of microbial biopolyesters polyhydroxyalkanoates. Artif Cells Blood Substit Biotechnol 37(1):1–12
Yang YH, Brigham CJ, Budde CF, Boccazzi P, Willis LB, Hassan MA, Yusof ZA, Rha C, Sinskey AJ (2010) Optimization of growth media components for polyhydroxyalkanoate (PHA) production from organic acids by Ralstonia eutropha. Appl Microbiol Biotechnol 87(6):2037–2045
York GM, Junker BH, Stubbe JA, Sinskey AJ (2001) Accumulation of the PhaP phasin of Ralstonia eutropha is dependent on production of polyhydroxybutyrate in cells. J Bacteriol 183(14):4217–4226
York GM, Lupberger J, Tian J, Lawrence AG, Stubbe J, Sinskey AJ (2003) Ralstonia eutropha H16 encodes two and possibly three intracellular poly[d-(−)-3-hydroxybutyrate] depolymerase genes. J Bacteriol 185(13):3788–3794
Acknowledgments
The work at Konkuk University was partially supported by the Energy Efficiency & Resources of the Korea Institute of Energy Technology Evaluation and Planning(KETEP) grant funded by the Korea Government Ministry of Trade, Industry and Energy (20133030000300). This research was also partially supported by Polar Academic Program (PAP, PD13010) from KOPRI and “Cooperative Research Program for Agriculture Science & Technology Development (Project title: Isolation and identification of rhizobacteria for indoor VOCs removal, Project No. 010205022014)” Rural Development Administration, Republic of Korea. We thank Mr. John W. Quimby for review of this manuscript prior to submission.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 156 kb)
Rights and permissions
About this article
Cite this article
Jeon, JM., Brigham, C.J., Kim, YH. et al. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (P(HB-co-HHx)) from butyrate using engineered Ralstonia eutropha . Appl Microbiol Biotechnol 98, 5461–5469 (2014). https://doi.org/10.1007/s00253-014-5617-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5617-7