Abstract
Enzymatic hydrolysis of cellulosic material is an essential step in the bioethanol production process. However, complete cellulose hydrolysis by cellulase is difficult due to the irreversible adsorption of cellulase onto cellulose. Thus, part of the cellulose remains in crystalline form after hydrolysis. In this study, after 96-h hydrolysis of Avicel crystalline cellulose, 47.1 % of the cellulase was adsorbed on the cellulose surface with 10.8 % crystalline cellulose remaining. In simultaneous saccharification and fermentation of 100 g/L Avicel with 1.0 filter paper unit/mL cellulase, a wild-type yeast strain produced 44.7 g/L ethanol after 96 h. The yield of ethanol was 79.7 % of the theoretical yield. On the other hand, a recombinant yeast strain displaying various cellulases, such as β-glucosidase, cellobiohydrolase, and endoglucanase, produced 48.9 g/L ethanol, which corresponds to 87.3 % of the theoretical yield. Higher ethanol production appears to be attributable to higher efficiency of cellulase displayed on the cell surface. These results suggest that cellulases displayed on the yeast cell surface improve hydrolysis of Avicel crystalline cellulose. Indeed, after the 96-h simultaneous saccharification and fermentation using the cellulase-displaying yeast, the amount of residual cellulose was 1.5 g/L, one quarter of the cellulose remaining using the wild-type strain, a result of the alleviation of irreversible adsorption of cellulases on the crystalline cellulose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous environmental and social benefits are anticipated from the replacement of petroleum-based transport fuels with bioethanol converted from lignocellulosic materials such as agricultural residues and industrial wastes. Establishing an economically feasible process for industrial cellulosic ethanol production requires markedly increased ethanol titers after fermentation due to the high energy demands of the subsequent ethanol distillation (Galbe and Zacchi 2007).
Enzymatic hydrolysis of biomass is an essential step in bioethanol production because sugars released by the hydrolysis are then fermented by microorganisms such as yeast. However, one of major drawbacks of the saccharification process is insufficient hydrolysis of crystalline cellulose. Cellulases such as cellobiohydrolase and endoglucanase are adsorbed onto the cellulose surface in hydrolyzing the β-glucoside bond of cellulose, but once adsorbed, the desorption of the enzyme is not easy (Otter et al. 1989; Palonen et al. 1999; Zhu et al. 2009). The binding is, at least, partly irreversible (Ma et al. 2008). It has been assumed that the extent to which a cellulase becomes denatured is correlated with its irreversible adsorption to the solid–liquid interface (Palonen et al. 1999; Zhang and Lynd 2004).
Irreversible protein adsorption on solid surfaces is reduced by increased protein stability (Karlsson et al. 2005). Immobilization of cellulases to solid supports increased protein stability at non-optimal reaction conditions including low temperature, organic solvent composition, and high and low pH (Lupoi and Smith 2011; Gole et al. 2001).
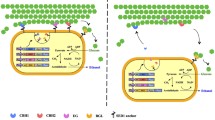
So far, recombinant yeast strains, which display cellulases on the cell surface, have produced ethanol from cellulosic materials via simultaneous cellulose hydrolysis and glucose utilization (Matano et al. 2012a; Yamada et al. 2011). Cellulases are genetically self-immobilized on the yeast cell surface so that the activities of the enzymes are retained as long as the yeast continues to grow (Ueda and Tanaka 2000). Thus, cellulases self-immobilized on the yeast cell surface were expected for alleviation of the irreversible adsorption.
In the present study, crystalline cellulose was fermented to ethanol by the combination of cellulase reagent and a cellulase-displaying yeast strain. The recombinant strain demonstrated higher ethanol production based on higher cellulose utilization compared with a wild-type strain, which would be caused by the alleviation of irreversible adsorption of the cellulase reagent on the crystalline cellulose.
Materials and methods
Yeast strains
Saccharomyces cerevisiae wild-type strain, NBRC1440, was obtained from the National Institute of Technology and Evaluation. A recombinant cellulase-displaying S. cerevisiae strain, NBRC1440/B-EC3, was created in a previous study (Matano et al. 2012a). The yeast strain was used for ethanol production from Avicel (PH-101, Fluka Chemie GmbH, Buchs, Switzerland) crystalline cellulose.
Measurement of cellulase activity
Cellulase activity was determined by the NREL procedure (Adney and Baker 1996; Ghose 1987).
Analysis of cellulase adsorption on Avicel surface
Enzymatic hydrolysis of Avicel was performed at 50 °C with a commercial cellulase, Cellic CTec2 (Novozymes Inc., Bagsvaerd, Denmark). Avicel of 50 or 100 g/L was hydrolyzed in 50 mM citric acid buffer (pH 5.0) and Cellic CTec2 in a 50 mL polypropylene tube (Corning Inc., NY). The tube was axially rotated at 35 rpm in a heat block (Thermo Block Rotator SN-06BN; Nissin, Tokyo, Japan) as described previously (Matano et al. 2012a). After 96-h hydrolysis, the hydrolysate and insoluble material were separated by centrifugation at 4,000 × g for 10 min at 4 °C. Cellulose content in the insoluble material was quantified by the method of NREL 2006 (Sluiter et al. 2008) with minor modifications, as described previously (Matano et al. 2012a). Protein concentration in the hydrolysate was analyzed by Bradford protein assay (Bradford 1976). The amount of irreversibly adsorbed protein was calculated by subtracting the amount of protein contained in the supernatant from the amount of total protein input (Gao et al. 2011; Kumar and Wyman 2009). Sugars contained in the hydrolysate were analyzed by high-performance liquid chromatography (HPLC), as described previously (Hasunuma et al. 2011).
Ethanol production
S. cerevisiae strains used for Avicel fermentation were propagated under aerobic conditions at 30 °C for 48 h in 10 g/L yeast extract, 20 g/L peptone, and 20 g/L dextrose media. The yeast cells were collected by centrifugation at 4,000 × g for 10 min at 4 °C and washed twice with distilled water. Fermentation of Avicel (50 or 100 g/L) was performed in a 50-mL polypropylene tube containing 10 g/L yeast extract, 20 g/L peptone, 50 mM citric acid buffer (pH 5.0), 100 g wet cell/L yeast cells, and Cellic CTec2 with a rotary fermentation system as described previously (Matano et al. 2012a). The fermentation tube was axially rotated at 35 rpm under a controlled temperature of 35 °C. Ethanol contained in the fermentation media was analyzed by HPLC, as described previously (Hasunuma et al. 2011). The ethanol yield was calculated as follows:
After 96-h fermentation, insoluble material remaining in the fermentation medium was collected as a pellet by centrifugation at 4,000 × g for 10 min. The residual cellulose was analyzed as described above.
Results
Cellulase adsorption on cellulose surface
The adsorption of proteins at a solid–liquid interface is a common phenomenon widely observed in various areas (Karlsson et al. 2005). Previously, the hydrolysis rate of cellulase was shown to decrease by irreversible adsorption of cellulases on cellulose (Eriksson et al. 2002; Karlsson et al. 2005; Ma et al. 2008). In Fig. 1, the amount of Cellic CTec2 adsorbed on Avicel crystalline cellulose was determined. Hydrolysis of 50 g/L Avicel was performed with 10 filter paper unit (FPU)/g cellulose Cellic CTec2 (0.5 FPU/mL) at 50 °C. The total added protein was 0.68 g/L. After 2 min of hydrolysis, the concentration of enzymes present as free proteins in the soluble fraction was 0.18 g/L. A part of Cellic CTec2 adsorbed was released from Avicel over time, reaching 0.36 g/L after 96 h. Glucose produced from Avicel reached a plateau after 72 h of hydrolysis. Glucose released by hydrolysis was 89.6 % of the cellulose, indicating that 5.7 g/L Avicel was retained as cellulose (Fig. 1a). A similar tendency was found in the 100 g/L Avicel hydrolyzed by 10 FPU/g cellulose Cellic CTec2 (1.0 FPU/mL) (Figs. 1c, d). In the presence of 50 and 100 g/L Avicel, 48.0 and 46.4 % of the total added Cellic CTec2 was adsorbed on the Avicel after 96 h, respectively.
Time course of protein (a, b) and glucose (c, d) in the supernatant of hydrolyzed solution. Initial Avicel concentrations were 50 g/L (black symbols) and 100 g/L (red symbols), respectively. Figures b and d were enlarged from figures a and c, respectively, to show time-dependent change of cellulase and glucose during 30-min saccharification. Data are expressed as the mean ± SD (error bars) of three independent experiments
Measurement of cellulose remaining after 96-h hydrolysis
In Fig. 2, the relationship between enzyme concentration and cellulose remaining after 96-h hydrolysis is plotted. For Cellic CTec2 concentrations of 0.05, 0.25, and 0.5 FPU/mL, 27.6, 12.1, and 4.2 g/L Avicel remained after the hydrolysis of 50 g/L Avicel, respectively, indicating that the residual cellulose decreased with increasing Cellic CTec2 concentration. On the other hand, more than 1.0 FPU/mL did not reduce the content of residual cellulose. Avicel was not completely hydrolyzed even if the concentration of Cellic CTec2 increased. The concentration of residual cellulose obtained from 100 g/L Avicel was higher than that from 50 g/L Avicel. According to a previous report (Xiao et al. 2004), incomplete hydrolysis of cellulose would be caused by the product inhibition of cellulase.
Simultaneous saccharification and fermentation of Avicel using the yeast strain NBRC1440
In order to consume glucose, which would inhibit cellulase activity, simultaneous saccharification and fermentation (SSF) of Avicel was performed using a suitable yeast strain. One hundred grams per liter Avicel was fermented by a laboratory S. cerevisiae strain, NBRC1440, in the presence of Cellic CTec2 at 35 °C. As shown in Fig. 3, in the presence of 0.1, 0.5, 1.0, 5.0, and 10.0 FPU/mL, 65.7, 29.3, 6.1, 6.5, and 6.6 g/L residual cellulose remained after 96-h SSF, respectively. The cellulose hydrolyzed by 1.0 FPU/mL cellulase was the same as that by 10.0 FPU/mL. Volumetric ethanol productivity was 2.0 g/L/h. The remaining cellulose decreased with the addition of yeast to the reaction. After 4 h of the SSF, glucose was not detected in the fermentation medium. These results indicate that product inhibition caused by glucose was avoided. However, some cellulose still remained after the SSF. Although SSF of 100 g/L Avicel was performed with a cellulase-secreting yeast strain, S. cerevisiae NBRC1440/ssB-ssEC3 (Matano et al. 2012b), ethanol produced and cellulose retained by NBRC1440/ssB-ssEC3 were the same with those by wild-type strain (data not shown).
Simultaneous saccharification and fermentation of Avicel using the cellulase-displaying yeast strain NBRC1440/B-EC3
Previously, display of fungal cellulases, such as Tricoderma reesei endoglucanase and cellobiohydrolase, and Aspergillus aculeatus β-glucosidase on the yeast cell surface effectively hydrolyzed hydrothermally pretreated rice straw, a lignocellulosic material (Matano et al. 2012a). In the present study, the cellulase-displaying yeast strain, NBRC1440/B-EC3, whose activity of phosphoric acid-swollen cellulose hydrolysis was 0.51 unit/g dry weight cell (Matano et al. 2012a), was used for the SSF of 100 g/L Avicel. In the presence of 0.1, 0.5, 1.0, 5.0, and 10.0 FPU/mL Cellic Ctec2, residual cellulose contents were 62.0, 26.6, 1.5, 4.8, and 7.5 g/L after 96 h, respectively (Fig. 3). Addition of 1.0 FPU/mL Cellic CTec2 enables utilization of 98.5 % of the initial cellulose. As shown in Fig. 2, increase of cellulase did not always increase cellulose hydrolysis. Meanwhile, addition of NBRC1440/B-EC3 strain improved cellulose hydrolysis, although the activity of cellulases displayed on the cell surface was lower than commercial enzymes. These results indicate that cellulases displayed on the yeast cell surface improve hydrolysis of Avicel crystalline cellulose.
Cellulase activity in the fermentation medium was determined after removal of insoluble materials by centrifugation to estimate amount of cellulase adsorbed on the cellulose as described previously (Gao et al. 2011). As shown in Table 1, the use of a cellulase-displaying yeast strain increased the activity of cellulase, which was not adsorbed on the cellulose during the SSF, compared to the wild-type yeast strain. These results suggest that adsorption of Cellic CTec2 was alleviated by cellulases present on the recombinant yeast strain.
Ethanol production from Avicel crystalline cellulose
The time course of ethanol production from Avicel is shown in Fig. 4. Volumetric ethanol productivity increased with increase in cellulase concentration. In the presence of 1.0 FPU/mL Cellic Ctec2, NBRC1440 produced 44.7 g/L ethanol from 100 g/L Avicel after 96-h fermentation. The yield of ethanol was 79.7 % of the theoretical yield. On the other hand, NBRC1440/B-EC3 produced 48.9 g/L ethanol, which corresponds to 87.3 % of the theoretical yield, in the presence of 1.0 FPU/mL cellulase. Volumetric ethanol productivity of NBRC1440B-EC3 was 2.5 g/L/h. Ethanol titer showed a positive correlation with the amount of cellulose utilized, as shown in Fig. 3. These results indicate that the cell surface display system alleviated adsorption of free cellulase onto the crystalline cellulose to improve hydrolysis of cellulose and ethanol production.
Time course of ethanol concentration in the SSF of 100 g/L Avicel by using NBRC1440 (closed symbols) and NBRC1440/B-EC3 (open symbols). The SSF was performed in the presence of 0.1 FPU/mL (black circle), 1.0 FPU/mL (red circle), and 10.0 FPU/mL (blue circle) Cellic CTec2. Data are expressed as the mean ± SD (error bars) of three independent experiments
Discussion
Improvement of cellulose hydrolysis efficiency is a significant target for realization of high titer ethanol production. However, it has been difficult to completely hydrolyze cellulose for the production of monosaccharides (Yang et al. 2006). The problem could be caused by both product inhibition of cellulase and irreversible adsorption on cellulose (Yang et al. 2010; Yang and Wyman 2006). As shown in Fig. 2, cellulose, which was not completely hydrolyzed by only Cellic Ctec2, was retained after the saccharification process. When 50 g/L Avicel was hydrolyzed with 5.0 FPU/mL Cellic CTec2, residual cellulose was 10.4 % of total input cellulose after 96 h (Fig. 2). In order to increase the ethanol yield from cellulosic biomass, enhancement of cellulose hydrolysis would be required. In the present study, in the SSF of 100 g/L Avicel, recombinant yeast strains displaying cellulases on the cell surface reduced residual cellulose to 1.5 % to produce 48.9 g/L ethanol, 87.3 % of the theoretical yield.
As shown in Fig. 1, Cellic CTec2 mixed with Avicel crystalline cellulose was immediately adsorbed on the cellulose. When 50 g/L Avicel was hydrolyzed with 0.68 g/L (0.5 FPU/mL) cellulase, the cellulase contained as free protein in the solution was 0.18 g/L after 2 min of hydrolysis (Fig. 1b). After 96-h hydrolysis, the free cellulase was 0.36 g/L, corresponding to 52.9 % of cellulase used in the reaction; 47.1 % of input cellulase was still adsorbed onto the crystalline cellulose surface. As shown in Fig. 2, Avicel was not completely hydrolyzed even when the concentration of Cellic CTec2 increased. Previously, hydrolysis of Avicel was inhibited by hydrolysis products such as cellobiose or glucose (Xiao et al. 2004). In order to avoid the product inhibition, SSF was performed with yeast S. cerevisiae. The cellulose was simultaneously hydrolyzed and fermented by wild-type yeast strains with 1.0 FPU/mL cellulase, whereas 5.6 % of cellulose still remained after the SSF (Fig. 3).
Recombinant yeast strains, which displayed three types of cellulases (cellobiohydrolase II, endoglucanase, and β-glucosidase) on the cell surface, improved ethanol production from the cellulose. In the presence of 1.0 FPU/mL Cellic CTec2, 48.9 g/L ethanol was produced by NBRC1440/B-EC3. Ethanol produced by NBRC1440/B-EC3 was 10 % higher than that by NBRC1440 (Fig. 4). After the SSF, the amount of residual cellulose was 1.5 g/L, which is one quarter of the cellulose remaining after the SSF using NBRC1440. The higher ethanol production would appear to be attributable to the highly efficient activity of cellulase displayed on the cell surface. These results suggest that cellulases displayed on the yeast cell surface improved hydrolysis of Avicel crystalline cellulose. Previously, ethanol production from rice straw hydrothermally pretreated was improved by a cellulase-displaying yeast strain (Matano et al. 2012a). The present study is the first to demonstrate that the display of cellulases on the yeast cell surface improves hydrolysis of crystalline cellulose by the alleviation of irreversible adsorption of cellulase onto the cellulose.
Previously, it was shown that reduction of the hydrolysis rate was caused by irreversible adsorption of cellulase (Desai and Converse 1997; Eriksson et al. 2002; Ma et al. 2008; Väljamäe et al. 1998). In the present study, volumetric ethanol productivity by NBRC1440/B-EC3 (2.5 g/L/h) was higher than that by NBRC1440 (2.0 g/L/h). These results also support the notion that the amount of irreversibly adsorbed cellulose is decreased by the cellulase-displaying yeast. Ma et al. (2008) reported that cellulases irreversibly adsorbed on cellulose were denatured. According to a previous report (Karlsson et al. 2005), increase in conformational stability of human carbonic anhydrase II through protein engineering reduced irreversible adsorption on gold particles.
Moreover, cellulase immobilized onto nanoparticle led to an increase in ethanol production from cellulose by the improvement of conformational stability of cellulases (Lupoi and Smith 2011). As described previously (Hasunuma and Kondo 2012; Ueda and Tanaka 2000), display of proteins on the yeast cell surface has stabilized their conformation. In the present study, amount of residual crystalline cellulose was reduced by using a cellulase-displaying yeast strain (Table 1). These results suggest that irreversible cellulase adsorption onto the crystalline cellulose would be alleviated by cellulases displayed on the yeast cell surface.
In SSF with NBRC1440/B-EC3 (Fig. 4), the highest ethanol titer was obtained in the presence of 1.0 FPU/mL cellulase. Interestingly, cellulase concentrations above 1.0 FPU/mL decreased the final ethanol titer. The amount of residual cellulose increased with increasing cellulase concentration (Figs. 3 and 4). These results suggest that excess amounts of commercial cellulase might first adsorb onto the cellulose to inhibit the activity of cellulases displayed on the recombinant yeast cell surface.
Surface-engineered yeast strains displaying cellulases have the following advantages (Hasunuma and Kondo 2012): (1) the close proximity of multiple cellulases on the cell surface enable synergistic hydrolysis of cellulose, which leads to increased sugar availability for ethanol production (Fujita et al. 2004; Matano et al. 2012a); (2) since the steady-state concentration of glucose in the medium can be maintained near zero, glucose repression, which prevents the uptake, catabolism, or both of non-glucose sugar, is alleviated to facilitate consumption of xylose (Nakamura et al. 2008); (3) reutilization of the yeast cells enables the reuse of the enzymes displayed on their cell surface without the need for reproduction of the yeast cells, which would reduce the cost of yeast propagation as well as enzyme addition (Kondo et al. 2002; Matano et al. 2012b), and (4) the display of cellulases on the yeast cell surface enables a reduction in the amount of added commercial cellulase (Matano et al. 2012a). In this study, enhancement of ethanol production from crystalline cellulose was achieved by the alleviation of irreversible desorption of cellulase with a cell surface-engineered yeast strain. To our knowledge, this is the first report of the effective hydrolysis of crystalline cellulose through a cellulase-displaying yeast strain. Application of cellulase-displaying yeast for various cellulosic biomasses would be promising because cellulases displayed on the cell surface positively impact the hydrolysis of crystalline cellulose. Moreover, pretreatment of lignocellulosic materials, which was usually performed at high pressure and temperature (Taherzadeh and Karimi 2008), could be performed under milder conditions because cellulase-displaying yeast strains can hydrolyze highly crystalline cellulosic materials. Finally, an environmentally benign process for bioethanol production is conceivable by the use of cell surface-engineered yeast strains.
References
Adney B, Baker J (1996) Measurement of cellulase activities (LAP). NREL TP-510-42628. National Renewable Energy Laboratory, Golden CO
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Desai SG, Converse AO (1997) Substrate reactivity as a function of the extent of reaction in the enzymatic hydrolysis of lignocellulose. Biotechnol Bioeng 56:650–655
Eriksson T, Karlsson J, Tjerneld F (2002) A model explaining declining rate in hydrolysis of lignocellulose substrates with cellobiohydrolase I (Cel7A) and endoglucanase I (Cel7B) of Trichoderma reesei. Appl Biochem Biotechnol 101:41–60
Fujita Y, Ito J, Ueda M, Fukuda H, Kondo A (2004) Synergistic saccharification, and direct fermentation to ethanol, of amorphous cellulose by use of an engineered yeast strain codisplaying three types of cellulolytic enzyme. Appl Environ Microbiol 70:1207–1212
Galbe M, Zacchi G (2007) Pretreatment of lignocellulosic materials for efficient bioethanol production. Adv Biochem Eng Biotechnol 108:41–65
Gao D, Chundawat SPS, Uppugundla N, Balan V, Dale BE (2011) Binding characteristics of Trichoderma reesei cellulases on untreated, ammonia fiber expansion (AFEX), and dilute-acid pretreated lignocellulosic biomass. Biotechnol Bioeng 108:1788–1800
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Gole A, Vyas S, Sainkar SR, Lachke A, Sastry M (2001) Enhanced temperature and pH stability of fatty amine-endoglucanase composites: fabrication, substrate protection, and biological activity. Langmuir 17:5964–5970
Hasunuma T, Kondo A (2012) Consolidated bioprocessing and simultaneous saccharification and fermentation of lignocellulose to ethanol with thermotolerant yeast strains. Process Biochem 47:1287–1294
Hasunuma T, Sung KM, Sanda T, Yoshimura K, Matsuda F, Kondo A (2011) Efficient fermentation of xylose to ethanol at high formic acid concentrations by metabolically engineered Saccharomyces cerevisiae. Appl Microbiol Biotechnol 90:997–1004
Karlsson M, Ekeroth J, Elwing H, Carlsson U (2005) Reduction of irreversible protein adsorption on solid surfaces by protein engineering for increased stability. J Biol Chem 280:25558–25564
Kondo A, Shigechi H, Abe M, Uyama K, Matsumoto T, Takahashi S, Ueda M, Tanaka A, Kishimoto M, Fukuda H (2002) High-level ethanol production from starch by a flocculent Saccharomyces cerevisiae strain displaying cell-surface glucoamylase. Appl Microbiol Biotechnol 58:291–296
Kumar R, Wyman CE (2009) Cellulase adsorption and relationship to features of corn stover solids produced by leading pretreatments. Biotechnol Bioeng 103:252–267
Lupoi JS, Smith EA (2011) Evaluation of nanoparticle-immobilized cellulase for improved ethanol yield in simultaneous saccharification and fermentation reactions. Biotechnol Bioeng 108:2835–2843
Ma A, Hu Q, Qu Y, Bai Z, Liu W, Zhuang G (2008) The enzymatic hydrolysis rate of cellulose decreases with irreversible adsorption of cellobiohydrolase I. Enzyme Microb Technol 42:543–547
Matano Y, Hasunuma T, Kondo A (2012a) Display of cellulases on the cell surface of Saccharomyces cerevisiae for high yield ethanol production from high-solid lignocellulosic biomass. Bioresour Technol 108:128–133
Matano Y, Hasunuma T, Kondo A (2012b) Cell recycle batch fermentation of high-solid lignocellulose using a recombinant cellulase-displaying yeast strain for high yield ethanol production in consolidated bioprocessing. Bioresour Technol (in press)
Nakamura N, Yamada R, Katahira S, Tanaka T, Fukuda H, Kondo A (2008) Effective xylose/cellobiose co-fermentation and ethanol production by xylose-assimilating S. cerevisiae via expression of β-glucosidase on its cell surface. Enzyme Microb Technol 43:233–236
Otter DE, Munro PA, Scott GK, Geddes R (1989) Desorption of Trichoderma reesei cellulase from cellulose by a range of desorbents. Biotechnol Bioeng 34:291–298
Palonen H, Tenkanen M, Linder M (1999) Dynamic interaction of Trichoderma reesei cellobiohydrolases Cel6A and Cel7A and cellulose at equilibrium and during hydrolysis. Appl Environ Microbiol 65:6–11
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton (2008) Determination of structural carbohydrates and lignin in biomass: laboratory 476 analytical procedure (LAP). NREL/TP-510-42618. National Renewable Energy Laboratory, Golden, CO
Taherzadeh MJ, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9:1621–1651
Ueda M, Tanaka A (2000) Cell surface engineering of yeast: construction of arming yeast with biocatalyst. J Biosci Bioeng 90:125–136
Väljamäe P, Sild V, Pettersson G, Johansson G (1998) The initial kinetics of hydrolysis by cellobiohydrolases I and II is consistent with a cellulose surface-erosion model. Eur J Biochem 253:469–475
Xiao Z, Zhang X, Gregg DJ, Saddler JN (2004) Effects of sugar inhibition on cellulases and beta-glucosidase during enzymatic hydrolysis of softwood substrates. Appl Biochem Biotechnol 113–116:1115–1126
Yamada R, Taniguchi N, Tanaka T, Ogino C, Fukuda H, Kondo A (2011) Direct ethanol production from cellulosic materials using a diploid strain of Saccharomyces cerevisiae with optimized cellulase expression. Biotechnol Biofuels 4:8
Yang B, Wyman CE (2006) BSA treatment to enhance enzymatic hydrolysis of cellulose in lignin containing substrates. Biotechnol Bioeng 94:611–617
Yang B, Willies DM, Wyman CE (2006) Changes in the enzymatic hydrolysis rate of avicel cellulose with conversion. Biotechnol Bioeng 94:1122–1128
Yang J, Zhang X, Yong Q, Yu S (2010) Three-stage hydrolysis to enhance enzymatic saccharification of steam-exploded corn stover. Bioresour Technol 101:4930–4935
Zhang YP, Lynd LR (2004) Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnol Bioeng 88:797–824
Zhu Z, Sathitsuksanoh N, Percival Zhang Y-H (2009) Direct quantitative determination of adsorbed cellulase on lignocellulosic biomass with its application to study cellulase desorption for potential recycling. Analyst 134:2267–2272
Acknowledgments
This work was supported through the project P07015 by the New Energy and Industrial Technology Development Organization (NEDO) under the sponsorship of the Ministry of Economy, Trade, and Industry (METI) of Japan. This work was also supported in part by Grants-in-Aid for Young Scientists (B) to TH from the Ministry of Education, Culture, Sports and Technology (MEXT) of Japan and a Special Coordination Fund for Promoting Science and Technology, Creation of Innovative Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe) from MEXT, Japan.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matano, Y., Hasunuma, T. & Kondo, A. Simultaneous improvement of saccharification and ethanol production from crystalline cellulose by alleviation of irreversible adsorption of cellulase with a cell surface-engineered yeast strain. Appl Microbiol Biotechnol 97, 2231–2237 (2013). https://doi.org/10.1007/s00253-012-4587-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4587-x