Abstract
Synthesis of cyanophycin (multi-l-arginyl-poly-l-aspartic acid, CGP) in recombinant organisms is an important option to obtain sufficiently large amounts of this polymer with a designed composition for use as putative precursors for biodegradable technically interesting chemicals. Therefore, derivates of CGP, harbouring a wider range of constituents, are of particular interest. As shown previously, cyanophycin synthetases with wide substrate ranges incorporate other amino acids than arginine. Therefore, using an organism, which produces the required supplement by itself, was the next logical step. Former studies showed that Pseudomonas putida strain ATCC 4359 is able to produce large amounts of l-citrulline from l-arginine. By expressing the cyanophycin synthetase of Synechocystis sp. PCC 6308, synthesis of CGP was observed in P. putida ATCC 4359. Using an optimised medium for cultivation, the strain was able to synthesise insoluble CGP amounting up to 14.7 ± 0.7% (w/w) and soluble CGP amounting up to 28.7 ± 0.8% (w/w) of the cell dry matter, resulting in a total CGP content of the cells of 43.4% (w/w). HPLC analysis of the soluble CGP showed that it was composed of 50.4 ± 1.3 mol % aspartic acid, 32.7 ± 2.8 mol % arginine, 8.7 ± 1.6 mol % citrulline and 8.3 ± 0.4 mol % lysine, whereas the insoluble CGP contained less than 1 mol % of citrulline. Using a mineral salt medium with 1.25 or 2% (w/v) sodium succinate, respectively, plus 23.7 mM l-arginine, the cells synthesised insoluble CGP amounting up to 25% to 29% of the CDM with only a very low citrulline content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The polyamide cyanophycin [multi-l-arginyl-poly-(l-aspartic acid)], also referred to as cyanophycin granule polypeptide (CGP), is a non-ribosomally biopolymer naturally synthesised nitrogen, energy and carbon storage compound of phototrophic and heterotrophic bacteria (Krehenbrink et al. 2002; Sallam et al. 2009; Ziegler et al. 2002). Research on CGP revealed that arginine in the side chain can be partially replaced by lysine. Consequently, recent research focused on the possibilities to obtain variants of the polymer consisting not only of aspartate, arginine and lysine, but also of other structurally related amino acids such as citrulline and ornithine, respectively, as shown in Fig. 1. Such polymer variants are of special interest for the production of bulk chemicals (Könst et al. 2009; Mooibroek et al. 2007; Sanders et al. 2007) or of pharmaceutically employed dipeptides derived from CGP (Sallam and Steinbüchel 2009, 2010). Applying mutants of Saccharomyces cerevisiae with defects in arginine biosynthesis, it was for the first time possible to obtain in vivo synthesised CGP derivatives containing up to 20 mol % citrulline or up to 8 mol % ornithine replacing arginine, respectively (Steinle et al. 2009). However, a major disadvantage was that considerable amounts of the respective compounds were incorporated only if the latter were supplied in the growth media. Therefore, we searched for alternative strains, and a natural citrulline producer was investigated for synthesis of citrulline-rich CGP.
Arginine deiminase (Adi, EC 3.5.3.6), encoded by arcA, catalyses the hydrolytic conversion of arginine to ammonia and citrulline and is part of the energy-producing arginine degradation pathway. The enzyme occurs in a wide range of bacteria such as in strains of pseudomonads (Pseudomonas putida, Pseudomonas aeruginosa, Pseudomonas fluorescens, Pseudomonas stutzeri), Lactococcus lactis, Streptococcus pyogenes, Mycobacterium smegmatis, and Rhodococcus opacus and few protists (Li et al. 2008). The catalytic mechanism of the enzyme from P. aeruginosa was characterised in detail (Galkin et al. 2005). However, for the present study, P. putida strain ATCC 4359 was chosen as it strongly expresses arcA under specific cultivation conditions and as it does not further convert citrulline due to the lack of ornithine transcarbamylase activity (Kakimoto et al. 1971). As a result, cells overproduce citrulline which was intended to be incorporated into the CGP polymer in our studies.
Previously, P. putida strains KT2440 and GPp104 were applied for heterologous expression of the cyanophycin synthetases from Synechocystis sp. strains PCC 6803 and PCC 6308, Synechococcus sp. strain MA19 and Anabaena sp. strain PCC 7120. Maximal CGP contents of 24.1% (w/w) were obtained after the expression of the enzyme from Anabaena sp. strain PCC 7120 (Aboulmagd et al. 2001b; Diniz et al. 2006; Voss et al. 2004). Applying cphA from Synechocystis sp. PCC 6308, maximal CGP contents of 10.0% (w/w) were detected (Voss et al. 2004). The latter was also applied in the present study as it is known for its wide in vitro and in vivo substrate range comprising also citrulline (Aboulmagd et al. 2001a; Steinle et al. 2009).
Materials and methods
Bacterial strains, plasmids and cultivation conditions
All bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli S17-1 (Simon et al. 1983) was used as donor for plasmid transfer by conjugation to Pseudomonas strains and was grown in lysogeny broth (LB) medium (Sambrook et al. 1989) containing 12.5 mg/l tetracycline (if required) overnight at 37°C and 120 rpm. P. putida ATCC 4359 was grown in a medium optimised for citrulline synthesis (Kakimoto et al. 1971), which was referred to as 3P medium, containing 20 g/l glucose, 5 g/l arginine (hydrochloride), 5 g/l peptone, 5 g/l (NH4)2HPO4, 5 g/l yeast extract, 1 g/l KH2PO4, 0.5 g/l MgSO4 × 7 H2O, 0.1 g/l MnSO4 × 4 H2O, 20 mg/l NaCl and 5 mg/l FeSO4 × 7 H2O. The pH value of the medium was set to 6.0. Cultures were grown in 500 ml Erlenmeyer flasks containing 220 ml 3P-media at 30°C and agitated at 120 rpm. Strains of Pseudomonas harbouring the vector pBBR1MCS3 or pBBR1MCS3::cphA 6308 were grown in a medium containing tetracycline (25.0 mg/l). P. putida was also grown in mineral salt media (Schlegel et al. 1961), which were referred to as MSM.
Transfer of DNA
E. coli S17-1 was transformed by the rubidium chloride procedure (Hanahan 1983) with the vector pBBR1MCS3::cphA6308 containing the cyanophycin synthetase gene from Synechocystis sp. PCC 6308. Transformants were selected on solid LB medium containing 12.5 mg/l tetracycline. The transformed E. coli S17-1 cells were used as a donor to transfer the vector to Pseudomonas strains by conjugation, using the method described by Friedrich et al. (1981). Transconjugants of P. putida were selected on solid mineral salt medium containing 2% Na-succinate as single carbon source and 25 mg/l tetracycline.
Cell harvest and preparation of samples for cyanophycin isolation
Bacterial cells were harvested in a bench centrifuge (30 min, 4,000×g, 4°C) and washed once with Tris–HCl (50 mM, pH 7.0). For determination of the cell dry matter (CDM), pellets were lyophilised for 24 h (Beta 1–16, Christ, Osterode, Germany), and the cell mass was gravimetrically determined. For cell disruption, the cell pellet was dissolved in 15 ml buffer (50 mM Tris–HCl, pH 7.0) per gramme fresh or dry cell mass and disrupted for 10 min by an ultrasonic probe (Sonoplus GM200, Bandelin GmbH, Berlin, Germany). Soluble cell fractions were obtained by centrifugation of crude cell extracts (30 min, 4,000×g, 4°C).
Isolation of cyanophycin
To isolate CGP, bacterial cells were disrupted as described above. Crude extracts obtained after cell disruption were centrifuged twice for 30 min (4,000×g) at 4°C. Water-soluble CGP was isolated from supernatants applying a modified method described by Steinle et al. (2009). After proteinase K digestion, two volumes of ice-cold ethanol were added to samples, and soluble CGP was precipitated. After one washing step with acetone and drying of CGP at 65°C, CGP was dissolved in 50 mM Tris–HCl (pH 7.0) and precipitated again. The washing steps were repeated three times to purify the polymer. Water-insoluble CGP was isolated from cell debris by resuspension in 0.1 M HCl, as described by Frey et al. (2002). After 30 min of centrifugation (4,000×g), the supernatant was neutralised, the polymer was sedimented by centrifugation and washed twice with demineralized water. To obtain pure CGP, the pellet was dissolved in 0.1 M HCl and precipitated alternately three times. After lyophilisation, the dry weight was determined gravimetrically.
High performance liquid chromatography for amino acid analysis
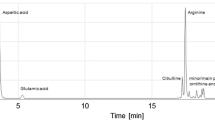
Amino acid constituents of isolated CGP were determined by high performance liquid chromatography (HPLC) using a Waters B801 (300 × 4 mm) column as described by Aboulmagd et al. (2000) and Steinle et al. (2009). Pre-column OPA-derivatisation was done by a Smartline autosampler 3900 as described in the manual (Knauer GmbH, Berlin, Germany). Calibration was done with samples from a reference kit for aspartate, arginine and lysine (Kollektion AS-10 from Serva Feinbiochemica, Heidelberg, Germany). Citrulline was purchased as monohydrochloride from Fluka.
Nuclear magnetic resonance for polymer analysis
1H nuclear magnetic resonance (NMR) analyses were performed with a Varian unity plus 600 MHz spectrometer (1D 1H, 599.54 MHz) as described by Steinle et al. (2009). An acquisition time of 4.7 s and a relaxation delay of 3 s were applied. The number of scans was 128. The measurements were carried out at 353 K with samples of 10 to 14 mg CGP solubilised in D2O (Aldrich).
SDS-PAGE for cyanophycin analysis
To analyse the purity and the molecular mass of isolated CGP, sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was carried out in 11.5% (w/v) polyacrylamide gels applying a standard method (Laemmli 1970). Standard molecular weight proteins were purchased from GE Healthcare. The applied mixture contained phosphorylase b from rabbit muscle (97.0 kDa), albumin from bovine serum (66.0 kDa), ovalbumin from chicken egg white (45.0 kDa), carbonic anhydrase from bovine erythrocyte (30.0 kDa), trypsin inhibitor from soybean (20.1 kDa) and α-lactalbumin from bovine milk (14.4 kDa).
Results
Selection of CphA and expression system for P. putida ATCC 4359
For the present study, P. putida ATCC 4359 was selected to achieve synthesis of CGP with a high content of citrulline. For this purpose, the rather unspecific cyanophycin synthetase from Synechocystis sp. strain PCC 6308 (CphA6308) was chosen (Aboulmagd et al. 2001a; Steinle et al. 2009). Furthermore, plasmid pBBR1MCS3::cphA 6308 (Voss et al. 2004) was used because it gave sufficient expression of CphA in previous experiments using P. putida KT2440 (Diniz et al. 2006).
Cultivation of P. putida ATCC 4359
For initial cultivation experiments, P. putida ATCC 4359 pBBR1MCS3::cphA 6308 was cultivated as described by Kakimoto et al. (1971). Erlenmeyer flasks (500 ml) containing 220 ml of 3P medium were inoculated with one loop full of fresh grown cells from solid LB medium. The cells were grown at 30°C and agitated at 120 rpm until they reached the stationary growth phase after about 25 h. In order to cover a broad range of growth conditions, the cells were further cultivated at different temperatures (30°C and 37°C, respectively) and also with and without addition of 110 g of l-arginine–HCl to the 220-ml medium (resulting in a final concentration of 2.1 M l-arginine), after reaching the stationary phase. These extremely high concentrations of arginine were used by Kakimoto et al. (1971). An elevation of the cultivation temperature was also described and used by Kakimoto et al. (1971), and their studies showed that 37°C was the compromise between the activity of the arginine deiminase and the stability of this enzyme. After another 48 h of cultivation, the cells were harvested, and the CDM and CGP content were measured. In addition, the CGP composition was analysed.
The presence of soluble and insoluble CGP in the cells depended on the cultivation conditions for the cells after they reached the stationary growth phase, as shown in Table 2. If 110 g of l-arginine–HCl had been added to the culture, no CGP could be isolated at all, irrespectively of the cultivation temperature. If the cultures were kept at 30°C, the cells accumulated about 3.7% (w/w of CDM) of insoluble CGP while the cells contained about 6.8% insoluble and 17.7% soluble CGP if the temperature was shifted to 37°C after the cells had reached the stationary growth phase. Therefore, in further cultivations, the cells were grown at 30°C to the stationary growth phase, and the temperature was then shifted to 37°C without adding additional l-arginine.
Next, the concentration of arginine in the normal 3P medium (23.7 mM) was varied to 0%, 50%, 100% or 200% of this concentration to investigate the effect on the amount of synthesised CGP. After reaching the stationary growth phase and the temperature shift to 37°C for 48 h, the cells were harvested. As shown in Table 3, cells grown in the presence of 100% arginine exhibited the highest CGP contents, containing 15.7% of insoluble and 26.0% of soluble CGP (w/w of CDM), respectively, at a cell density of 1.01 g/l. By doubling the amount of arginine to 200%, the cells did not synthesise soluble CGP, and the cell density was strongly diminished. Without supplementation of the medium with arginine, the cells did not synthesise soluble CGP and contained also less insoluble CGP.
Since inoculation of the main cultures by a loop full of cells as described by Kakimoto et al. (1971) did not provide a good level of reproducibility, the cultures were inoculated from liquid LB precultures. Using 440 μl of an overnight LB preculture for inoculation of a 220-ml 3P medium gave good results and matched the growth behaviour of cultures inoculated by a loop full of cells.
Four 3P cultures were independently cultivated, and samples of the cultures were analysed for optical density and by HPLC to determine the concentrations of arginine and citrulline. When the cells had reached the stationary growth phase after about 21 h (Fig. 2), one of the four cultures was harvested. Cells harvested after 21 h contained only 11.0% of insoluble CGP, soluble CGP could not be detected at all, and the culture had reached a cell density of 0.87 g/l. The temperature of the other three cultures was shifted to 37°C. The arginine concentration in the medium started to decrease rapidly afterwards, and citrulline began to accumulate in the medium after about 12 h and continued to increase significantly after the temperature shift. After about 45 h, the arginine level reached a minimum, but citrulline formation was observed until the very end of the cultivation. After 69 h also, these cells were harvested as described, CDM and CGP content were measured, and the CGP composition was analysed. As shown in Table 5, the cells (1.09 ± 0.05 g/l) contained about 14.7 ± 0.7% of insoluble and 28.7 ± 0.8% of soluble CGP (w/w of CDM).
Growth and cultivation of P. putida ATCC 4359 in the 3P medium and conversion of arginine into citrulline. Cells were grown at 30°C until reaching the stationary growth phase after 21 h; cultivation was then continued at 37°C. The optical density (filled diamond) was measured photometrically at 600nm. The concentrations of arginine (filled square) and citrulline (filled triangle) were measured by HPLC
The noticeable opacity of the colonies of P. putida pBBR1MCS3::cphA 6308 on solid mineral salt medium indicated high contents of insoluble CGP of the cells. P. putida was therefore grown in MSM containing 1.25% or 2.0% sodium succinate. The cells were cultivated entirely at 30°C, since the cultivations in the 3P medium indicated that insoluble CGP is synthesised mostly at this temperature. Additionally, the cultures were inoculated using 440 μl of an overnight preculture or one loop full of fresh grown cells from solid media as described by Kakimoto et al. (1971), respectively. The cultures were cultivated for 96 h, and the average of three independent cultures was determined. As shown in Table 4, the cells showed a high content of insoluble CGP. The amount of about 25% (w/w; CGP/CDM) was independent of the amount of sodium succinate and the method of inoculation, and so was the CDM of the cultures. Soluble CGP could not be isolated from these cells.
Determination of the amino acid composition of the cyanophycin by HPLC and NMR
The amino acid compositions of the isolated CGP samples were determined by HPLC. As shown in Table 5, the isolated soluble CGP from the three cultures in the 3P medium exhibited an average composition of 50.4 ± 1.3 mol % aspartate, 32.7 ± 2.8 mol % arginine, 8.7 ± 1.6 mol % citrulline and 8.3 ± 0.4 mol % lysine. In contrast, the insoluble CGP of the 3P cultures contained on average 1.0 ± 0.1 mol % citrulline. The CGP isolated from the culture harvested after 21 h showed 0.4 mol % of citrulline. In both insoluble CGP samples, no lysine was detectable. The NMR analysis showed significant similarities to the NMR profile of citrulline-rich CGP from S. cerevisiae published by Steinle et al. (2009), but slight variations due to the different content of citrulline and the presence of lysine.
The insoluble CGP isolated from the cells grown in MSM was composed of the two major components aspartate and arginine, harbouring only insignificant amounts of less than 1.5% citrulline and lysine (Table 4).
Analysis of cyanophycin by SDS-PAGE
To determine the molecular weights of the CGP samples exhibiting different citrulline and lysine contents, insoluble and soluble CGP samples isolated from several cultivations in the 3P medium were analysed by SDS-PAGE. For this, each 60 μg of CGP was solubilised and applied onto an SDS polyacrylamide gel. Insoluble CGP from cells cultivated at 30°C, from cells also cultivated at 30°C but provided with additional arginine at the beginning of the stationary phase, and from cells of a culture that was shifted to 37°C after reaching the stationary phase were compared, as well as the soluble CGP isolated from cells cultivated at 37°C. The insoluble CGPs synthesised at 30°C exhibited an average molecular weight of about 25 kDa and a weight distribution between 20 and 30 kDa (Fig. 3). The insoluble and soluble CGP, isolated from cells of a culture grown at 37°C, exhibited the same average weight, but a larger polydisperse area of up to about 66 kDa in comparison to the CGP from 30°C cultures.
Analysis of CGP isolated from cells of P. putida ATCC 4359 by SDS polyacrylamide gel electrophoresis. Sixty microgrammes of each polymer was solubilised and applied to the gel. Lane 1 insoluble CGP from cells cultivated in the LB medium at 30°C, lane 2 insoluble CGP from cells cultivated in the 3P medium at 30°C, lane 3 insoluble CGP from cells cultivated in the 3P medium at 37°C, lane 4 soluble CGP from cells cultivated in the 3P medium at 37°C, lane M molecular weight marker proteins. The 37°C cultures were grown at 30°C until they reached the stationary growth phase before the temperature was raised to 37°C
Discussion
As the analysis showed, the strategy of this study, to achieve synthesis of a citrulline-rich CGP using a citrulline-producing strain, was successful. The content of almost 9 mol % citrulline in the soluble CGP as well as the high concentration of the soluble CGP itself are promising for further experiments, since the cultivation has not yet been optimised with regard to CGP synthesis and citrulline incorporation into the polymer. Noticeable is also the fact that P. putida accumulated both soluble and insoluble CGP in high amounts, resulting in a total CGP content of 43.4% (w/w) of the CDM (14.7% insoluble and 28.7% soluble CGP). This content is comparable with those obtained by recombinant bacteria in fermentation processes, which were optimised for synthesis and accumulation of GCP like Acinetobacter baylyi ADP1 or Ralstonia eutropha with CGP contents of up to 40% (w/w) of CDM (Elbahloul and Steinbüchel 2006; Voss and Steinbüchel 2006).
Another interesting observation was that a high citrulline content occurred almost only in the soluble CGP (8.7 mol %), while the insoluble CGP showed only insignificant amounts of citrulline not exceeding 1.0 to 1.5 mol %. These observations match the results of experiments with S. cerevisiae (Steinle et al. 2009), where high fractions of citrulline and ornithine in the polymer were also restricted to the soluble CGP, which showed five to six times higher amounts than the insoluble CGP isolated from the same cells. The reasons for this have yet to be investigated, but obviously, synthesis of soluble CGP seems to be more accessible for incorporation of alternative constituents than synthesis of the insoluble CGP. Another explanation for these observations might be a post-synthetic modification of CGP by arginine deiminase, thereby converting the arginine constituents of CGP into citrulline. This could also explain the higher citrulline content in the soluble CGP, as it is likely that, due to its solubility, this CGP form is better accessible for the arginine deiminase than the insoluble form. A post-synthetic modification was not yet investigated but is an interesting aspect for further research.
A major not understood issue is still the lack of information on the mechanisms and conditions, leading to the synthesis of soluble instead of insoluble CGP in some organisms. Since Steinle et al. (2009) detected in soluble CGP only aspartic acid and arginine, the high amounts of alternative constituents like citrulline and ornithine could not be the reason for the unusual solubility behaviour of this CGP. These observations are supported by NMR and HPLC analyses, which showed no structural differences between the two CGPs (Füser and Steinbüchel 2005; Steinle et al. 2009; Ziegler et al. 2002).
However, the observations made with P. putida ATCC 4359 showed that the synthesis of soluble CGP seemed to be entirely dependent on the temperature at which this strain is cultivated. If the cells are grown in the 3P medium entirely at 30°C, they synthesised only insoluble CGP. However, when the temperature of the culture is shifted to 37°C after the cells have reached the stationary growth phase, the strain almost stops the production of insoluble CGP and starts to synthesise soluble CGP instead. Since P. putida normally does not grow at temperatures above 32°C, it is most likely that the cultivation at 37°C has a large influence on the metabolism and on the milieu of the cells, which might be responsible for the synthesis of soluble CGP and which may also influence the incorporation rate of citrulline. However, our data suggest that the incorporation rate is more dependent on the concentration of citrulline, which is also higher at 37°C due to the increased arginine deiminase activity in comparison to 30°C. This behaviour could be the base for further investigations on critical elements controlling the synthesis of either soluble or insoluble CGP. By measuring essential parameters of the culture and of the cells, and by comparing the proteome of the cells, differences between these two cultivation temperatures may be identified and further investigated for their influence on the synthesis of CGP.
References
Aboulmagd E, Oppermann-Sanio FB, Steinbüchel A (2000) Molecular characterization of the cyanophycin synthetase from Synechocystis sp. strain PCC 6308. Arch Microbiol 174:297–306
Aboulmagd E, Oppermann-Sanio FB, Steinbüchel A (2001a) Purification of Synechocystis sp. PCC6308 cyanophycin synthetase and its characterization with respect to substrate and primer specificity. Appl Environ Microbiol 67:2176–2182
Aboulmagd E, Voss I, Oppermann-Sanio FB, Steinbüchel A (2001b) Heterologous expression of cyanophycin synthetase and cyanophycin synthesis in the industrial relevant bacteria Corynebacterium glutamicum and Ralstonia eutropha and in Pseudomonas putida. Biomacromolecules 2:1338–1342
Diniz CS, Voss I, Steinbüchel A (2006) Optimization of cyanophycin production in recombinant strains of Pseudomonas putida and Ralstonia eutropha employing elementary mode analysis and statistical experimental design. Biotechnol Bioeng 93:698–717
Elbahloul Y, Steinbüchel A (2006) Engineering the genotype of Acinetobacter sp. strain ADP1 to enhance biosynthesis of cyanophycin. Appl Environ Microbiol 72:1410–1419
Frey KM, Oppermann-Sanio FB, Schmidt H, Steinbüchel A (2002) Technical-scale production of cyanophycin with recombinant strains of Escherichia coli. Appl Environ Microbiol 68:3377–3384
Friedrich B, Hogrefe C, Schlegel HG (1981) Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J Bacteriol 147:198–205
Füser G, Steinbüchel A (2005) Investigations on the solubility behavior of cyanophycin. Solubility of cyanophycin in solutions of simple inorganic salts. Biomacromolecules 6:1367–1374
Galkin A, Lu X, Dunaway-Mariano D, Herzberg O (2005) Crystal structures representing the Michaelis complex and the thiouronium reaction intermediate of Pseudomonas aeruginosa arginine deiminase. J Biol Chem 280:34080–34087
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Kakimoto T, Shibatani T, Nishimura N, Chibata I (1971) Enzymatic production of l-citrulline by Pseudomonas putida. Appl Microbiol 22:992–999
Könst P, Franssen MCR, Scott EL, Sanders JPM (2009) A study on the applicability of l-aspartate α-decarboxylase in the biobased production of nitrogen containing chemicals. Green Chem 11:1646–1652
Kovach ME (1995) Four new derivates of the broad host range cloning vector pBBR1MCS, carrying different antibiotic resistance cassettes. Gene 166:175–176
Krehenbrink M, Oppermann-Sanio FB, Steinbüchel A (2002) Evaluation of non-cyanobacterial genome sequences for occurrence of genes encoding proteins homologous to cyanophycin synthetase and cloning of an active cyanophycin synthetase from Acinetobacter sp. strain DSM 587. Arch Microbiol 177:371–380
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li L, Li Z, Chen D, Lu X, Feng X, Wright EC, Solberg NO, Dunaway-Mariano D, Mariano PS, Galkin A, Kulakova L, Herzberg O, Green-Church KB, Zhang L (2008) Inactivation of microbial arginine deiminases by l-canavanine. J Am Chem Soc 130:1918–1931
Mooibroek H, Oosterhuis N, Giuseppin M, Toonen M, Franssen H, Scott E, Sanders J, Steinbüchel A (2007) Assessment of technological options and economical feasibility for cyanophycin biopolymer and high-value amino acid production. Appl Microbiol Biotechnol 77:257–267
Sallam A, Steinbüchel A (2009) Cyanophycin-degrading bacteria in digestive tracts of mammals, birds and fish and consequences for possible applications of cyanophycin and its dipeptides in nutrition and therapy. J Appl Microbiol 107:474–484
Sallam A, Steinbüchel A (2010) Dipeptides in nutrition and therapy: cyanophycin-derived dipeptides as natural alternatives and their biotechnological production. Appl Microbiol Biotechnol 87:815–828
Sallam A, Steinle A, Steinbüchel A (2009) Cyanophycin: biosynthesis and applications. In: Rehm BHA (ed) Microbial production of biopolymers and polymer precursors. Caister Academic Press, Norfolk, UK
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Springer Harbor Laboratory, Cold Spring Harbor, NY
Sanders J, Scott E, Weusthuis R, Mooibroek H (2007) Bio-refinery as the bio-inspired process to bulk chemicals. Macomol Biosci 7:105–117
Schlegel HG, Kaltwasser H, Gottschalk G (1961) A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies. Arch Mikrobiol 38:209–222
Simon RD, Priefer U, Pühler A (1983) A broad host range mobilization system for in vitro genetic engineering: transposon mutagenesis in gram-negative bacteria. Biotechnology 1:784–791
Steinle A, Bergander K, Steinbüchel A (2009) Metabolic engineering of Saccharomyces cerevisiae for production of novel cyanophycins with an extended range of constituent amino acids. Appl Environ Microbiol 75:3437–3446
Voss I, Steinbüchel A (2006) Application of a KDPG-aldolase gene-dependent addiction system for enhanced production of cyanophycin in Ralstonia eutropha strain H16. Metab Eng 8:66–78
Voss I, Diniz CS, Aboulmagd E, Steinbüchel A (2004) Identification of the Anabaena sp. strain PCC 7120 cyanophycin synthetase as suitable enzyme for production of cyanophycin in gram-negative bacteria like Pseudomonas putida and Ralstonia eutropha. Biomacromolecules 5:1588–1595
Ziegler K, Deutzmann R, Lockau W (2002) Cyanophycin synthetase-like enzymes of non-cyanobacterial eubacteria: characterization of the polymer produced by a recombinant synthetase of Desulfitobacterium hafniense. Z Naturforsch C 57:522–529
Acknowledgement
NMR analysis of the polymer samples was carried out by Klaus Bergander (Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster) and is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wiefel, L., Bröker, A. & Steinbüchel, A. Synthesis of a citrulline-rich cyanophycin by use of Pseudomonas putida ATCC 4359. Appl Microbiol Biotechnol 90, 1755–1762 (2011). https://doi.org/10.1007/s00253-011-3224-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3224-4