Abstract
Among the laccases produced by the white-rot fungus Pleurotus ostreatus, there are two closely related atypical isoenzymes, POXA3a and POXA3b. These isoenzymes are endowed with quaternary structure, consisting of two subunits very different in size. The POXA3 large subunit is clearly homologous to other known laccases, while the small subunit does not show significant homology with any protein in data banks. To investigate on the singular structure of the POXA3 complex, a new system for recombinant expression of heterodimer proteins in the yeast Kluyveromyces lactis has been set up. A unique expression vector has been used and the cDNAs encoding the two subunits have been cloned under the control of the same bi-directionally acting promoter. Expression of the large subunit alone and co-expression of both subunits in the same host have been demonstrated and the properties of the recombinant proteins have been compared. Clones expressing the large subunit alone exhibited always notably lower activity than those expressing both subunits. In addition to the activity increase, the presence of the small subunit led to a significant increase of laccase stability. Therefore, a role of the small subunit in POXA3 stabilisation is suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
White rot fungi produce several oxidative extracellular enzymes such as laccases and peroxidases that are able to degrade lignin, but can also degrade different synthetic chemicals usually recalcitrant to biodegradation. Among the laccases produced by the white-rot fungus Pleurotus ostreatus, the POXA3 isoenzymes (Palmieri et al. 2003; Giardina et al. 2007) exhibit unusual structural features, being heterodimeric enzymes, differently from most of the known laccases that are monomers or sometimes homodimers (Min et al. 2001; Wahleithner et al. 1996; Yaver et al. 1996). The POXA3 subunits have different sizes (molecular masses of 67 and 16 or 18 kDa). Moreover, both the large and the small subunits are heterogeneous, providing a complex fungal isoenzyme pattern. Two closely related POXA3 isoforms (POXA3a and POXA3b), differing for the occurrence of two amino acid substitutions in their small subunits, can be separated. Furthermore, each small subunit is found as two forms, the 18-kDa subunit resulting from glycosylation of the 16-kDa polypeptide. Moreover, the large subunit of each isoenzyme contains two forms resulting from alternative splicing of the transcript, giving rise to an insertion of four amino acids between Pro287 and Leu288 (Giardina et al. 2007). The amino-acid sequence of the POXA3 large subunit is clearly homologous to other known laccase sequences, and contains all the putative copper-binding residues (Palmieri et al. 2003). On the contrary, the small subunits do not show any sequence homology neither with laccases or laccase-associated domains nor with any other protein in data banks; therefore, no indication on the function of these subunits can be deduced from their primary structures. It is worth to note that the small subunit is found associated only with POXA3 large subunit among the various laccase isoenzymes produced in the same P. ostreatus culture.
The complex heterogeneity and the singular heterodimeric structure of POXA3 complexes led us to develop a system exploitable for heterologous expression of heterodimers to investigate on POXA3 peculiar properties.
So far, heterologous expression of laccases has been attained for example in Saccharomyces cerevisiae (Kiiskinen and Saloheimo 2004; Klonowska et al. 2005), Pichia pastoris (Liu et al. 2003; Soden et al. 2002), Yarrowia lipolytica (Jolivalt et al. 2005), Trichoderma reesei (Kiiskinen et al. 2004), and several Aspergillus sp. (Sigoillot et al. 2004; Larrondo et al. 2003; Record et al. 2002). Kluyveromyces lactis was also shown to be an appropriate host for recombinant expression of P. ostreatus POXC and POXA1b laccases, providing a higher expression yield of secreted laccases in comparison with S. cerevisiae without hyperglycosylation of proteins (Piscitelli et al. 2005).
Recombinant expression of hetero-oligomeric proteins in yeasts—mainly P. pastoris and S. cerevisiae—has been mostly performed by means of co-transformation with monomer subunit expression vectors (Sen Gupta and Dighe 1999; Beggah et al. 2000; Pakkanen et al. 2003; Hoyt et al. 2003; Keizer-Gunnink et al. 2000; Salo et al. 2005). However, an example of recombinant protein expression by means of bi-directional promoter has been reported in S. cerevisiae (Miller et al. 1998).
In this paper, a new system for recombinant expression of heterodimeric proteins in the yeast K. lactis is reported, by using an unique expression vector, where monomer subunits are cloned under the control of the same bi-directionally acting promoter. Expression of the POXA3 large subunit alone and co-expression of both subunits in the same host provided us a tool to investigate on the role of POXA3 small subunit, by comparing the properties of the recombinant proteins.
Materials and methods
Heterologous POXA3 expression
The K. lactis strain used for heterologous expression was CMK5 (a thr lys pgi1 adh3 adh1::URA3 adh2::URA3) (Saliola et al. 1999). The plasmid pYG132 was engineered from pKD1, a natural plasmid originally isolated from Kluyveromyces drosophilarum (Falcone et al. 1986) that can stably replicate in K. lactis. Expression of inserts is controlled by the ethanol-inducible 1200 nucleotides extended KlADH4 promoter (Mazzoni et al. 1992) and the S. cerevisiae phosphoglycerate kinase terminator. To insert a HindIII site upstream and downstream of the cDNA, the 5′ and 3′ regions of poxa3 large subunit cDNA (EMBL accession number AJ344434) were amplified by means of Phusion polymerase (Stratagene) with fwpoxa3HindIII/revpoxa3BamHI and fwpoxa3BamHI/revpoxa3HindIII primers (Table 1), respectively, using pGEM7Zf (Promega, Italy) containing poxa3 large subunit cDNA (EMBL accession number AJ344434) as template. The amplified regions were re-ligated in pGEM vector, and checked by sequencing. Then, the complete cDNA fragment was cloned in HindIII site of pYG132-HSA vector giving the vector A3L (Fig. 1a). cDNA encoding for POXA3b small subunit including its signal peptide (EMBL accession number AM409319) was amplified with sspoxa3 5′SalI/sspoxa3 3′SalI primers, in order to insert a SalI site upstream and downstream of the cDNA. The amplified cDNA was initially cloned into pUC18 vector. The SalI cDNA fragment was then cloned in the SalI site (1,200 nt upstream of HindIII site) of the vector A3L providing the vector A3LS (Fig. 1b).
a The vector A3L for recombinant expression of POXA3 large subunit, consisting of pYG132 vector containing cDNA encoding for the POXA3 large subunit with its signal peptide (pre l-poxa3) in HindIII site. b A3LS vector for recombinant co-expression of the POXA3 large and small subunits, prepared by cloning the cDNA encoding for the POXA3 small subunit with its signal peptide (pre s-poxa3) into the SalI site of A3L vector. Selection markers [aminoglycoside 3-phosphotransferase (aph) gene and beta-lactamase (bla) gene], promoter and terminator regions and replication origins are indicated
K. lactis transformation was performed by electroporation with a Bio-Rad Micro-Pulser apparatus, as specified by the manufacturer. The cells were spread on YPPG (10 g l−1 yeast extract, 40 g l−1 bacto tryptone, 20 g l−1 galactose) medium containing 100 μg ml−1 of geneticin G418, after an over night incubation at 28°C in YPPG liquid broth. Agar plate assays on YPPG supplemented with 100 μg ml−1 of geneticin G418, 0.5% ethanol, 1 mM CuSO4 and 0.2 mM ABTS were used for transformant selection. Plates were incubated inverted at 28°C and checked for the development of colour. Approximately 100 μl of ethanol was added each day to the lid of the plate to compensate its evaporation. Transformed clones were screened for laccase production by growing them in 10-ml selective medium (geneticin containing YPPG) supplemented with 1 mM CuSO4 and 0.5% ethanol, at 28°C on a rotary shaker (150 rpm). Ethanol (0.5%) was daily added and samples were withdrawn at intervals for optical density and laccase activity determination. The best laccase producing clone was chosen to study laccase production in 250 ml flasks containing 50 ml of selective YPPD (10 g l−1 yeast extract, 40 g l−1 bacto tryptone, 20 g l−1 glucose) or YPPG medium, containing 0.5 or 1% ethanol, and 0.5 or 1 mM CuSO4, starting from 0.06 OD600. Cultures were grown at 28 or 20°C on a rotary shaker. Culture aliquots (1 ml) were collected daily and cells were pelleted by centrifugation (12,000 × g for 2 min at 4°C). Laccase activity in the culture supernatant was assayed at 25°C by monitoring the oxidation of ABTS at 420 nm (ɛ 420 = 3.6 × 104 M−1 cm−1). The assay mixture contained 2 mM ABTS, 0.1 M sodium citrate buffer (pH 3.0).
Small subunit recombinant expression in Escherichia coli
The pUC18 plasmid (Fermentas Life Sciences) containing the POXA3b small subunit encoding sequence was used as template for the amplification experiment performed with the oligonucleotides sspoxa3 5′NdeI and sspoxa3 3′EcoRI (Table 1) as primers. To subclone it into the T7 expression vector pET-22b(+) (Invitrogen), NdeI and EcoRI sites are needed at the 5′ and 3′ ends, respectively, of the amplified fragment. In addition, a starting codon (ATG) has to be introduced just before the first codon of the mature protein. The sequence of the final, ligated expression vector was confirmed by DNA sequencing. This vector was used to transform E. coli strain BL21(DE3) (Invitrogen). A single colony was selected and grown overnight at 37°C in 10 ml of Luria–Bertani broth (LB) containing 100 mg l−1 ampicillin. The starting culture was used to inoculate 1 l of LB medium with ampicillin at 37°C until an A 600 nm of 0.6–0.8 was attained. At this stage the cells were induced for 3 h by addition of IPTG to a final concentration of 0.4 mM. Cells were harvested by centrifugation, resuspended in 25 mM Tris/HCl pH 7.3, 5 mM EDTA, 0.5 mM PMSF and lysed by sonication.
The protein was purified from the supernatant by loading on DEAE Sepharose Fast Flow cromatography (GE Healthcare) and eluting with NaCl linear gradient (0–300 mM) in 25 mM Tris/HCl, pH 7.3. The last step of the purification procedure was a P-30 gel-filtration chromatography in 150 mM ammonium bicarbonate, pH 7.8. Protein homogeneity was verified by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and matrix assisted laser desorption/ionization (MALDI) time-of-flight mass spectra. Antibodies anti recombinant POXA3 small subunit were produced by PRIMM (Milan, Italy).
Protein deglycosylation was performed by incubating samples with 1 mU of endoglycosidase H (Boehringer Mannheim) in 20 mM sodium acetate, pH 5.2, 0.01% SDS, 5 mM dithiothreitol over night at 37°C.
Western blots
Protein production by similarly grown cultures, was analysed on SDS-PAGE (12.5%) loading 130 μl of culture broths or intracellular samples corresponding to 0.3 OD600. Proteins were then transferred to a PVDF membrane using an electroblotting transfer apparatus (Trans-Blot Semi-Dry Transfer Cell, Bio-Rad, USA). Proteins were detected by using anti-POXA1b (Giardina et al. 1999) antibodies (cross-reacting with POXA3) at a 1:2,000 ratio or anti-small subunit antibodies at 1:1,000 ratio, and peroxidase-conjugated anti-rabbit secondary antisera (1:40,000) (Sigma). The membranes were developed by using SuperSignal West Femto Maximum Sensitivity Substrate detection kit (Pierce).
Enzyme stability
Culture broth samples (containing 0.3 and 0.5 U laccase activities expressed by A3L of A3LS, respectively) were incubated at room temperature and their laccase activities were followed at least for 3 h. Experiments were also carried out on samples whose protein concentration had been increased by addition of BSA up to 0.5 mg ml−1 or up to 1 mg ml−1.
Approximately 0.8 μg of POXA3 small subunit isolated from P. ostreatus (eluted at the end of the gradient of the Phenyl Superose chromatography, Giardina et al. 2007) were added to concentrated culture broth samples (containing 0.3 and 0.5 U laccase activities expressed by A3L of A3LS, respectively) and their laccase activities were followed at least for 3 h.
Protein concentration was determined using the Bio-Rad protein assay with bovine serum albumin as the standard.
Results
The heterologous recombinant expression of the POXA3 large subunit alone and the recombinant co-expression of the POXA3 large and small subunits were performed in K. lactis, a yeast recombinant expression system already successfully set up to express POXC and POXA1b laccase isoenzymes from P. ostreatus (Piscitelli et al. 2005). K. lactis was transformed with the vector A3L (Fig. 1a), containing the cDNA encoding the POXA3 large subunit, to express it under the control of the KlADH4 promoter. The co-expression of the POXA3 large and small subunits was performed using the same expression system by exploiting the bidirectional functioning of the KlADH4 promoter (C. Mazzoni, personal communication). The cDNA encoding the POXA3b small subunit was chosen for these experiments because of the higher stability of the POXA3b complex compared to that of the POXA3a complex observed previously in culture medium of P. ostreatus (Palmieri et al. 2003). The cDNA was inserted in the vector A3L, upstream of KlADH4 promoter generating the vector A3LS (Fig. 1b).
Production of recombinant laccase was firstly tested by assaying the enzymatic activity on plates containing ABTS as substrate. All the transformants carrying the vector A3LS secreted laccase activity, whereas no detectable activity was secreted by any transformant carrying the vector A3L. About 20 recombinant clones from each transformation were grown in liquid culture at 28°C and their laccase activity production was tested. After 10 days, extracellular laccase activity produced by the A3LS transformants ranged from 5 to 10 mU ml−1, whilst no laccase activity was detectable in culture supernatants of any A3L transformant.
The best producing A3LS clone was selected to study the time course of recombinant laccase activity production and the effect of culture conditions on the expression levels. The laccase activity was not detectable until the fifth day and the maximum laccase activity production was observed between the 14th and the 17th day (20 mU ml−1).
To improve laccase production, cultures of the best producing A3LS clone were carried out in the presence of different carbon sources (20 g l−1 glucose or galactose), ethanol (0.5 or 1%), and copper concentrations (0.5 or 1 mM CuSO4), and at different incubation temperatures (20 or 28°C).
The analysed clone was grown in YPPG geneticine medium in the presence of 0.5 mM CuSO4 at different ethanol concentrations: the presence of 0.5% ethanol induced production of laccase activity (10 mU ml−1 after 10 days) more effectively than 1% ethanol (4 mU ml−1 after 10 days). Copper concentration was then varied in the YPPG geneticine and 0.5% ethanol containing medium and the activity increased with copper concentration, reaching 18 mU ml−1 after 10 days in the presence of 1 mM CuSO4. Copper should only affect protein stabilisation, having no effect on yeast growth and protein production, as already demonstrated for other recombinant laccases in K. lactis (Piscitelli et al. 2005).
Influence of the carbon source (20 g l−1 glucose or galactose) on laccase production was then evaluated in the presence of 0.5% ethanol and 1 mM CuSO4, observing a higher production of laccase activity (30 mU ml−1 after 10 days) in glucose-containing medium (YPPD).
Addition of PMSF (0.1 mM) to the culture broth in the optimized conditions did not lead to any change of laccase activity, thus suggesting that proteolysis does not affect protein production.
The effect of the growth temperature on laccase activity production was analysed, performing cultures in the optimized growth medium (geneticine YPPD medium containing 1 mM CuSO4 and 0.5% ethanol) at 20 and 28°C (Fig. 2). Decreasing the growth temperature led to a further increase of laccase activity production (80 mU ml−1 after 10 days). Comparison of growth time courses at the two temperatures showed only a slight delay of yeast growth due to the temperature decrease.
In the optimized conditions for laccase production by the A3LS clone, laccase activity was also detectable in A3L transformants, even if at lower levels (Fig. 2). In fact, the best producing A3L clone exhibited a laccase activity, at the 14th day, that is eightfold lower than that of the A3LS clone (Fig. 2), whereas protein concentration in the culture broths for both clones was comparable (0.16 mg ml−1).
Expression of POXA3 small subunit was also performed in E. coli, producing a soluble form of the protein. The recombinant protein was purified, its identity ascertained by SDS-PAGE and MALDI mass spectra, and used to produce specific antibodies. These antibodies were able to recognize the POXA3 small subunits in P. ostreatus cultures (data not shown).
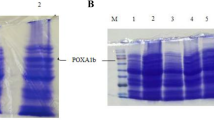
Production and cellular localization of small and large subunits expressed by K. lactis were investigated by Western blot analyses of cellular extracts and culture supernatants withdrawn at the 4th, 7th and 11th growth days of A3L and A3LS clones. As far as the large subunit is concerned, bands of similar intensity were observed analysing the culture supernatants of both clones, while the cellular extracts of A3L clone produced notably more intense bands with respect to those of A3LS clone (Fig. 3a, b). Therefore, it seems that the recombinant large subunit was produced at higher level by the A3L clone, but the protein was poorly secreted. The secreted protein exhibited an apparent molecular mass (Mr) slightly higher than that of the native protein in both A3LS and A3L clones possibly due to different glycosylation patterns, whilst the intracellular forms have similar Mr values. As far as the small subunit is concerned, its expression by A3LS clone was verified by Western blot analyses, thus demonstrating the effective bidirectional functioning of the KlADH4 promoter (Fig. 3c). Two isoforms were detected, differing for their glycosylation moiety, as demonstrated by endo-glycosidase treatment of the protein (lane marked with LSd). The recombinant small subunit was totally secreted, as it was not detectable in the cellular extracts (data not shown).
Western blot analyses: a) cellular extracts (corresponding to 0.3 OD600); b) and c) supernatants (130 ml of similarly grown cultures), withdrawn at different growth days of A3L (lanes marked with L) and A3LS (lanes marked with LS) clones. Antibodies cross-reacting with POXA3 large subunit (a, b) or POXA3 small subunit (c) were used. Lane marked with LSd: culture supernatant of A3LS clone after endo-glycosidase treatment
Laccase activity of A3L and A3LS culture broth samples incubated at room temperature was measured (at 0.16 mg ml−1 protein concentration), revealing a significant higher stability of the A3LS with respect to the A3L samples (residual activity after three hours of 70 and 35%, respectively). Similar behaviours were observed when experiments were carried out on samples whose protein concentration had been increased by addition of BSA up to 0.5 mg ml−1 (residual activity after 3 h of 77 and 48%, for A3LS and A3L, respectively) or up to 1 mg ml−1 (residual activity after 3 h of 94 and 68%, for A3LS and A3L, respectively; Fig. 4).
Time course of residual laccase activity of culture broth samples, incubated at room temperature, of A3L ( ), A3LS (
), A3LS ( ), A3L amended with P. ostreatus POXA3 small subunit (
), A3L amended with P. ostreatus POXA3 small subunit ( ) and A3LS amended with P. ostreatus POXA3 small subunit (
) and A3LS amended with P. ostreatus POXA3 small subunit ( ). Protein concentration was adjusted to 1 mg ml-1. Reported data are the mean values of at least two experiments
). Protein concentration was adjusted to 1 mg ml-1. Reported data are the mean values of at least two experiments
When POXA3 small subunit isolated from P. ostreatus and not showing any laccase activity, (Giardina et al. 2007) was added to the A3L culture broth, an increase of the enzyme stability was observed (Fig. 4). Nevertheless, the addition of the small subunit did not lead to variation of enzyme stability in A3LS culture broth (Fig. 4) and did not increase laccase activity of both A3L and A3LS culture broth samples. These results support the hypothesis that the higher stability of A3LS with respect to the A3L samples is due to the presence of the small subunit.
Discussion
A system for recombinant expression of heterodimeric proteins in the yeast K. lactis has been developed by using an unique expression vector under the transcription control of KlADH4 promoter. Preliminary data from C. Mazzoni and co-workers had suggested a possible bidirectional functioning of the KlADH4 promoter. Successful co-expression of the subunits constituting the heterodimeric laccase POXA3 under the control of this promoter has been verified by immunoblot analyses, thus demonstrating the peculiar performance of the KlADH4 promoter and providing a simple system for co-expression of proteins. Such a system can represent a general tool to study structure–function relationships of heterodimeric proteins.
The singular heterodimeric structure of POXA3 laccase has been investigated, taking advantage of this system. Any attempt aimed at obtaining separation of POXA3 large and small subunits from P. ostreatus had been unsuccessful. Therefore, heterologous expression of the large subunit alone and co-expression of both subunits in K. lactis were chosen as an alternative strategy to compare the properties of the complex to those of the large subunit alone. This approach allowed us to analyse the effect of the presence of the small subunit on the activity and the stability of recombinant POXA3.
The availability of specific antibodies against each POXA3 subunit provided us a tool to compare the amount of the expressed proteins and to determine their cellular localization. The only slightly higher molecular mass of the recombinant secreted large subunit respect to that of the native protein confirmed the low tendency of K. lactis to hyperglycosylate with respect to other yeast hosts. Two forms of the small subunit secreted by K. lactis have been recognised by the specific antibodies, and the smaller form showed an electrophoretic mobility similar to that of the 16 kDa subunit from P. ostreatus (data not shown). The recombinant forms differ in their glycosylation moieties, as demonstrated by de-glycosylation experiments (Fig. 4). It is worth noting that in P. ostreatus small subunits are secreted as two forms, one of which (the 18 kDa subunit) results from glycosylation of the corresponding 16 kDa polypeptide (Giardina et al. 2007). Therefore the same type of heterogeneity due to the occurrence of differently glycosylated forms was also found for the recombinant protein.
Analyses of production and cellular localization of the small and large subunits in A3L and A3LS clones suggested that the large subunit is expressed at higher level by A3L than A3LS. This finding could be due to a better functioning of the promoter working mono-directionally with respect to that promoting expression in both directions.
In optimized growth conditions, laccase activity can be detected in both A3L and A3LS transformed yeast cultures, but the clone expressing the small subunit together with the large one exhibited always notably higher activity than that expressing the large subunit alone. A3LS culture broth samples and A3L culture broth samples amended with POXA3 small subunit from P. ostreatus (Giardina et al. 2007) showed significantly higher stability of laccase activity with respect to that of A3L (Fig. 4). All these findings suggest a role of the small subunit in POXA3 stabilisation. In fact quick loss of the large subunit native structure in the absence of the small subunit could cause the lower activity determined for A3L.
References
Beggah S, Lechenne B, Reichard U, Foundling S, Monod M (2000) Intra- and intermolecular events direct the propeptide-mediated maturation of the Candida albicans secreted aspartic proteinase Sap1p. Microbiology 146:2765–2773
Falcone C, Saliola M, Chen XJ, Frontali L, Fukuhara H (1986) Analysis of a 1.6-micron circular plasmid from the yeast Kluyveromyces drosophilarum: structure and molecular dimorphism. Plasmid 15:248–252
Giardina P, Palmieri G, Scaloni A, Fontanella B, Faraco V, Cennamo G, Sannia G (1999) Protein and gene structure of a blue laccase from Pleurotus ostreatus. Biochem J 341:655–663
Giardina P, Autore F, Faraco V, Festa G, Palmieri G, Piscitelli A, Sannia G (2007) Structural characterization of heterodimeric laccases from Pleurotus ostreatus. Appl Microbiol Biotechnol 75:1293–1300
Hoyt MA, Zhang M, Coffino P (2003) Ubiquitin-independent mechanisms of mouse ornithine decarboxylase degradation are conserved between mammalian and fungal cells. J Biol Chem 278:12135–12143
Jolivalt C, Madzak C, Brault A, Caminade E, Malosse C, Mougin C (2005) Expression of laccase IIIb from the white-rot fungus Trametes versicolor in the yeast Yarrowia lipolytica for environmental applications. Appl Microbiol Biotechnol 66:450–456
Keizer-Gunnink I, Vuorela A, Myllyharju J, Pihlajaniemi T, Kivirikko KI, Veenhuis M (2000) Accumulation of properly folded human type III procollagen molecules in specific intracellular membranous compartments in the yeast Pichia pastoris. Matrix Biology 19:29–36
Kiiskinen LL, Saloheimo M (2004) Molecular cloning and expression in Saccharomyces cerevisiae of a laccase gene from the ascomycete Melanocarpus albomyces. Appl Environ Microbiol 70:137–144
Kiiskinen LL, Kruus K, Bailey M, Ylosmaki E, Siika-Aho M, Saloheimo M (2004) Expression of Melanocarpus albomyces laccase in Trichoderma reesei and characterisation of the purified enzyme. Microbiology 150:3065–3074
Klonowska A, Gaudin C, Asso M, Fournel A, Reglier M, Tron T (2005) LAC3, a new low redox potential laccase from Trametes sp strain C30 obtained as recombinant protein in yeasts. Enzyme Microb Technol 36:34–41
Larrondo LF, Avila M, Salas L, Cullen D, Vicuna R (2003) Heterologous expression of laccase cDNA from Ceriporiopsis subvermispora yields copper-activated apoprotein and complex isoform patterns. Microbiology 149:1177–1182
Liu W, Chao Y, Liu S, Bao H, Qian S (2003) Molecular cloning and characterization of a laccase gene from the basidiomycete Fome lignosus and expression in Pichia pastoris. Appl Microbiol Biotechnol 63:174–181
Mazzoni C, Saliola M, Falcone C (1992) Ethanol-induced and glucose-insensitive alcohol dehydrogenase activity in the yeast Kluyveromyces lactis. Mol Microbiol 6:2279–2286
Miller CA, Martinat MA, Hyman LE (1998) Assessment of aryl hydrocarbon receptor complex interactions using pBEVY plasmids: expression vectors with bi-directional promoters for use in Saccharomyces cerevisiae. Nucleic Acids Res 26:3577–3583
Min KL, Kim YH, Kim YW, Jung HS, Hah YC (2001) Characterization of a novel laccase produced by the wood-rotting fungus Phellinus ribis. Arch Biochem Biophys 392:279–286
Pakkanen O, Hämäläinen ER, Kivirikko KI, Myllyharju J (2003) Assembly of Stable Human Type I and III Collagen Molecules from Hydroxylated Recombinant Chains in the Yeast Pichia pastoris. Effect of an engineered C-terminal oligomerization domain foldon. J Biol Chem 278:32478–32483
Palmieri G, Cennamo G, Faraco V, Amoresano A, Sannia G, Giardina P (2003) Atypical laccase isoenzymes from copper supplemented Pleurotus ostreatus cultures. Enzyme Microb Technol 33:220–230
Piscitelli A, Giardina P, Mazzoni C, Sannia G (2005) Recombinant expression of Pleurotus ostreatus laccases in Kluyveromyces lactis and Saccharomyces cerevisiae. Appl Microbiol Biotech 69:428–439
Record E, Punt PJ, Chamkha M, Labat M, van Den Hondel CA, Asther M (2002) Expression of the Pycnoporus cinnabarinus laccase gene in Aspergillus niger and characterization of the recombinant enzyme. Eur J Biochem 269:602–609
Saliola M, Mazzoni C, Solimando N, Crisà A, Falcone C, Jung G, Fleer R (1999) Use of the KlADH4 promoter for ethanol dependent production of recombinant human serum albumin in Kluyveromyces lactis. Appl Environ Microbiol 65:53–60
Salo H, Sievi E, Suntio T, Mecklin M, Mattila P, Renkonen R, Makarow M (2005) Co-expression of two mammalian glycosyltransferases in the yeast cell wall allows synthesis of sLex. FEMS Yeast Research 5:341–350
Sen Gupta C, Dighe RR (1999) Hyperexpression of biologically active human chorionic gonadotropin using the methylotropic yeast, Pichia pastoris. J Mol Endocrinol 22:273–283
Sigoillot C, Record E, Belle V, Robert JL, Levasseur A, Punt PJ, Van Den Hondel CA, Fournel A, Sigoillot JC, Asther M (2004) Natural and recombinant fungal laccases for paper pulp bleaching. Appl Microbiol Biotechnol 64:346–352
Soden DM, O’Callaghan J, Dobson AD (2002) Molecular cloning of a laccase isozyme gene from Pleurotus sajor-caju and expression in the heterologous Pichia pastoris host. Microbiology 148:4003–4014
Wahleithner JA, Xu F, Brown KM, Brown SH, Golightly EJ, Halkier T, Kauppinen S, Pederson A, Schneider P (1996) The identification and characterization of four laccases from the plant pathogenic fungus Rhizoctonia solani. Curr Genet 29:395–403
Yaver DS, Xu F, Golightly EJ, Brown KM, Brown SH, Rey MW, Schneider P, Halkier T, Mondorf K, Dalboge H (1996) Purification characterization, molecular cloning, and expression of two laccase genes from the white rot basidiomycete Trametes villosa. Appl Environ Microbiol 62:834–841
Acknowledgment
This work was supported by the European Commission, Sixth Framework Program (SOPHIED contract NMP2-CT2004-505899), by grants from the Ministero dell’Università e della Ricerca Scientifica (Progetti di Rilevante Interesse Nazionale, PRIN), INTAS (International Association for the promotion of cooperation with scientists from the New Independent States of the former Soviet Union, ref. no. 03-51-5889) and from Centro Regionale di Competenza BioTekNet. The authors thank Prof. Claudio Falcone for kindly making available K. lactis strain and plasmid, and Dr Cristina Mazzoni for her personal communication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faraco, V., Ercole, C., Festa, G. et al. Heterologous expression of heterodimeric laccase from Pleurotus ostreatus in Kluyveromyces lactis . Appl Microbiol Biotechnol 77, 1329–1335 (2008). https://doi.org/10.1007/s00253-007-1265-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1265-5






 ) and 20°C (
) and 20°C ( ) and the A3L clone at 20°C (
) and the A3L clone at 20°C ( ). No laccase activity was detected in culture supernatants of A3L clones at 28°C. Reported data are the mean values of at least two experiments
). No laccase activity was detected in culture supernatants of A3L clones at 28°C. Reported data are the mean values of at least two experiments
