Abstract
Artemisinin isolated from the aerial parts of Artemisia annua L. is a promising and potent antimalarial drug which has a remarkable activity against chloroquine-resistant and chloroquine-sensitive strains of Plasmodium falciparum, and is useful in treatment of cerebral malaria. Because the low content (0.01–1 %) of artemisinin in A. annua is a limitation to the commercial production of the drug, many research groups have been focusing their researches on enhancing the production of artemisinin in tissue culture or in the whole plant of A. annua. This review mainly focuses on the progresses made in the production of artemisinin from A. annua by biotechnological strategies including in vitro tissue culture, metabolic regulation of artemisinin biosynthesis, genetic engineering, and bioreactor technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Being the world’s most severe parasitic infection, malaria caused more than a million deaths and 500 million cases annually. Despite tremendous efforts for the control of malaria, the global morbidity and mortality have not been significantly changed in the last 50 years (Riley 1995). The key problem is the failure to find effective medicines against malaria. Obtained from a Chinese medicinal plant Artemisia annua L., artemisinin, a sesquiterpene lactone containing an endoperoxide bridge (Fig. 1), has become increasingly popular as an effective and safe alternative therapy against malaria (Luo and Shen 1987). Artemisinin and its derivatives are effective against multidrug-resistant Plasmodium falciparum strains mainly in Southeast Asia and more recently in Africa, without any reported cases of resistance (Krishna et al. 2004). In the treatment of severe malaria, intravenous artesunate is more rapidly acting than intravenous quinine in terms of parasite clearance, and it can reduce mortality (Dondorp et al. 2006). Artemisinin is acting rapidly to the asexual stages of P. falciparum, the most malignant form of malaria. Because of the emerging resistance of P. falciparum to conventional antimalarial drugs (quinine and chloroquine), artesunate plus amodiaquine (ASAQ) and artemether–lumefantrine (AL) are the main artemisinin-based combination therapy (ACT) candidates for the treatment of drug-resistant malaria in Africa (Mårtensson et al. 2005). Meanwhile, a clear understanding of the mechanism of artemisinin action is an ongoing pursuit. Specific reaction of artemisinin with TCTP (translationally controlled tumor protein), inhibition of the SERCA (sarco/endoplasmic reticulum Ca2+-ATPase) orthologue (PfATP6) of P. falciparum, and inhibition of P. falciparum cysteine proteases have been proposed to contribute to its drug activity (Kannan et al. 2005). PfATP6 is thought to be the real molecular target of artemisinin in spite of some disagreements (Eskstein-Ludwig et al. 2003). Jung et al. (2005) reported the three-dimensional structure of PfATP6 by using homology modeling. A single amino acid (Leu263) in transmembrane segment 3 of SERCAs is proposed to determine susceptibility to artemisinin (Uhlemann et al. 2005). Artemisinin has been shown to selectively kill cancer cells and human cancer. In the recent study, the efficacy of a combined treatment of dihydroartemisinin (DHA artemisinin analog) and butyric acid at low doses in killing cancer cells offered a less toxic, inexpensive, and effective cancer chemotherapy (Singh and Lai 2005). Because artemisinin is a complex molecule and its synthesis is not economic, many efforts have been made to prepare simpler antimalarial molecules based on the trioxane ring of artemisinin. The most promising compounds up to date, OZ 277, consist of a trioxane ring attached to an adamantine ring (Vennerstrom et al. 2004; Dong et al. 2005).
A. annua, belonging to Compositae family, is an annual herb native to China and grows naturally as a part of steppe vegetation in northern parts of China at 1,000–1,500 m above the sea level (Wang 1961). It is single stemmed with alternate branches reaching more than 2 m in height. Its leaves are deeply dissected and range from 2–5 cm in length. The earliest report on the use of A. annua was in the Recipes for 52 kinds of diseases found in the Mawangdui Han Dynasty Tomb dating from 168 B.C. Later, a decoction of A. annua was suggested in the Wenbing Tiaobian in 1798 as a treatment for malaria (Abdin et al. 2003). The isolation and characterization of artemisinin from A. annua are considered as one of the most novel discoveries in recent medicinal plant research (Charles et al. 1990). Qinghaosu Antimalaria Coordinating Research Group (1979) reported that they treated 2,099 cases of malaria with different dosage forms of artemisinin, leading to the clinical cure of all patients. In addition, 143 cases of chloroquine-resistant falciparum malaria and 141 cases of cerebral malaria were treated with good results. Nowadays, artemisinin and its derivatives are considered as part of the treatment for malaria in Africa by WHO (Shetty 2004). Because artemisinin production by organic synthesis is complicated with low yields and high cost (Liu et al. 2005), many researchers focus their studies on enhancing the production of artemisinin in cell/tissue culture or whole plants of A. annua. Most recently, efforts to produce artemisinin in recombinant bacteria by fermentation have also been explored.
Biosynthesis of artemisinin
Artemisinin has been reported to accumulate in the leaves, small green stems, buds, flowers, and seeds of A. annua. Its content varies with the plant developmental stages. There are mainly two suggestions on the stage of the highest content of artemisinin in plant development (Abdin et al. 2003): one is that the highest content of artemisinin is reached before plant flowering, while the other is that it is reached in the full flowering period. Wang et al. (2004) studied on the effects of fpf1 gene (flowering promoting factor1) on A. annua flowering time and the linkage between flowering and artemisinin biosynthesis. They found that flowering was not a necessary factor for increasing the artemisinin content, and the best harvest time was the period between the later vegetative growth stage and the occurrence of flowering bud.
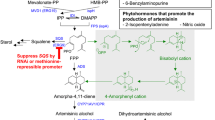
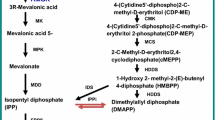
Artemisinin is an endoperoxide sesquiterpene lactone which belongs to isoprenoid group of compounds. The isoprenoid pathway is one of the most important biosynthetic pathways in plants (Fig. 2). Terpenoids are derived from two common precursors, isopentenyl diphosphate (IPP) and its isomer, dimethylallyl diphosphate (DMAPP). It has been established that higher plants have two independent biosynthetic pathways leading to the formation of IPP: the cytosolic mevalonate pathway and the plastid-localized mevalonate-independent pathway (Liu et al. 2005). Recently, two gene clones encoding for deoxy-d-xylulose-5-phosphate synthase (DXPS) and deoxy-d-xylulose-5-phosphate reductoisomerase (DXPR) isolated from transformed hairy roots of A. annua demonstrated the plastid-localized terpenoid biosynthetic pathway (Krushkal et al. 2003; Souret et al. 2002). As a result, the mevalonate pathway is not thought to be the sole route to the synthesis of artemisinin in A. annua. In the mevalonate pathway, three molecules of acetyl-coenzyme A couple together to yield 3-hydroxy-3-methylglutaryl CoA(HMG-CoA), which is subsequently reduced by the enzyme HMG-CoA reductase (HMGR) to yield mevalonic acid (MVA). Then, under the catalysis of mevalonate kinase, mevalonate 5-diphosphate is subsequently decarboxylated to yield IPP (Newman and Chappell 1999). Upon the synthesis of IPP and DMAPP by either MVA or DXP pathways, the next step is chain elongation. The carbonium ion is a potent alkylating agent that can react with IPP, giving geranyl diphosphate (GPP). GPP has the active allylic phosphate group and can further react with IPP to produce farnesyl diphosphate (FPP). FPP takes part in a cyclization reaction catalyzed by cyclases to produce various isoprenoid final products such as artemisinin (Barkovich and Liao 2001). After FPP synthesis, the biochemical reactions and enzymes involved have not been fully understood and well characterized. Akhila et al. (1987) proposed a complete biosynthetic pathway for artemisinin, starting from mevalonic acid and IPP. The following biosynthetic pathway was suggested: farnesyl pyrophosphate (FPP), germacrane skeleton, dihydrocostunolide, cadinanolide, arteannuin B, and artemisinin. The content of artemisinic acid is eight to ten times of artemisinin content in A. annua. Therefore, it has been suggested that artemisinic acid is a possible biosynthesis precursor for both artemisinin B and artemisinin. Sangwan et al. (1993) reported the transformation of artemisinic acid to artemisinin B and artemisinin both in vivo and in a cell free system. EI-Feraly et al. (1986) converted artemisinic acid to artemisinin B by single oxygen generated through sensitized photo-oxygenation. Artemisinin B has been considered as another precursor for artemisinin because it was converted to artemisinin using crude and semipurified cell-free extracts of the leaf homogenate of A. annua (Nair and Basile 1993; Martinez and Staba 1988). Wallaart et al. (1999a,b) isolated dihydroartemisinic acid and dihydroartemisinic acid hydroperoxide in A. annua with yields of 66 and 29 %, respectively. Dihydroartemisinic acid could be chemically converted to artemisinin under conditions that may also be present in the living plant. The presence of dihydroartemisinic acid hydroperoxide and dihydroartemisinic acid in the plant and the conditions under which dihydroartemisinic acid can be converted into dihydroartemisinic acid hydroperoxide that can very easily be oxidized to artemisinin provide an evidence for a nonenzymatic, photochemical conversion of dihydroartemisinic acid into artemisinin.
Proposed pathway of artemisinin biosynthesis (Liu et al. 2005; Bertea et al. 2005). CMK 4-(Cytidine 5′-diphospho)-2-C-methyl-d-erythritol kinase, CMS 2-C-methyl-d-erythritol 4-phosphate cytidyl transferase, DXR 1-deoxy-d-xylulose 5-phosphate reductoisomerase, DXS 1-deoxy-d-xylulose 5-phosphate synthase, FPPS farnesyl diphosphate synthetase, GPPS geranyl diphosphate synthase, HMGR 3-hydroxy-3-methylglutaryl coenzyme A(HMG-CoA) reductase; HMGS HMG-CoA synthase; IDS isopentenyl diphosphate synthase, MCS 2-C-methyl-d-erythritol 2,4-cyclodiphosphate synthase, MDD mevalonate diphosphate decarboxylase, MK mevalonate kinase, MPK mevalonate-5-phosphate kinase, SES sesquiterpene synthase
Bertea et al. (2005) identified intermediates and enzymes involved in the biosynthesis of artemisinin in A. annua. They hypothesized that the early steps in artemisinin biosynthesis involved amorpha-4,11-diene hydroxylation to artemisinic alcohol, followed by oxidation to artemisinic aldehyde, and reduction of the C11–C13 double bond to dihydroartemisinic aldehyde and oxidation to dihydroartemisinic acid. A cDNA clone encoding a cytochrome P450 designated CYP71AV1 was characterized to catalyze the oxidation of the biosynthetic intermediates amorpha-4,11-diene, artemisinic alcohol, and artemisinic aldehyde in 2006 (Teoh et al. 2006). Several key enzymes involved in the biosynthesis of artemisinin have been discovered (Liu et al. 2006), such as 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR), farnesyl diphosphate synthase (FPPS), and sesquiterpene synthase (SES). HMGR catalyzed HMG-CoA to yield MVA. Because mevalonate synthesis is an irreversible reaction, the step catalyzed by HMGR is usually considered to be rate limiting in both plant and animal. There have been many reports on the correlation of synthesis of sesquiterpene and the activity of HMGR which determines the direction of carbon flow (Chappell et al. 1995). The genes coding for HMGR have also been cloned (Kang et al., Genbank Accession No. U14624, U1465). FPPS is another key enzyme which belongs to 1,4-isoprenoid-transforming enzyme. It catalyzes geranyl diphosphate and isopentenyl diphosphate to yield farnesyl diphosphate. The gene for FPPS was cloned in 1996 which encodes for 343 amino acids (39.42 kDa; Matsushita et al. 1996). SES is a key branch point enzyme in the biosynthesis of sesquiterpenes from isoprenoid intermediate farnesyl diphosphate. In A. annua, there is a gene family coding for sesquiterpene cyclases. FPP is cyclized by the action of sesquiterpene cyclase to amorpha-4,11-diene, a possible biosynthetic route from FPP to artemisinin. Bouwmeester et al. (1999) first isolated amorpha-4,11-diene synthetase from pentane extract of A. annua leaves. This partially purified enzyme shows the typical characteristics of SES, such as a broad pH optimum around 6.5–7.0 and a molecular mass of 56 kDa. In 2000, the gene for amorpha-4,11-diene synthetase was cloned and expressed in Escherichia coli (Mercke et al. 2000). Dhingra and Narasu (2001) partially purified and characterized the enzyme which carried out the peroxidation reaction involved in artemisinin biosynthesis of arteannuin B to artemisinin. The enzyme has a K m of 0.5 mM for arteannuin B and a pH optima between 7.0 and 7.2.
Biotechnological production of artemisinin
The commercial sources of most artemisinin are from field-grown leaves and flowering tops of A. annua, which are subjected to seasonal and somatic variation and infestation of bacteria, fungi, and insects that can affect the functional medicinal content of this plant (Klayman 1985; Luo and Shen 1987). The total organic synthesis is very complicated with low yields, and economically unattractive (Avery et al. 1992; Xu et al. 1986). In view of these problems, artemisinin production from in vitro plant tissue culture has been considered as an attractive alternative. The biosynthesis of artemisinin was studied in the calli, suspension cells, shoots, and hairy roots of A. annua during their cultivation in vitro (He et al. 1983; Tawfiq et al. 1989; Weathers et al. 1994; Paniego and Giulietti 1996; Liu et al. 1997; Nair et al. 1986; Teo et al. 1995).
A certain degree of differentiation of A. annua tissue cultures is a prerequisite for the synthesis of artemisinin. Paniego and Giuletti (1994) reported that no artemisinin was found in cell suspension cultures of A. annua, whereas trace amounts were found in the multiple shoot cultures. Woerdenbag et al. (1993) reported a high percentage of artemisinin content in A. annua shoots cultured on 1/2 MS medium supplemented with 0.05 mg/l naphthaleneacetic acid, 0.2 mg/l benzyladenine (BA), and 2 % sucrose. The flowering of A. annua was observed in vitro by supplementing with gibberellic acid (GA3) where artemisinin content reached 0.1 % in A. annua plantlets, and the highest artemisinin content in the plantlets was observed in full bloom (Gulati et al. 1996). Most groups did not find artemisinin in root part of A. annua plant. However, artemisinin content in the shoot part of cultured plantlet was higher than that in the cultured shoots without roots (Ferreira and Janick 1996; Martinez and Staba 1988).
Attempts were also made to improve the artemisinin production by optimizing chemical and physical environmental factors. Wang and Tan (2002) reported the influence of the ratio of NO3/NH4 and total initial nitrogen concentration on the artemisinin yield in hairy roots. With the ratio of NO3/NH4 at 5:1(w/w), the optimum concentration of total nitrogen for artemisinin production was 20 mM. Under this concentration, artemisinin production was 57 % higher than that in the standard MS medium. Weathers’ research group investigated the effects of media sterilization method and types of sugar on growth and artemisinin accumulation of A. annua hairy roots. They found that biomass from filter-sterilized medium was greater than that from autoclaved medium, but artemisinin accumulation from filter-sterilized medium was less than that from autoclaved medium. Growth of hairy roots in the medium with sucrose (3.99 g DW/l) was equivalent to the growth in the medium with fructose (3.75 g DW/l) and significantly better than in the medium with glucose (2.16 g DW/l), while the roots that grew in glucose showed a dramatic stimulation in artemisinin content which is three- and twofold higher than that in medium with sucrose and fructose (Weathers et al. 2004). Casein hydrolysate, a source of amino acids and oligopeptides, at low concentration enhances artemisinin production in A. annua shoot cultures (Woerdenbag et al. 1993). A combination of BA and kinetin increased the yields of artemisinin in cultured shoots by 3.6 and 2.6 fold (Whipkey et al. 1992). GA3, a plant hormone that can induce blooming, has been reported to improve growth and artemisinin biosynthesis in shoot cultures, root cultures, and plantlets of A. annua (Fulzele et al. 1995; Charles et al. 1990; Smith et al. 1997; Weathers et al. 2005). The effects of light irradiation on growth and production of artemisinin were studied in hairy root cultures of A. annua L. by Liu et al. (2002). They found that when the hairy roots were cultured under illumination of 3,000 lx for 16 h using several cool-white fluorescent lamps, the dry weight and artemisinin concentration reached 13.8 g/l and 244.5 mg/l, respectively (Liu et al. 2002). Wang et al. (2001) investigated the dependence of biomass of hairy roots and artemisinin content on the light spectrum. They found that the highest biomass (5.73 g DW/l) and artemisinin content (31 mg/g) were obtained under red light at 660 nm which were 17 and 67 % higher than those obtained under white light, respectively. Temperature in the range of 15–35 °C also affected growth and artemisinin biosynthesis in the cultured A. annua hairy roots. The maximum hairy root growth was found at 25 °C. However, the highest artemisinin content in the root cultures was observed at 30 °C (Guo et al. 2004).
Enhancing the artemisinin production by precursor feeding was also investigated. Addition of artemisinin precursors to the medium used for tissue cultures of A. annua resulted in a fourfold increase of artemisinin in the tissue and an 11-fold increase of artemisinin in the spent medium (Weathers et al. 1994). The feeding of mevalonic acid alone, however, did not induce an enhancement of artemisinin production Woerdenbag et al. 1993. But the addition of some compounds such as naphtiphine (an inhibitor of the enzyme squalene epoxidase) to the medium improved the artemisinin production. Other additions, such as 5-azacytidine (a gene regulator), colchicine (a gene regulator), miconazole (an inhibitor of sterol demethylase), and terbinaphine (an inhibitor of the enzyme squalene epoxidase), were too toxic for the cultures to induce an enhancement of the artemisinin production (Woerdenbag et al. 1993). Kudakasseril et al. (1987), however, reported a concentration-dependent increase in the levels of artemisinin and growth of shoot cultures with miconazole. Other sterol inhibitors, such as chlorocholine chloride, 2-isopropyl-4-(trimethylammonium chloride)-5-methylphenylpiperidinecarboxylate, and 4-chloro-2-(2-diethylaminoethoxyphenyl)-2-(4-methyl-phenyl)-benzene-ethanol, increased both the incorporation of 14C-IPP into artemisinin by cell-free extracts and the production of artemisinin in shoot culture of A. annua. Sterol inhibitors inhibited the enzyme in the mevalonate pathway, resulting in increased terpenoid production rather than sterol production.
To develop more potent antimalarial agents with improved in vivo stability, tremendous efforts have been made toward structure modification of artemisinin and analogue synthesis. Due to the difficulties of structural modification by conventional chemical methods, microbial transformation serves as a valuable tool that comes to play an important role in the modification. To date, a number of oxidating products of artemisinin at different positions of artemisinin structure have been reported. These transformations include conversion to 3α-hydroxy-deoxyartemisinin and deoxyartemisinin, conversion to 9β-hydroxy-artemisinin and 3α-hydroxy-artemisinin, and conversion to 10-hydroxy-artemisinin and 9β-hydroxy-11α-artemisinin. In addition, microbial transformations on some artemisinin analogue, such as artemether, arteether, artemisitene, and 12-deoxoartemisinin, have been reported to produce oxidative products by different microorganisms (Liu et al. 2006).
Genetic engineering of A. annua
It is attractive to produce transgenic plants of A. annua, which ensures a constant high production of artemisinin by overexpressing the enzymes in the terpene biosynthetic pathway or by inhibiting an enzyme of another pathway competing for artemisinin precursors. In recent years, there is remarkable progress in the molecular regulation of artemisinin biosynthesis (Bouwmeester et al. 1999). The genes of the key enzymes involved in the biosynthesis of artemisinin, such as farnesyl diphosphate synthase (FPS), amorpha-4,11-diene synthase (AMS), and the genes of the enzymes relevant to the biosynthesis of artemisinin including squalene synthase (SQS), have been cloned from A. annua (Matsushita et al. 1996; Mercke et al. 2000; Wallaart et al. 2001; Liu et al. 2003b). The enzyme involved in biochemical transformation of arteannuin B to artemisinin was purified by Dhingra and Narasu (2001). By genetic engineering, the key enzymes involved in biosynthesis of artemisinin, such as FPS and AMS, can be overexpressed, and artemisinin production was significantly enhanced in the transgenic high-yield A. annua.
In 1994, Weathers et al. (1994) and Qin et al. (1994) induced hairy root from A. annua with Agrobacterium rhizogenes; furthermore, the factors influencing A. rhizogenes were explored to optimize the transformation system by Liu et al. (1998a). Xie et al. (2001) induced hairy root from A. annua leaf blade pieces and petiole segments infected with A. rhizogenes strain 1601. A clone with high content of 1.195 mg/g DW was obtained. In 1996, Vergauwe et al. (1996) developed an Agrobacterium tumefaciens-mediated transformation system for A. annua L. plants with high transformation rates (75 % regenerants harboring foreign gene). Artemisinin content in the leaves of regenerated plant was 0.17 % DW, a little bit higher than that presented in the leaves of normally cultured plants (0.11 % DW). In 1998, they investigated the factors influencing A. tumefaciens-mediated transformation of A. annua, which included the age of the explant, A. tumefaciens strain, and plant genotype (Vergauwe et al. 1998). Han et al. (2005) established a system of high efficiency of genetic transformation and regeneration of A. annua via A. tumefaciens. They investigated the factors, such as composition of infection bacterium suspension, methods of co-cultivation, and co-cultivation period, to optimize the transformation system. Transgenic frequency of fascicled shoots reached 4–10 %. In 1999, Chen et al. (1999) reported transferring green fluorescent protein (GFP) reporter gene into A. annua with A. tumefaciens and regenerating transgenic plants. They induced fascicled shoot from the leaves, buds, and flowers. They found that artemisinin production with the shoot cultures induced from flower organ explants was two times of that from the leaves (Geng et al. 2001a).
Artemisinic acid is one of the precursors of artemisinin, which has the cadinene structure. Chen et al. (1998) transformed a cotton cadinene synthase cDNA into the leaf explants of A. annua using A. rhizogenes. The enhanced artemisinin accumulation was observed in the transgenic plantlets. In the isoprenoid biosynthesis pathway, farnesyl diphosphate synthase (FDS) catalyzes condensation of IPP with DMAPP (Cane 1990). Recently, cDNAs encoding FDS have been isolated from a number of plant species, including Arabidopsis thaliana (Delourme et al. 1994) and Lupinu albus (Attucci et al. 1995). Because 15-carbon FPP can be catalyzed by sesquiterpene cyclases to form cyclic sesquiterpenoids, overexpressing FDS by introducing a foreign gene into A. annua holds the possibility of affecting accumulation of artemisinin. A cDNA encoding cotton FPPS placed under a CaMV 35S promoter was transferred into A. annua via A. tumefaciens strain LBA 4404 or A. rhizogenes strain ATCC 15834 by Chen et al. (1999, 2000). In the transgenic plants, the concentration of artemisinin is approximately 8–10 mg/g DW, which is two- to threefold higher than that in the control.
Cytokinins are a group of plant hormones that regulate many aspects of plant growth and development, including cell division, cell enlargement, chloroplast development, senescence, and cell differentiation (Binns 1994; Mok and Mok 1994). The A. tumefaciens cytokinin biosynthetic gene codes for the enzyme isopentenyl transferase (ipt), and this enzyme catalyzes the condensation of isopentenyl pyrophosphate and adenosine monophosphate (AMP) giving isopentenyl AMP (Akiyoshi et al. 1984). Isopentenyl transferase may represent the rate-limiting step in cytokinin biosynthesis in tumorous plant tissue (Akiyoshi et al. 1983). Many reports have shown that the overexpression of ipt gene was a feasible way to elevate cytokinin levels in transgenic plants (Mckenzie et al. 1998). Previous studies indicated that capitate glands on the leaf surface (Duke et al. 1994) and specialized chloroplasts of the capitate gland appeared to play very important roles in artemisinin biosynthesis (Duke and Paul 1993). The effect of isopentenyl transferase gene expression on the physiological and biochemical characteristics of A. annua L. was studied by Geng et al. (2001b). It was found, in the ipt gene expression, that cytokinins, chlorophyll, and artemisinin contents increased by different degrees. Content of cytokinins was elevated two- to threefold, chlorophyll 20–60 %, and artemisinin 30–70 % compared with the control plants. A direct correlation was found between the content of cytokinins, chlorophyll, and artemisinin.
Because plant tissue extractions typically yield low terpenoid concentrations, many researchers sought an alternative method to produce terpenoid compounds in a microbial host. Several research groups have described the engineering of the DXP pathway to increase the supply of isoprenoid precursors needed for high-level production of carotenoids in E. coli (Farmer and Liao 2001; Kajiwara et al. 1997; Kim and Keasling 2001). Balancing the pool of glyceraldehyde-3-phosphate and pyruvate, or increasing the expression of 1-deoxy-d-xylulose 5-phosphate synthase (DXS; encoded by the gene dxs) and IPP isomerase (encoded by idi), resulted in increased carotenoid buildup in the cell. Martin et al. (2003) engineered the expression of a synthetic amorpha-4,11-diene synthase gene and the mevalonate isoprenoid pathway from Saccharomyces cerevisiae in E. coli. Concentrations of amorphadiene, the sesquiterpene olefin precursor to artemisinin, reached 24 μg caryophyllene equivalent/ml. Although total biosynthesis of artemisinin was not achieved in their study, the engineered biochemical pathway could be extended to produce artemisinic acid which can then be converted easily to artemisinin by the method of chemical synthesis. The simultaneous expression of a synthetic amorphadiene synthase gene in their engineered strain resulted in high-level production of amorphadiene and alleviated growth inhibition. Because IPP and DMAPP are the universal precursors to all isoprenoids, the strains they reported can serve as platform hosts for the production of any terpenoid compound for which the biosynthetic genes are available.
Artemisinin production in bioreactor
Hairy roots, the result of genetic transformation by A. rhizogenes, have attractive properties for secondary metabolite production. Hairy root cultures provide a promising alternative to the biotechnological exploitation of plant cell cultures. Their characteristic biosynthetic capacity for secondary metabolite production, inherent genetic stability reflected in stable productivity, and the possibility of genetic manipulation to increase biosynthetic capacity have attracted considerable attentions, both as a fundamental research tool and as a source of valuable products (Kim et al. 2002). Conversely, one of the most important limitations for the commercial exploitation of hairy roots is the development of technologies for large-scale culture, specifically the design of bioreactors that permit the growth of interconnected and nonhomogeneous root cultures which are unevenly distributed throughout the vessel.
In 1998, our research group developed different types of bioreactors to produce artemisinin from A. annua L. hairy roots and compared the root growth and artemisinin accumulation in the bubble column, modified bubble column, and modified inner-loop airlift bioreactor with three stainless steel meshes with 2-mm pore size fixed along the height of the column (Liu et al. 1998b,c). Experimental results showed that the hairy root cultures in the modified inner-loop airlift bioreactor grew more homogeneously between the two meshes than those in other bioreactors. The growth rate of the hairy roots and the productivity of artemisinin were higher than those in flasks. Recently, Weathers’ research group developed nutrient mist bioreactor using ultrasonic transducers to produce nearly three times as much artemisinin as the hairy roots grown in bubble column bioreactors (Kim et al. 2001, 2002). To improve the content of artemisinin, mist cycle, carrier gas, and nutrient compositions were optimized (Wyslouzil et al. 2000). In 2003, Souret et al. (2003) investigated the expression levels of four key terpenoid biosynthetic genes: HMGR, DXs, DXR, and FPS in a mist reactor while a combination of multiple factors were involved.
Some experimental bioreactors for shoot cultures have been developed with the aim of reducing production costs and maximizing plant growth (Woo and Park 1993; Liu et al. 2004). However, the use of bioreactors for large-scale cultivation is limited because of the high costs and abnormal shoot morphogenesis associated with liquid culture (Aitken-Christie et al. 1995). Most shoot cultures are sensitive to shearing stress (Liu et al. 2000) and tend toward vitrification when they are in liquid medium over a prolonged period of time. Therefore, it is necessary to select a bioreactor configuration that can satisfy the biological requirements and engineering needs for the shoot culture. Liu et al. (2003a) found a significant difference correlated with reactor type and cultivation mode in the growth and artemisinin biosynthesis of A. annua L. shoot cultures. The shoot cultures in both the nutrient mist bioreactor and the multiplate radius-flow bioreactor showed excellent growth, but those in the modified inner-loop airlift bioreactor resulted in hyperhydration due to the poor respiration and severe physical stress of shoots submerged in liquid media for a long time.
Outlook
In the last 25 years or so, a number of significant advances have been made to control malaria. The discovery of artemisinin and its analogues as potent antimalarial agents is of immense importance (Wright 2005). Numerous investigations have been carried out to explain the mechanism of action, determine efficient clinical uses, and discover new drugs with better pharmacological properties. Several strategies have been developed for the design of semisynthetic and synthetic endoperoxides with greater metabolic and hydrolytic stability than artemisinin itself (Begue and Bonnet 2005). A better understanding of mechanisms of (multi-) drug resistance is urgent to monitor. There are many recent advances in the identification of molecular markers that can be employed in predicting in vitro and in vivo resistance in Southeast Asia (Uhlemann and Krishna 2005). Artemisinin-based combination therapies (ACTs) have been long considered more effective than the existing drugs. ACTs are much more expensive than other drugs because of the relatively low yields of artemisinin in A. annua. Therefore, there have been many efforts to enhance the production of artemisinin in vivo and in vitro by biotechnology. Even though viable methods of increasing artemisinin content, e.g., A. annua organ culture, hormone medium, and metabolic manipulation, have been investigated, and show potential for future development, the improvements delivered by these methods have not yet met the demand. To increase the yield of artemisinin by biotechnology, it is necessary to study the enzymatic pathway. Enzymes and precursors involved in the artemisinin biosynthesis have to be isolated and characterized. In recent years, many researchers have focused their efforts on investigating the molecular regulation of artemisinin biosynthesis and the genes coding for the key enzymes involved in the artemisinin biosynthesis. The high efficiency of genetic transformation and regeneration procedure developed by Han et al. (2005) allows the manipulation of artemisinin biosynthesis by genetic methods. By genetic engineering, we can overexpress the key enzymes involved in biosynthesis of artemisinin, or inhibit the enzymes involved in other pathways competing for its precursors to obtain transgenic high-yield A. annua. Although greatly improved yields were obtained by combining the expression of a synthetic sesquiterpene synthase with a recombinant mevalonate pathway, the data suggest that a maximum yield was not attained. Furthermore, in vitro evolution and combinatorial biosynthesis of sesquiterpene biochemical pathways in microbes may lead to artemisinin derivatives or even new sesquiterpene compounds. Appropriate bioreactor technology is essential to produce artemisinin in large scale. Although different types of bioreactor have been used to produce artemisinin, there are relatively few detailed studies relating oxygen and nutrient transfer, hydrodynamic behavior, and mixing characteristics in the plant organ and tissue culture. Studies in both genetic engineering to increase artemisinin content and novel bioreactors for producing artemisinin in large scale are urgently needed.
References
Abdin MZ, Israr M, Rehman RU, Jsin SK (2003) Artemisinin, a novel antimalarial drug: biochemical and molecular approaches for enhanced production. Planta Med 69:289–299
Aitken-Christie J, Kozai T, Takayama S (1995) Automation in plant tissue culture-general introduction and overview. In: Aitken-Christie J, Kozai T, Smith MAL (eds) Automation and environmental control in plant tissue culture. Kluwer, Boston, MA, pp 1–18
Akhila A, Thakur RS, Popli SP (1987) Biosynthesis of artemisinin in Artemisia annua. Phytochemistry 16:1927–1930
Akiyoshi DE, Morris RO, Mischke BS, Kosuge T, Garfinkel D, Gordon MP, Nester EW (1983) Cytokinin auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc Natl Acad Sci USA 80:407–411
Akiyoshi DE, Klee H, Amasino R, Nester EW, Gordon MP (1984) T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci USA 81:5994–5998
Attucci S, Aitken SM, Ibrahim RK, Gulick PJA (1995) cDNA encoding farnesyl pyrophosphate synthesis in white lupine. Plant Physiol 108:835–836
Avery MA, Chong WKM, Jennings-White C (1992) Stereoselective total synthesis of (+)-artemisinin, the antimalarial constituent of Artemisia annua L. J Am Chem Soc 114:974–979
Barkovich R, Liao JC (2001) Metabolic engineering of isoprenoids. Metab Eng 3:27–39
Begue JP, Bonnet DD (2005) The future of antimalarials: artemisinins and synthetic endoperoxides. Drugs Future 30:509–518
Bertea CM, Freije JR, van der Woude H, Verstappen FWA, Perk L, Marquez V, DeKraker JW, Posthumus MA, Jansen BJM, de Groot A, Franssen MCR, Bouwmeester HJ (2005) Identification of intermediates and enzymes involved in the early steps of artemisinin biosynthesis in Artemisia annua. Planta Med 71:40–47
Binns AN (1994) Cytokinin accumulation and action: biochemical, genetic, and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 45:173–196
Bouwmeester HJ, Wallaart TE, Janssen MHA, Loo B, Jansen BJM, Schmidt MAPCO, Kraker JWD, König WA, Franssen MCR (1999) Amorpha-4,11-diene synthase catalyses the first probable step in artemisinin biosynthesis. Phytochemistry 52:843–854
Cane DE (1990) Enzymatic formation of sesquiterpenes. Chem Rev 90:1089–1103
Chappell J, Wolf F, Proulx J, Cuellar R, Saunders C (1995) Is the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase a rate-limiting step for isoprenoid biosynthesis in plants? Plant Physiol 109:1337–1343
Charles DJ, Simon JE, Wood KV, Heinsten P (1990) Germplasm variation in artemisinin content of Artemisinin annua using an alternative method of artemisinin analysis from crude plant extracts. J Nat Prod 53:157–160
Chen DH, Meng Y, Ye HC, Li GF, Chen XY (1998) Cultures of transgenic Artemisia annua hairy root with cadinene synthase gene. Acta Bot Sin 40:711–714
Chen DH, Ye HC, Li GF (1999) Expression of green fluorescent protein gene in transgenic shoots of Artemisia annua. Acta Bot Sin 41:490–493
Chen DH, Ye HC, Li GF (2000) Expression of a chimeric farnesyl diphosphate synthase gene in Artemisia annua L. transgenic plants via Agrobacterium tumefaciens-mediated transformation. Plant Sci 155:179–185
Delourme D, Lacroute F, Karst F (1994) Cloning of an Arabidopsis thaliana cDNA coding for farnesyl diphosphate synthase by function complementation in yeast. Plant Mol Biol 26:1867–1873
Dhingra V, Narasu ML (2001) Purification and characterization of an enzyme involved in biochemical transformation of arteannuin B to artemisinin from Artemisia annua. Biochem Biophys Res Commun 281:558–561
Dondorp A, Nosten F, Stepniewska K, Day N, White N (2006) Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 9487:717–725
Dong YX, Chollet J, Matile H, Charman SA, Chiu FCK, Charman WN, Scorneaux B, Urwyler H, Tomas JS, Scheurer C, Snyder C, Dorn A, Wang XF, Karle JM, Tang YQ, Wittlin S, Brun R, Vennerstrom JL (2005) Spiro and dispiro-1,2,4-trioxolanes as antimalarial peroxides: charting a workable structure–activity relationship using simple prototypes. J Med Chem 48:4953–4961
Duke SO, Paul RN (1993) Development and fine structure of the glandular trichomes of Artemisia annua L. Int J Plant Sci 154:107–118
Duke MV, Paul RN, Elsohly HN, Sturtz G, Duke SO (1994) Localization of artemisinin and artemisitene in foliar tissues of glanded and glandless biotypes of Artemisia annua L. Int J Plant Sci 155:365–372
EI-Feraly FS, AIMeshal IA, Alyahya MA, Hifnawy MS (1986) On the possible role of qinghao acid in the biosynthesis of artemisinin. Phytochemistry 25:2777–2778
Eskstein-Ludwig U, Webb RJ, van Goethem IDA, East JM, Lee AG, Kimura M, O’Neill PM, Bray PG, Ward SA, Krishna S (2003) Artemisinins target the SERCA of Plasmodium falciparum. Nature 424:957–961
Farmer WR, Liao JC (2001) Precursor balancing for metabolic engineering of lycopene production in Escherichia coli. Biotechnol Prog 17:57–61
Ferreira JF, Janick J (1996) Immunoquantitative analysis of artemisinin from A. annua using polyclonal antibodies. Phytochemistry 41:97–104
Fulzele DP, Heble MR, Rao PS (1995) Production of terpenoid from Artemisia annua L. plantlet cultures in bioreactor. J Biotechnol 40:139–143
Geng S, Ye HC, Li GF (2001a) Flowering of Artemisia annua L. test-tube plantlets and artemisinin production with shoot cultures induced from flower organ explants. Chin Appl Environ Biol 7:201–206
Geng S, Ma M, Ye HC, Liu BY, Li GF, Kang C (2001b) Effect of ipt gene expression on the physiological and chemical characteristics of Artemisia annua L. Plant Sci 160:691–698
Gulati A, Bharel S, Jain SK, Abdin MZ, Srivastava PS (1996) In vitro micropropagation and flowering in Artemisia annua. J Plant Biochem Biotech 5:31–35
Guo C, Liu CZ, Ye HC, Li GF (2004) Effect of temperature on growth and artemisinin biosynthesis in hairy root cultures of Artemisia annua. Acta Bot Boreal-Occident Sin 24:1828–1831
Han JL, Wang H, Ye HC, Liu Y, Li ZQ, Zhang Y, Zhang YS, Yan F, Li GF (2005) High efficiency of genetic transformation and regeneration of Artemisia annua L. via Agrobacterium tumefaciens-mediated procedure. Plant Sci 168:73–80
He XC, Zeng MY, Li GF, Liang Z (1983) Callus induction and regeneration of plantlets from Artemisia annua and changes of qinghaosu contents. Acta Bot Sin 25:87–90
Jung M, Kim H, Nam KY, No TK (2005) Three-dimensional structure of Plasmodium falciparum Ca2+-ATPase (PfATP6) and docking of artemisinin derivatives to PfATP6. Bioorg Med Chem Lett 15:2994–2997
Kajiwara S, Fraser PD, Kondo K, Misawa N (1997) Expression of an exogenous isopentenyl diphosphate isomerase gene enhances isoprenoid biosynthesis in Escherichia coli. Biochem J 324:421–426
Kannan R, Kumar K, Sahal D, Kukreti S, Chauhan VS (2005) Reaction of artemisinin with haemoglobin: implications for antimalarial activity. Biochem J 385:409–418
Klayman DL (1985) Qinghaosu (artemisinin): an antimalarial drug from China. Science 228:1049–1055
Kim SW, Keasling JD (2001) Metabolic engineering of the nonmevalonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotechnol Bioeng 72:408–415
Kim Y, Wyslouzil BE, Weathers PJ (2001) A comparative study of mist and bubble column reactors in the in vitro production of artemisinin. Plant Cell Rep 20:451–455
Kim Y, Wyslouzil BE, Weathers PJ (2002) Secondary metabolism of hairy root cultures in bioreactors. In Vitro Cell Dev Biol Plant 38:1–10
Krishna S, Uhlemann AC, Haynes RK (2004) Artemisinins: mechanisms of action and potential for resistance. Drug Resist Updat 7:233–244
Krushkal J, Pistilli M, Ferrell KM, Souret FF, Weathers PJ (2003) Computational analysis of the evolution of the structure and function of 1-deoxy-D-xylulose-5-phosphate synthase, a key regulation of the mevalonate-independent pathway in plants. Gene 313:127–138
Kudakasseril GJ, Lam L, Staba EJ (1987) Effect of sterol inhibitors on the incorporation of 14C-isopentenyl pyrophosphate into artemisinin by a cell-free system from Artemisia annua tissue cultures and plants. Planta Med 53:280–284
Liu CZ, Wang YC, Ouyang F, Ye HC, Li GF (1997) Production of artemisinin by hairy root cultures of Artermisia annua L. Biotechnol Lett 19:927–930
Liu BY, Ye HC, Li GF (1998a) Studies on dynamic of growth and artemisinin biosynthesis of hairy root of Artemisia annua L. Chin J Biotechnol 14:401–404
Liu CZ, Wang YC, Guo C, Ouyang F, Ye HC, Li GF (1998b) Enhanced production of artemisinin by Artemisia annua L. hairy root cultures in a modified inner-loop airlift bioreactor. Bioprocess Eng 19:389–392
Liu CZ, Wang YC, Ouyang F, Ye HC, Li GF (1998c) Production of artemisinin by hairy root cultures of Artemisia annua L. in bioreactor. Biotechnol Lett 20:265–268
Liu CZ, Moon KH, Honda H, Kobayashi T (2000) Immobilization of rice (Oryza sativa L.) callus in polyurethane foam using a turbine blade reactor. Biochem Eng J 4:169–175
Liu CZ, Guo C, Wang YC, Ouyang F (2002) Effect of light irradiation on hairy root growth and artemisinin biosynthesis of Artemisia annua L. Process Biochem 38:581–585
Liu CZ, Guo C, Wang YC, Ouyang F (2003a) Comparison of various bioreactors on growth and artemisinin biosynthesis of Artemisia annua L. shoot cultures. Process Biochem 39:45–49
Liu Y, Ye HC, Wang H, Li GF (2003b) Molecular cloning, Escherichiacoli expression and genomic organization of squalene synthase gene from Artemisia annua. Acta Bot Sin 45:608–613
Liu CZ, Murch S, El-Demerdash M, Saxena PK (2004) Artemisia judaica L.: mass propagation and antioxidant potential. J Biotechnol 110:63–71
Liu Y, Wang H, Ye HC, Li GF (2005) Advances in the plant isoprenoid biosynthesis pathway and its metabolic engineering. J Integr Plant Biol 47:769–782
Liu JH, Chen YG, Yu BY, Chen YJ (2006) A novel ketone derivative of artemisinin biotransformed by Streptomyces griseus ATCC 13273. Bioorg Med Chem Lett 16:1909–1912
Luo XD, Shen CC (1987) The chemistry, pharmacology and clinical applications of Qinghaosu (artemisinin) and its derivatives. Med Res Rev 7:29–52
Mårtensson A, Strömberg J, Sisowath C, Msellem MI, Gil JP, Montgomery SM, Olliaro P, Ali AS, Björkman A (2005) Efficacy of artesunate plus amodiaquine versus that of artemether–lumefantrine for the treatment of uncomplicated childhood Plasmodium falciparum malaria in Zanzibar, Tanzania. Clin Infect Dis 41:1079–1086
Martin VJJ, Pitera DJ, Withers ST, Newman JD, Keasling JD (2003) Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol 21:796–802
Martinez BC, Staba EJ (1988) The production of artemisinin in Artemisia annua L. tissue cultures. Adv Cell Cult 6:69–87
Matsushita Y, Kang WY, Charlwood BV (1996) Cloning and analysis of a cDNA encoding farnesyl diphosphate synthase from Artemisia annua. Gene 172:207–209
Mckenzie MJ, Mett V, Reynolds PHS, Jameson PE (1998) Controlled cytokinin production in transgenic tobacco using a copper-inducible promoter. Plant Physiol 116:969–977
Mercke P, Bengtsson M, Bouwmeester HJ, Posthumus MA, Brodelius PE (2000) Molecular cloning, expression and characterization of amorpha-4,11-diene synthase, a key enzyme of artemisinin biosynthesis in Artemisia annua L. Arch Biochem Biophys 381:173–180
Mok DWS, Mok MC (1994) Cytokinins: chemistry, activity and function. CRC Press, Boca Raton, FL
Nair MSR, Basile DV (1993) Bioconversion of arteannuin B to artemisinin. J Nat Prod 56:1559–1566
Nair MSR, Acton N, Klayman DL (1986) Production of artemisinin in tissue cultures of Artemisia annua. J Nat Prod 49:504–507
Newman JD, Chappell J (1999) Isoprenoid biosynthesis in plants: carbon partitioning within the cytoplasmic pathway. Crit Rev Biochem Mol Biol 34:95–106
Paniego NB, Giuletti AM (1994) Artemisia annua L. dedifferentiated and differentiated cultures. Plant Cell Tissue Organ Cult 36:163–168
Paniego NB, Giulietti AM (1996) Artemisinin production by Artemisia annua L-transformed organ cultures. Enzyme Microb Technol 18:526–530
Qin MB, Li GZ, Yun Y, Ye HC, Li GF (1994) Induction of hairy root from Artemisia annua with Agrobacterium rhizogenes and its culture in vitro. Acta Bot Sin 36:165–170 (Suppl)
Qinghaosu Antimalaria Coordinating Research Group (1979) Antimalaria studies on qinghaosu. Chin Med J (Engl) 92:811–816
Riley EM (1995) The London School of Tropical Medicine: a new century of malarial research. Mem Inst Oswaldo Cruz 95:25–32
Sangwan RS, Agarwal K, Luthra R, Thakur RS, Singh SN (1993) Biotransformation of artemisinic acid into arteannuin B and artemisinin in Artemisia annua. Phytochemistry 34:1301–1302
Shetty P (2004) Global fund switches to artemisinin. Lancet Infect Dis 4:477
Singh NP, Lai HC (2005) Synergistic cytotoxicity of artemisinin and sodium butyrate on human cancer cells. Anticancer Res 25:4325–4331
Smith TC, Weathers PJ, Cheetham RC (1997) Effect of gibberellic acid on hair root cultures of Artemisia annua growth and artemisinin production. In vitro Cell Dev Biol Plant 33:75–79
Souret FF, Weathers PJ, Wobbe KK (2002) The mevalonate-independent pathway is expressed in transformed roots of Artemisia annua and regulated by light and culture age. In Vitro cell Dev Biol Plant 38:581–588
Souret FF, Kim Y, Wyslouzil BE, Wobbe KK, Weathers PJ (2003) Scale-up of Artemisia annua L. hairy root cultures produces complex patterns of terpenoid gene expression. Biotechnol Bioeng 83:653–667
Tawfiq NK, Anderson LA, Roberts MF, Phillipson JD, Bray DH, Warhurst DC (1989) Antiplasmodial activity of Artemisia annua plant cell cultures. Plant Cell Rep 8:425–428
Teo CKH, Yap AW, Chan KL, Gan EK, Tanaka M (1995) Artemisinin production from callus of Artemisia annua cultured in fluorocarbon polymer film culture bag. Asia-Pac J Mol Biol Biotechnol 3:317–321
Teoh KH, Polichuk DR, Reed DW, Nowak G, Covello PS (2006) Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett 580:1411–1416
Uhlemann AC, Krishna S (2005) Antimalarial multi-drug resistance in Asia: mechanisms and assessment. Curr Top Microbiol Immunol 295:39–53
Uhlemann AC, Cameron A, Eckstein-Ludwig U, Fischbarg J, Iserovich P, Zuniga FA, East M, Lee A, Brady L, Haynes RK, Krishna S (2005) A single amino acid residue can determine the sensitivity of SERCAs to artemisinins. Nat Struct Mol Biol 12:628–629
Vennerstrom JL, Arbe-Barnes S, Brun R, Charman SA, Chiu FCK, Chollet J, Dong YX, Dorn A, Hunziker D, Matile H, Mclntosh K, Padmanilayam M, Tomas JS, Scheurer C, Scorneaux B, Tang YQ, Urwyler H, Wittlin S, Charman WN (2004) Identification of an antimalarial synthetic trioxolane drug development candidate. Nature 430:900–904
Vergauwe A, Cammaert R, Vandenberghe D, Genetello C, Van Montagu M, Vanden Eeckhout E (1996) Agrobacterium tumefaciens-mediated transformation of Artemisia annua L. and regeneration of transgenic plant. Plant Cell Rep 15:929–937
Vergauwe A, Van GE, Inze D, Van DEE (1998) Factor influencing Agrobacterium tumefaciens-mediated transformation of Artemisia annua L. Plant Cell Rep 18:105–110
Wallaart TE, Pras N, Quax WJ (1999a) Isolation and identification of dihydroartemisinic acid hydroperoxide from Artemisia annua: a novel biosynthetic precursor of artemisinin. J Nat Prod 62:1160–1162
Wallaart TE, van Uden W, Lubberink HGM, Woerdenbag HJ, Pras N, Quax WJ (1999b) Isolation and identification of dihydroartemisinic acid from Artemisia annua and its possible role in the biosynthesis of artemisinin. J Nat Prod 62:40–42
Wallaart TE, Bouwmeester HJ, Hille J, Poppinga L, Maijers NCA (2001) Amorpha-4,11-diene synthase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta 212:460–465
Wang CW (1961) The forest of China with a survey of grassland and desert vegetation In: Harvard University Maria moors cabot foundation, vol 5. Harvard University, Cambridge, MA, pp 171–187
Wang JW, Tan RX (2002) Artemisinin production in Artemisia annua hair root cultures with improved growth by altering the nitrogen source in the medium. Biotechnol Lett 24:1153–1156
Wang YC, Zhang HX, Zhao B, Yuan XF (2001) Improved growth of Artemisia annua L. hairy roots and artemisinin production under red light conditions. Biotechnol Lett 23:1971–1973
Wang H, Ge L, Ye HC, Chong K, Liu BY, Li GF (2004) Studies on the effects of fpf1 gene on Artemisiaannua flowering time and on the linkage between flowering and artemisinin biosynthesis. Planta Med 70:347–352
Weathers PJ, Cheethan RD, Follansbee E, Theohairides K (1994) Artemisinin production by transformed roots of Artemisia annua. Biotechnol Lett 16:1281–1286
Weathers PJ, DeJesus-Gonzalez L, Kim YJ (2004) Alteration of biomass and artemisinin production in Artemisia annua hairy roots by media sterilization method and sugars. Plant Cell Rep 23:414–418
Weathers PJ, Bunk G, Mccoy MC (2005) The effect of phytohormones on growth and artemisinin production in Artemisia annua hairy roots. In Vitro Cell Dev Biol Plant 41:47–53
Whipkey A, Simon JE, Charles DJ, Janick J (1992) In vitro production of artemisinin from Artemisia annua L. Phytother Res 1:15–25
Woerdenbag HJ, Luers JFJ, Van Uden W, Pras N, Malingre TH, Alfermann AW (1993) Production of the new antimalarial drug artemisinin in shoot cultures of Artemisia-annua L. Plant Cell Tissue Organ Cult 32:247–257
Woo SH, Park JM (1993) Multiple shoot culture of Dianthus caryophyllus using mist culture system. Biotechnol Tech 7:697–702
Wright WC (2005) Traditional antimalarials and the development of novel antimalarial drugs. J Ethnopharmacol 100:67–71
Wyslouzil BE, Waterbury RG, Weathers PJ (2000) The growth of single roots of Artemisia annua in nutrient mist reactors. Biotechnol Bioeng 70:143–150
Xie DY, Zou ZR, Ye HC, Li GF, Guo ZC (2001) Selection of hairy root clones of Artemisia annua L. for artemisinin production. Isr J Plant Sci 49:129–134
Xu XX, Zhu J, Huang DZ, Zhou WS (1986) Total synthesis of arteannuin and deoxyarteannuin. Tetrahedron 42:819–828
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, C., Zhao, Y. & Wang, Y. Artemisinin: current state and perspectives for biotechnological production of an antimalarial drug. Appl Microbiol Biotechnol 72, 11–20 (2006). https://doi.org/10.1007/s00253-006-0452-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0452-0