Abstract
Artemisia annua L. is an innocuous medicinal plant that is suddenly found at the forefront of global efforts aimed at the eradication of malaria. The plant is also seen as an effective treatment against several other infectious diseases and human cancer cell lines, and this has been correlated with its richness in several bioactive compounds including artemisinin, other sesquiterpenes, and antioxidants. Undoubtedly, this is a development that has drastically increased artemisinin demand worldwide. Up until now, A. annua L. remains the only commercial source for the supply of this vital antimalarial drug to the international market. Recent advances in biotechnology, however, such as have been demonstrated in the production of isoprenoid precursors of artemisinin in yeast, and bacteria are feasible complementary strategies that would help reduce artemisinin cost in the future. The key genes encoding for enzymes regulating the biosynthesis of artemisinin in planta are fully understood to enable metabolic engineering of the pathway, and results from pilot genetic engineering studies in microbial strains thus far are very inspiring. This current treatise, therefore, explores the status of artemisinin and other plant metabolites for use in both human and animal healthcare and highlights the implications of in planta production of artemisinin in comparison with that from synthetic biology. Overall, these two methods need not be mutually exclusive and can be made complementary to each other depending on the location of production. Aside from artemisinin, required for saving the lives of countless patients in malaria-stricken societies, the plant also contains several other secondary metabolites with enormous benefits in the promotion of human and animal health. Consequently, keeping in planta production of artemisinin would play a pivotal role in providing artemisinin for ACTs as well as maintaining profit margins to local and regional economies in countries where malaria is endemic, especially in Africa where A. annua has been cultivated for the past 10 years.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
15.1 Introduction
Malaria is the most deadly parasitic disease known to man today, especially in the developing world where it imposes an enormous burden of morbidity and mortality. It is caused by protozoan parasites, notably Plasmodium falciparum, which is spread following the bite of infected female Anopheles mosquitoes. At the moment, over 40 % of the world population is under some risk of contracting malaria, with recent estimates suggesting several hundred million cases of clinical episodes and about 800,000 deaths per year (Kappe et al. 2010). Unfortunately, about 90 % of malaria-related mortality usually occurs among children less than five years of age and pregnant women in sub-Saharan Africa (Rinaldi 2004), where a large proportion of the entire population has no access to proper healthcare services. In addition, the drain on local African economies is inconceivable, often estimated to be more than US$12 billion each year in lost gross domestic product (WHO 2002). Threat of malaria is also seen as a deterrent to tourism and internal trade, further constituting a serious obstacle to socio-economic development that perpetuates a cycle of poverty in the continent.
In the past, quinoline-based drugs were the main choice for the prevention and treatment for malaria. Unfortunately, the emergence, through mutation, of drug-resistant Plasmodium species in many parts of the world, has rendered these traditional and low-cost antimalarial medicines, such as chloroquine, ineffective. Presently, the best hope for a replacement treatment lies with drugs based on artemisinin, to which Plasmodium parasites have not yet developed resistance (Gordi et al. 2002; Xu et al. 1986; Schmid and Hofheinz 1983), except in an area in western Cambodia (Cheeseman et al. 2012). Thus, artemisinin-based drugs remain the primary weapon for reducing the burden of disease in individuals and general populations in malaria-endemic societies.
Artemisinin is a cadinene-type sesquiterpene lactone with a crucial endoperoxide bridge. It is produced and sequestered in glandular trichomes that are found on leaves, floral buds, and flowers (Ferreira and Janick 1995; Tellez et al. 1999) of a weedy plant called annual wormwood (Artemisia annua L). Commercial production of this sesquiterpenoid compound, which is highly potent and effective against all Plasmodium species, including multidrug-resistant strains, requires that it be extracted from the aerial parts of this herb.
With its semi-synthetic derivatives such as dihydroartemisinin, artesunate, and artemether, artemisinin has displayed unique pharmacological activities against a wide range of other parasitic organisms including Schistosoma species (Mishina et al. 2007; Xiao et al. 2001; Utzinger et al. 2001), Leishmania donovani (Yang and Liew 1993; Ma et al. 2004), Toxoplasma gondii (Jones-Brando et al. 2006), Pneumocystis carinii (Merali and Meshnick 1991), and the pathogens responsible several neglected diseases including for cryptosporidiosis, amoebiasis, giardiasis, clonorchiasis, and leishmaniasis (Ma et al. 2004; Yang and Liew 1993). Artemisinin has also been recently indicated as having antiviral activities (Romero et al. 2006) and has the potential to be used in the treatment of hepatitis B, C, and others (Efferth et al. 2008). More compelling and of greater pharmacological significance, even to those in industrially developed societies, is the fact that artemisinin or its semi-synthetic derivatives have been demonstrated to be novel antitumour agents for some of the deadliest cancers known to man. For example, artemisinin derivatives have been shown to be very effective against radiation-resistant breast cancer cells in vitro (Singh and Lai 2001), drug-resistant small-cell lung carcinoma cells (Sadava et al. 2002), human leukaemia cell lines (Lai and Singh 1995), colon cancer, and active melanomas (Efferth et al. 2001). Collectively, these parasitic diseases and cancers appear to afflict over a billion people each year in different parts of the world and can be successfully treated with artemisinin or its semi-synthetic derivatives, where an adequate source of the drug is guaranteed and at an affordable cost. Invariably, these developments have attracted a very high degree of attention; a situation that has led to the source plant of the drug to be rated as one of the top ten industrial crops of the modern world (Sangwan et al. 1998).
In spite of the enormous pharmacological importance of artemisinin in both human and animal healthcare (Efferth et al. 2011; Brisibe et al. 2008a, b; Turner and Ferreira 2005; Ferreira 2009; Ferreira et al. 2005), its availability, especially as a key active ingredient in the production of the world’s most effective antimalarial drugs, artemisinin-based combination therapies (ACTs), is limited not only by low yield in A. annua but even more so by the uncertainty of farmers and producers in the current debate, in which plant-derived artemisinin stands to be replaced by its bioengineered counterpart (http://www.malariaworld.org/poll/semi-synthetic-artemisinin-production-through-bioengineered-yeast-great-step-forward-cover#comment-1244). Not surprisingly, this situation can lead to a more unstable supply of plant-derived artemisinin, resulting in shortages and price fluctuations that will further complicate production planning by ACT manufacturers. Coupled with the added cost of the secondary antimalarial drug partner(s) in the ACT, this has become a major hindrance to the availability of affordable ACTs to patients with the need, especially in countries where the use of ACTs has been encouraged (WHO 2006), further fuelling an increased demand within the past 10 years. Consequently, there is the need for a stable source of affordable artemisinin that will be sufficient to meet the current market demand. For example, artemisinin demand for 2013, as reported by the UNITAID-supported and BCG-managed ACT forecasting consortium, is between 101 and 119 metric tons, depending on the different scenarios (http://www.a2s2.org/upload/4.NewsandEvents/Newsletter3Oct2012/A2S2MarketUpdateOctober2012.pdf).
15.2 Production of Artemisinin In Planta
Although de novo chemical synthesis of artemisinin is possible (Zhu and Cook 2012), the process is very complex with many reaction steps, resulting in low yields. The chemical analogues produced are thus not economically competitive with that synthesized in planta in A. annua (Ferreira et al. 2005; Xu et al. 1986; Schmid and Holheinz 1983), which means its solvent extraction from the leafy biomass of the plant invariably appears to be the most viable option for producing cheap and large quantities of the drug. At the moment, increased cultivation of the crop in smallholder fields in Asia and Africa, which are usually less than 1 ha, and the improvement in extraction methods are the most effective strategies for producing artemisinin. However, one of the major shortcomings on the production of sesquiterpenoid compounds via A. annua, especially in tropical countries, is the relatively lengthy growing cycle required to obtain appreciable yields (g/100 g dry weight). So far, the best commercial varieties, when harvested multiple times in the same year, are estimated to produce 70 kg of artemisinin/ha (Kumar et al. 2004). Usually, the period from time of planting to artemisinin extraction from the plant is approximately 5–8 months. Not surprisingly, the yields derived from dried leafy biomass after such a lengthy period are considered low for commercial production, where a full ton of plant materials can produce between 6 and 18 kg of purified artemisinin (Brisibe et al. 2012). This low yield thus appears to be one of the most intractable problems related to in planta production and use of artemisinin-derived drugs against malaria, especially in Africa where the cultivation of the crop is found in only about five countries.
Presently, about 95 % of artemisinin used in the formulation of ACTs for malaria treatment is produced in China and Vietnam, while the reminder comes from Africa and India. However, in many regions of sub-Saharan Africa with a high incidence of malaria, local populations, against the advice of WHO, continue to drink extracts of A. annua leaves as a tea or take the fresh leaves of the plant directly not only in the treatment for malaria fever but also other ailments such as hyperglycaemia (Brisibe et al. 2011a) and HIV (Lubbe et al. 2012). Undeniably, both hot water extracts and the fresh plant material consumed would contain not only artemisinin but other bioactive compounds, including polymethoxylated flavonoids such as artemetin, casticin, chrysosplenetin, chrysosplenol D, cirsilineol, and eupatorin and more than a dozen other sesquiterpenes that abound in the leaves, which have been indicated as important compounds with antimalarial (Elfawal et al. 2012; Willcox 2009) and potential anticancer activities. Synergistic benefits may also be derived from the presence of other antimalarial compounds such as dehydrosilibin and dimethylallyl campferide. Aside from this, it has been reported that the traditional Artemisia tea therapy contained artemisinin as well as some antioxidant compounds mostly flavonoids (Rath et al. 2004; Willcox et al. 2007). In addition to their bioavailability, these compounds such as phenols, saponins, flavonoids, alkaloids, and tannins act to reduce parasitaemia independent of artemisinin (Liu et al. 1992). The presence of other compounds in A. annua leaves has thus raised suspicion as to the possibility of their synergistic role with artemisinin in malaria and cancer treatment (Ferreira et al. 2010). These in planta constituents potentiate and enhance the overall activity of artemisinin (Elford et al. 1987), the reason given for the long-term use of the plant as a tea in China even before the discovery of artemisinin (Ferreira et al. 2010). Consequently, given the complex nature of A. annua and the many bioactive components and nutrients present in its tissues (Bhakuni et al. 2001; Brisibe et al. 2009), it would be simplistic to consider the consumption of either the traditional tea or whole-plant material essentially as a monotherapy, an understandable fear expressed by many people. However, this worry appears to be misplaced. Some studies have actually shown that there may be less chance of resistance occurring from the combined use of numerous plant constituents, which enhances the overall activity of artemisinin and can prevent Plasmodium or any other microbial parasite from developing resistance to the compound.
Now, considering that some plant secondary metabolites appear to have a more synergistic effect when provided in planta than in a purified form (Gilbert and Alves, 2003), an edible form of Artemisia leaf biomass via a compacted capsule in combination with an ACT partner has also been offered as a reliable, safe, and inexpensive mode to deliver the drug (Elfawal et al. 2012). In fact, it would be very tempting to consider the whole-plant treatment as an alternative delivery mechanism for artemisinin. This is supported by the results of Weathers et al. (2011) and Elfawal et al. (2012), which have provided strong evidence to suggest that the parasite-killing substances present in the whole-plant material may be acting through their potentiation of artemisinin that renders whole-plant consumption as an innovative plant-based artemisinin combination therapy (pACT). In one of their recent studies, Weathers et al. (2011) actually demonstrated that mice fed with dried whole-plant material of A. annua had about 40 times more artemisinin in their bloodstream than those fed with a corresponding amount of the pure drug. This amount exceeded by eightfold the minimum concentration of serum artemisinin (10 μg/l) required against P. falciparum (Alin and Bjorkman 1994), which suggests that the active ingredients contained in the whole-plant material were delivered faster and in greater quantity than those from pure drug treatments.
Though plant-based supply of active pharmaceutical ingredients (in this case, artemisinin) is not in agreement with the preference of modern pharmaceutical industry for single-ingredient drugs; nonetheless, this method would dramatically reduce the cost of healthcare not only in developing countries, but perhaps also in more developed nations where a holistic approach to disease treatment with herbal products has recently become fashionable. There are several examples that illustrate the synergistic benefits of drug delivery using complex botanical materials in preference to that in an isolated form (Raskin et al. 2002; Gilbert and Alves 2003). We, therefore, completely agree with the proposal of Weathers et al. (2011) that loading of capsules with compacted A. annua leaf powder of a known dosage artemisinin to which the ACT drug partner can be added or administered separately could be another cost-effective, inexpensive, and reliable method of artemisinin delivery in resource-poor settings, especially in Africa where the scourge of malaria has its highest toll of mortality. The processing facility for such inexpensive artemisinin delivery route could be centred within an area where local farmers currently grow the plant such that the entire process could be self-sustaining.
This proposition will not only strengthen local health, as confirmed by the efficacy of the plant-derived artemisinin and the ethanol extract from locally cultivated plants in in vitro evaluation studies, but also the local economy. For example, Table 15.1 shows the IC50 of ethanol, methanol, and hexane fractions of two Artemisia species as well as pure artemisinin extracted from the locally cultivated A. annua plants in Nigeria in comparison with the values for chloroquine and artemisinin purchased from a commercial source in China. It is obvious that the in vitro activity against chloroquine-resistant (KI) strain of Plasmodium falciparum from artemisinin locally extracted was also very high. Besides, the fact that the ethanol extract from the locally cultivated A. annua plants was very efficacious against the KI strain of the parasite in the study suggests the presence of artemisinin and other compounds, further confirming the use of plant material as an effective alternative mode of delivery of artemisinin (Weathers et al. 2011).
Taken together, these observations strong support that apart from the use of WHO-recommended ACTs, some researchers have vigorously campaigned in favour of either re-establishing the use of traditional Artemisia tea (Van der Koov and Verpoorte 2011; De Ridder et al. 2008; Hsu 2006) or using fresh whole leaves (Brisibe and Daniel 2013) or encapsulated dried leaves (Elfawal et al. 2012; Weather et al. 2011), with the caveat that the plant material used has high or clinical levels of artemisinin in remote areas where malaria is endemic. Considering that the onset of cerebral malaria and malaria-induced coma is fast and the nearest hospital or clinic may be 2–3 days away, the use of the plant material (in whole or as tea) should be investigated seriously and, hopefully, permitted to sustain a malaria patient to reach a health centre stocked with antimalarial drugs (Ferreira et al. 2010). ‘Plant materials (pACTs) may not be as perfect as the ideal doses administered in active pharmaceutical formulations, however they may be better than no treatment at all. These treatment methods will not only save precious lives but also have several advantages. Firstly, they are inexpensive. Secondly, they are in forms that most resource-poor societies can rely on. It can be construed from the above that continuous cultivation of A. annua, not solely for the purpose of artemisinin extraction, but also for the significance of the plant in its multipurpose therapeutic potential and holistic treatment for malaria and a variety of other diseases and neglected parasitic ailments, must be encouraged.
15.3 Artemisia annua and Some of Its Pharmacological Activities
A. annua is an annual weed with an aggressive and vigorous growth habit. It is considered to have originated, and occurs naturally, as part of the steppe vegetation in Northern China (Ferreira et al. 2005). However, it now grows effectively in other climatic conditions. In Asia, for example, it is well distributed and extends as a native into southern Siberia, Vietnam, and northern India. Outside of Asia, the plant has adapted ubiquitously to different growth conditions as seen in many parts of Europe, USA, Australia, and Argentina (Ferreira et al. 2005). In Africa, it has been introduced into commercial-scale cultivation in Tanzania, Kenya, Uganda, and Madagascar within the past 10 years and more recently in Nigeria (Brisibe et al. 2012), where evaluation of optimal agronomic practices and mass selection for late-flowering and high artemisinin-yielding lines were evaluated with interesting results. For these studies, seeds were obtained from six different countries—Brazil, China, Vietnam, India, Germany, and USA. Some of these, especially the hybrid populations from Brazil, originated plants that had a growth span of about 192 days before flowering and were up to 2.84 m in height (Fig. 15.1) with an average leaf biomass yield of 324 g/plant and artemisinin concentrations as high as 1.0975 % (on a g/100 g dry weight basis) under humid lowland tropical conditions (Brisibe et al. 2012).
As a crop with a rich ethnopharmacological significance, it is surprising that A. annua has been relatively undeveloped over several millennia, despite its depiction in the Chinese Materia Medica as a therapeutic tea for malaria and fever (Hsu 2006) and its uses, a non-trivial matter considering its bitter taste, as a condiment by various Asian cultures (Weathers et al. 2011). It has been documented that the artemisinin content in the dry leaf of varieties from different geographical origins varies considerably, ranging from 0.01 to 1.9 % (Brisibe et al. 2012; Delabays et al. 2001; Ferreira and Gonzales 2009). To a large extent, such variations can be attributed to differences in field management practices that must be adapted to specific environments and local costs, level of intensification, and interactions between methods of cultivation and variety. Other factors that are equally known to affect artemisinin content in the plant include periods of harvest and environmental components such as temperature and nutrient availability (Ferreira 2007; Delabays et al. 2002; Delabays et al. 2001). Aside from these, artemisinin content has also been shown to be highly heritable, indicating that a strong genetic component contributes to the variation seen in the cultivated crop (Delabays et al. 2001; Graham et al. 2010). Such genetic components and their interactions with environmental factors have been exploited for breeding purposes to produce improved hybrid lines (Townsend et al. 2013), which can boost artemisinin supplies from smallholders in Asia and Africa.
In recent years, there has been an increase in the number of scientific investigations that have validated the potential of A. annua and its extracts both as dietary feed supplement and to treat a variety of ailments afflicting both humans and livestock (Almeida et al. 2012; Brisibe et al. 2008a, 2011a; Cherian et al. 2013; Drăgan et al. 2010; Ferreira 2009). In addition to artemisinin, the plant is a storehouse of many biologically active compounds including more than 40 different flavonoids, phenolics, purines, lipids, aliphatic compounds, antioxidants, and others (Brisibe et al. 2009; Ferreira et al. 2010). The relatively high amino acid and vitamin profiles coupled with the very low and often negligible levels of inherent antinutritive factors, especially in the leaves, which are far below levels considered toxic, establish A. annua also as a good reservoir of nutrients and antioxidants that favour its use as an important supplementary or phytogenic feed additive (Brisibe et al. 2008a; Cherian et al. 2013) for livestock production systems and a potential herbal tonic for humans (Brisibe et al. 2009).
Aside from these, recent research works have continued to buttress the need for further investigations on the use of A. annua and its extracts. For example, ethanol extract of the plant showed immunosuppressive effect on autoimmune diseases such as lupus erythematosus and rheumatoid arthritis (Zhang and Sun 2009), while SM905, a water-soluble artemisinin derivative, also obtained from A. annua, ameliorates collagen-induced arthritis by the suppression of inflammatory and Th17 responses. Oral treatment with SM905 not only delayed disease onset, reduced arthritis incidence and severity, but also suppressed the enhanced expression of pro-inflammatory cytokines, chemokines, and chemokine receptors in draining lymph nodes. In established arthritis, SM905 profoundly inhibited disease progression, reduced IL-17A, and RORgt mRNA expression and suppressed pro-inflammatory mediator expression in arthritic joints (Wang et al. 2008). Similarly, as the incidence of HIV/AIDS becomes more prevalent in different parts of the world with varying consequences, a lot of new drugs (both natural and synthetic) are presently evaluated as lead molecules in the fight against HIV/AIDS. So far, A. annua has been identified as one of the few medicinal plants to show great promise in this regard (Lubbe et al. 2012). Intuitively, this will be a major pharmacological novelty once the anti-HIV effects of A. annua are confirmed in humans.
Now apart from its commonly known traditional uses, several research groups and stakeholders are presently seeking for alternative uses and therapies for Artemisia that are efficient, affordable, accessible, and widely available. Some of those highlighted so far include its use for immune boosting, production of scopoletin (by extraction), insect repellents, perfumes from essential oils, and flavouring for alcoholic beverages. The plant also has an important role in agriculture, where its anticoccidial, anthelminthic, allelopathic, antifungal, and insecticidal activities have been identified in livestock (Almeida et al. 2012; Brisibe et al. 2008b; Ferreira 2009; Hart et al. 2007), fishery (Ekanem and Brisibe 2010) and crop production (Tang et al. 2000), respectively. It is also a highly effective plant growth inhibitor with great potential as an organic herbicide (Abate et al. 2011) or pesticide in stored grains (Brisibe et al. 2011b). All of these are capacities conferred on the plant due to its numerous bioactive compounds. This, in itself, is not surprising as many natural products in plants are multifunctional molecules that protect them from bacterial, viral, and other microbial infections, or even from herbivores such as insects and worms. Against this backdrop, therefore, there should be no single usage intended for A. annua, but a range of treatment possibilities provided by the plant ingredients. These are further indications that highlight the need for expanding the scope of cultivating the plant and thereby potentially increasing artemisinin supply and reducing its cost of production.
15.4 Strategies for Increased Production of Artemisinin
In 2012, with an increased A. annua planting and good weather conditions during the growing season, the global production of artemisinin increased considerably and was predicted to be sufficient to meet the most optimistic ACT forecasts for the 2012/2013 production season. However, there was considerable uncertainty throughout 2012 as to the future of the Affordable Medicines Facility—malaria (AMFm) programme, and therefore, the earlier demand for a timely ACT forecast has not been met (Malcolm Cuttler, Kenya Artemisinin Conference, Jan 2013). Considering that the drug must be produced cheaply, and in much greater quantities than currently available to meet this short- and medium-term demand, several strategies must be adopted for improving its supply. Some of these include an improvement in the agronomic practices that increase leaf biomass yield as well as an increase in the land area devoted to the cultivation of A. annua, following a genetic approach as it has been demonstrated previously (Debruner et al. 1996; Delabays 1994). Recently, this approach has been supported by the identification of the loci associated with artemisinin production (Graham et al. 2010). Other strategies include the induced production of artemisinic acid in Nicotiana benthamiana (van Harpen et al. 2010), production of artemisinin precursors in heterologous systems such as microorganisms (Paddon et al. 2013; Ro et al. 2006; Teoh et al. 2006), and the semi-synthesis of artemisinin from two of its precursors, artemisinic acid and dihydroartemisinic acid, which are usually discarded in the extraction process (Brisibe et al. 2008b). Some of these strategies are highlighted below.
15.4.1 Optimization of Agro-technologies for Enhanced Production of Artemisinin
The availability and cost of artemisinin are largely functions of its yield in A. annua cultivars, which has significant effect on the dynamics of supply that is currently a key cost driver for the production of ACTs. However, global supply of plant-derived (or natural) artemisinin has lately experienced boom-and-bust cycles that has led to ACT drugs being priced out of reach for poor people. It is not surprising, therefore, that there is a current surge in the cultivation of the plant around the world, most notably in Africa, where farmers have pioneered the commercial cultivation of A. annua since the late 1990s with high artemisinin-yielding lines. However, African regions mostly afflicted by malaria are within the tropics, where day lengths are short, thus likely to induce most cultivars which are not adapted to the tropics to flower early without the accumulation of sufficient leafy biomass (Ferreira et al. 2005). Ostensibly, there are currently genotypes that have been developed by Mediplant in Switzerland (Delabays 1994) and by the Chemical, Biological and Agricultural Research Centre (Debrunner et al. 1996), University of Campinas, Campinas, Brazil (in collaboration with Mediplant), which are late-flowering and produce sufficient leafy biomass that appear most suitable for cultivation in the tropics. The interpretation that these varieties, especially those from Brazil, can perform well within the tropics has support from our recent studies in Nigeria (Fig. 15.1), which showed that these populations can produce on average 1.0975 % artemisinin (Table 15.2) and can be selected further for adaptation to lower latitudes quite close to the equator (Brisibe et al. 2012). Plants from this Brazilian selection have produced as high as 1.5 % artemisinin when tested in West Virginia, USA (Jorge Ferreira, pers. comm.).
Artemisia is well suited to both smallholder and plantation agriculture. However, the most significant bottleneck for feasible commercial production of artemisinin anywhere in the world presently is the availability of seed stocks of lines suitable for the local conditions which can produce high leafy biomass and artemisinin yields. Once the problem associated with seed production has been conquered, other agronomic practices that seek to maximize the yield of leaf biomass and artemisinin per unit area of land need to be optimized. One of such factors is discussed here.
15.4.1.1 Selective Breeding and Cultivation of Genetically Superior and High-Yielding Hybrid Lines
Leafy biomass yields could be vastly improved by cultivating new strains of the crop where, on average, one kilogram of its dried leaves produces about 8 g of crystalline artemisinin. Lately, however, researchers in the United Kingdom have used selective breeding to create hybrid populations that produce up to 24 g. These plants are now being grown and harvested commercially in Madagascar, while trials are currently ongoing in South Africa, Uganda, Zimbabwe, the United States, and Britain.
In an alternative approach, Graham and colleagues at the University of York, United Kingdom, identified key genes that could be manipulated to optimize agricultural yields, robustness, or other desirable traits when Artemisia is grown in different regions of the world (Graham et al. 2010; Bowles et al. 2008). The work has helped to create plants that produce up to 50 % more artemisinin per kilogram of leaf biomass than the best commercial varieties presently in cultivation. This is a major breakthrough as the interaction between improved genetic material and good field management practices in the right environment with adequate soil moisture and nutrients leads to the production of an A. annua crop with high leafy biomass and artemisinin content, as we have demonstrated in Nigeria (Brisibe et al. 2012; Brisibe 2006).
Generally, Artemisia seeds are very small, and usually, commercial cultivation involves transplanting of vigorous nursery-grown seedlings to the field at the 3–5 leaf stage when they are about 10–15 cm in height. However, in localities where labour is scarce or expensive, seedlings can be raised directly in the field after the preparation of a fine seedbed. We observed in several trials that transplanting was clearly inferior in terms of agronomic performance and artemisinin yield of plants when raising seedlings in a nursery prior to field cultivation was compared with direct seeding in the field (Brisibe et al. 2012; Ferreira et al. 2005). However, irrespective of the method of establishment, it is always preferable to plant after the rains have started. This would mean that the soil has high moisture content since any moisture stress in the early- and mid-vegetative growth stages of the plant tends to induce premature flowering or leaf atrophy (Brisibe et al. 2012). Our preliminary studies in Nigeria have also demonstrated that leaf biomass yield and artemisinin production have a wider variation in plants generated from seeds than in those generated from asexual propagation methods such as cuttings or in vitro culture. Although this has not been evaluated on a large scale, however, a recent study using plants generated by cloning plants donated by Mediplant has proven that 0.9 % artemisinin and an average of 450 g dry leafy biomass per plant can be obtained with low variability when compared to a Chinese seed-generated crop that produced an average of 0.6 % artemisinin (Ferreira, unpublished). The same clone from Mediplant, tested on potassium-deficient soil maintained the average concentration of 0.9 % artemisinin when potassium was supplied, but produced 1.6 % artemisinin when potassium was withheld (Ferreira 2007). Against this backdrop, crop establishment from cloned plants looks like an attractive option if the source plant is rich in artemisinin content, such as 1.5 % that has been reported recently (Graham et al. 2010). Such vegetative propagation methods will be equally useful for maintaining genetic fecundity. However, the cost benefits of crop establishment from seeds versus asexually propagated plantlets also need to be evaluated.
Meanwhile, there is great hope that global artemisinin requirements can be met by in planta production as potential new plant sources of artemisinin, apart from A. annua, have been identified (Mannam et al. 2010). This development is of great pharmacological importance since these Artemisia species are widely distributed and are abundant in many parts of Asia. Thus far, some of these diverse Artemisia species including A. sieversiana, A. dracunculus var. dracunculus, and others have shown that they can produce artemisinin, although at significantly lower concentrations than A. annua (Fig. 15.2). Consequently, these plants can be collected from the wild and screened further for genetic improvement and biological activity against the same diseases, which are being used to evaluate the efficacy of A. annua and artemisinin.
Comparison of whole-plant parts (flowers, leaves, stem, and roots) artemisinin concentration among all seventeen Artemisia species (Bar represents the mean values of artemisinin in each treatment, and the alphabets above represent the LSD ranking of these values at a = 0.05) (Culled from Mannan et al. 2010)
15.4.2 Biotechnology of A. annua and Enhanced Production of Artemisinin
Considering that A. annua is the only viable source of artemisinin at the moment, there is understandably a great degree of interest in enhancing its production. And although effective, the agronomic platform as the main production strategy seems unlikely to solve the problem of global artemisinin availability due to the boom-and-bust cycles that its production has become associated with lately. As there are growing concerns that current artemisinin supply chain will be unable to meet future requirements, it is obvious that there is need for an additional source of artemisinin which supply will be consistent, reliable, and inexpensive. Consequently, a multifaceted approach using several strategies, including the utilization of the advanced techniques emerging from classical molecular biology, industrial fermentation, and genetic engineering research, would be of great interest. Some of these strategies include, but are not necessarily limited to the following.
15.4.2.1 Artificial Polyploidization of Artemisia annua
Artificial polyploidization is generally known to give rise to larger reproductive and vegetative organs (Adaniya and Shira 2001). It has also been shown to increase the production of important medicinal compounds and other secondary metabolites over those of their diploid counterparts (Griesbach and Kamo 1996). With this in mind, Wallaart et al. (1999) successfully induced tetraploid whole plants (2n = 4x = 36) from the diploid A. annua plants using colchicine. They reported a polyploidy production efficiency of 20 and a 30 % higher artemisinin yield in the tetraploid plants. And although the increased yields of these tetraploid clones did not reach commercially useful quantities (mg/g dry weight) of artemisinin, the work showed that there are certainly some advantages in selecting for high-yielding polyploids.
15.4.2.2 Metabolic Engineering of the Artemisinin Biosynthesis Pathway
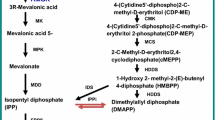
In recent years, the use of genetic engineering techniques to alter the metabolic pathway of artemisinin biosynthesis in transgenic A. annua has been attempted (Arsenault et al. 2008; Liu et al. 2011). This has been achieved mainly through the introduction of key genes encoding for enzymes regulating the biosynthetic pathway leading to the formation of artemisinin in planta. In this connection, the role of certain genes, especially those involving key enzymes in the biosynthesis of artemisinin such as farnesyl diphosphate synthase (FDS) and amorpha-4,11-diene synthase (AMS), readily comes to mind. It could be speculated that genes controlling these key enzymes can be manipulated such that the enzymes become overexpressed in A. annua. Alternatively, other enzymes that are involved in pathways competing for precursors of artemisinin, for example, squalene synthase (SQS) can be inhibited through genetic engineering such that the genetically modified plants produce more artemisinin.
Efforts are equally geared towards the development of transgenic plants by introducing the gene for artemisinin production (from A. annua) into a much faster-growing plant species, for example, chicory or tobacco (Nicotiana tabacum) with a proportionately higher leaf biomass, possibly, to enhance higher artemisinin yield at a very low cost. Such efforts already appear to be largely rewarding as demonstrated recently where the introduction of a gene into N. tabacum resulted in the expression of an active enzyme and the accumulation of the first-dedicated precursor of artemisinin (amorpha-4,11-diene) ranging from 0.2 to 1.7 ng/g fresh weight of leaf tissue (Wallaart et al. 2001). Some studies have also transformed a cDNA encoding cotton FDS (farnesyl diphosphate synthase) under the control of CaMV 35S promoter into A. annua via A. tumefaciens or A. rhizogenes. By overexpressing FDS, a key enzyme in the biosynthesis of artemisinin, the content of the sesquiterpenoid antimalarial drug was increased by about 0.8–1 % dry weight in the transgenic plants (Chen et al. 2000). Lately, N. benthamiana has also been deployed at commercial scale for rapid production of several pharmaceutical precursors of artemisinin (van Harpen et al. 2010), further opening up the vista of opportunities that can be utilized for the production of this essential antimalarial drug.
15.4.2.3 Up-Scaling of Ex Planta Semi-synthesis of Artemisinin in Microbial Systems
Surprisingly, this feature does not seem to be unique to plants alone. Recent advances using recombinant microbes circumvented the poor performance of plant terpene cyclases by expressing a codon-optimized fold (Martin et al. 2003). In a remarkable series of metabolic engineering experiments, these authors equally used engineered mevalonate pathway gene from the yeast eukaryotic system, which was about 30 to 90 times more efficient than the normal pathway in E. coli. This combined approach highlights an increased production of amorpha-4,11-diene by approximately 1,000-fold (Martin et al. 2003), which taken further into the pathway would possibly lead to the production of artemisinic acid. In a more facile approach, a cytochrome P450 monooxygenase gene (CYP71AV1) isolated directly from glandular trichomes of A. annua (Teoh et al. 2006) and inserted in yeast cells performed a three-step oxidation of amorpha- 4,11-diene that allowed its conversion into artemisinic acid in yields that appear suitable for large-scale fermentation (Ro et al. 2006). These authors successfully added or tweaked a dozen genes in yeast in commercial fermentation tanks to produce artemisinic acid. Coming on the footsteps of this development, it is of special pharmacological interest that efforts are currently underway to optimize the CYP71AV1 gene expression system in several prokaryotic strains in order to sustain high-level production of amorpha-4,11-diene that can be easily converted to artemisinic acid, which can be subsequently oxidized to yield artemisinin (Hale et al. 2007). The hallmark in all of these studies was the desire to modify the genomes of bacteria and yeast which can be fermented in huge bioreactors to yield a plentiful and inexpensive supply of artemisinic acid. This metabolically synthesized artemisinic acid can be obtained easily through a simple purification process, which can be converted to artemisinin through a few inexpensive chemical steps in the laboratory. The artemisinin thus produced can be further converted through simple downstream chemistry into derivatives such as dihydroartemisinin, artesunate, or artemether for possible integration with other antimalarial drugs for the production of low-cost, life-saving ACTs with a great impact on malaria mortality or the treatment for several neglected parasitic diseases in the tropics.
Production of artemisinin in large fermentation vessels through microbial engineering and simple chemistry, as illustrated in Fig. 15.3, may pave the way for an industrial process capable of supplementing the global supply of the drug from a second source, independent of the boom-and-bust uncertainties associated with in planta production (Paddon et al. 2013), which have had a highly negative impact both on the producers and on health outcomes. This ex planta approach came as a promise to increase supplies of high-quality artemisinin and, overall, lower the cost of ACTs in the near future (Ro et al. 2006). However, seven years down the road, this has not yet materialized because the science-related logistics are still beset with a lot of problems as the process has only recently moved into commercial production and distribution. Consequently, it is expected that production from the crop will remain a crucial source of artemisinin for the foreseeable future, though promise of the arrival of semi-synthetic artemisinin to international commerce has put considerable pressure on prices of the plant-derived compound, which fell from US$ 800—950/kg to US$420—550/kg in October 2012. Meanwhile, factory produced ex planta-derived artemisinin, when the process becomes commercially successful, could serve as a supplemental source of the drug and not necessarily as the single magic bullet for its production. This is especially so as artemisinin semi-synthesized in microbial systems in fermentation tanks might not be any cheaper than the in planta-derived version. In this regard, artemisinin derived from ex planta sources could be used to simply smooth shortfalls that are presently experienced in agricultural production. This will be inevitable, as the loss of a child every 40 s to malaria (Bowles et al. 2008; Sachs and Malaney 2002) in parts of the world should prompt everyone to focus on enhancing the present supply of plant-derived artemisinin by cultivating genetically improved varieties and increasing the land area dedicated to the crop. The above scenario has actually led the Royal Tropical Institute of The Netherlands to assert that sufficient supplies of artemisinin could be met by increasing cultivation of A. annua in suitable regions of the developing world, especially Africa, where farmers have become quite used to its field management (Heemskerk et al. 2006). Now, since approximately 90 % of deaths from malaria occur in Africa, malaria is an African ‘problem’. Thus, it is not surprising that African producers of the crop also have a strong incentive to remain in the forefront of the development of artemisinin-based drugs, which can be considered a locally based remedy for a locally based disease (Ellman 2010). Such developments will certainly bring immediate benefits to the existing artemisinin supply chain by reducing production costs, stabilizing supplies, and improving grower confidence in the crop.
The process for the microbial production of artemisinin (Adapted from Hale et al. 2007). Using synthetic biology, the metabolism of the microbe is engineered to produce artemisinic acid, a precursor to artemisinin. Starting from acetyl-CoA (an abundant product of the central metabolism of many microbes), the microbes produce, in turn, mevalonate, farnesyl pyrophosphate (FPP), amorphadiene, and, finally, artemisinic acid. The artemisinic acid is released from the microbes and purified from the culture media. The artemisinic acid is chemically converted to artemisinin. Once the artemisinin is produced, it must be further chemically converted into a derivative such as artesunate or artemether, which are integrated into ACTs for the treatment for malaria
15.4.3 Enhanced Semi-synthesis of Artemisinin Through Conversion of Sesquiterpenic Precursors of Artemisinin
Apart from in planta approach in A. annua and biotechnological means for enhancing the production of artemisinin in microbial systems, a new and efficient method being touted involves the establishment of commercial-scale extraction of artemisinic acid and dihydroartemisinic acid, two major sesquiterpenoid precursors of artemisinin, which have been identified from most commercial cultivars of the plant. A Chinese cultivar that was cultivated in 2006 in a West Virginia, USA, field and analysed for artemisinin, dihydroartemisinic acid, and artemisinic acid by HPLC throughout the growing season showed a peak in artemisinin production between August 28th and September 1st (Ferreira 2008). These plants had 0.93 % artemisinin, 1.6 % dihydroartemisinic acid, and 0.28 % artemisinic acid, respectively. The author suggested that artemisinin production could, at least, be doubled by using both dihydroartemisinic acid and artemisinic acid eliminated in the by-product of artemisinin production (Jorge Ferreira, pers. comm.). Also, approximate quantifications for these sesquiterpenoid precursors indicate that there were about 24 % of dihydroartemisinic acid and 5 % of artemisinic acid, respectively, from the high artemisinin-containing cultivar Artemis (Ferreira, unpublished). Unfortunately, both dihydroartemisinic acid and artemisinic acid, which are usually extracted with refluxing in the extraction solvent, are presently discarded in the artemisinin purification steps, where artemisinin is pooled into non-polar fractions. It is, therefore, of immense economic importance that methods for extraction and conversion of dihydroartemisinic acid and artemisinic acid into artemisinin are optimized. This can potentially increase the final artemisinin profile derivable from a given quantity of dry leafy biomass by approximately 30 % (Brisibe et al. 2008b), especially against the backdrop of a recent finding where a photochemistry-based method, developed by researchers at the Max Plank Institute, Germany transformed dihydroartemisinin into artemisinin without enzymes but just with the use of light and oxygen (Lévesque and Seeberger 2012). It is obvious, therefore, that this approach is feasible and can be used to increase artemisinin production from the crop.
15.5 Conclusion and Future Perspective
Thomas Jefferson in 1813 wrote that ‘The greatest service which can be rendered any country is to add a useful plant to its culture’. There is no doubt that the spotlight on international malaria therapy is presently focused on the availability of artemisinin and the supply of ACTs from a seemingly simple, yet versatile plant of Asian origin that is suddenly found at the forefront of global efforts aimed at the eradication of malaria. In the current setting, it is essential that the production of artemisinin and its use as the key active ingredient in the manufacture of ACTs should be seen as the central focus (Brisibe et al. 2008b). Not surprisingly, the heavy demand placed on artemisinin due to its huge pharmacological benefits, especially in the treatment for malaria, would necessitate that possible alternatives for its larger-scale production, apart from in planta extraction, be considered. Under this circumstance, ex planta synthesis in microbial systems to produce some of its isoprenoid precursors such as dihydroartemisinic acid and artemisinic acid, which can subsequently be converted to artemisinin through inexpensive chemical procedures (Roth and Acton 1989), holds some promise. Still, this approach clearly has its own limitations of producing only artemisinin. The corollary implications of the ex planta method of artemisinin production include eliminating the natural germplasm of A. annua and the impact this would have on biodiversity and on the livelihoods of small- and medium-scale farmers already invested in the agricultural production of A. annua for its artemisinin-rich dried leaves. This is aside the unnecessary limitations that would be placed on other potential medicinal and industrial benefits of the plant that are conferred on it by the rich portfolio of biologically active compounds such as antioxidants, which in turn, synergize with artemisinin to enhance its potency. In addition to artemisinin, other bioactive components including other antimalarial compounds present in the leaves may become available in the traditional Artemisia tea therapy, indicating that the plant is already a combination drug that may offer a cost-effective and affordable solution for malaria for low-income patients in developing countries. Currently, there are already pointers in this direction. All that would be required is for thoroughly controlled clinical studies to be conducted on the effectiveness and potential risks of treatment with the Artemisia tea, encapsulated dried biomass or fresh plant material.
A 2006 report of The Netherland’s Tropical Institute actually warned that the prospect of synthetic production of artemisinin could further destabilize a very young market for natural artemisinin derived from A. annua, undermining the security of farmers just beginning to plant it for the first time. Surely a stable and adequate source of artemisinin supply would be fundamentally important in the global fight against malaria (Assured Artemisinin Supply System 2012—http://www.a2s2.org/index.php?id=50) and many other neglected tropical diseases. However, both plant-derived and ex planta production of artemisinin should be encouraged and adequately supported as they will collectively play a pivotal role in the supply of artemisinin for ACT production as well as bring immediate benefits to the existing artemisinin supply chain by reducing production costs, stabilizing supplies, and improving grower confidence in the crop in countries such as Kenya, Tanzania, Madagascar, Uganda, and Nigeria, where A. annua has been cultivated by smallholders for the past 10 years. Presently, an estimated land area of 2,000 ha in Kenya (all processed by East African Botanicals), 800 ha in Madagascar, 200 ha in Uganda, 20 ha in Tanzania (Malcolm Cutler, pers. comm.) and 156 ha in Nigeria are devoted to the cultivation of A. annua by small-scale, rural-based farmers, who take a net revenue of about US$ 600 from the dried leaf biomass per ha of land. The figures may be similar in other African countries where Artemisia cultivation has started. The income derived by rural families from the cultivation of the crop is very beneficial and help to solve several socio-economic problems in rural communities. Consequently, encouragement of ex planta production of artemisinin to the detriment of agricultural production can disrupt the livelihoods of thousands of farmers in Africa, who cultivate A. annua and earn reasonable incomes by supplying dried leaves to companies that specialize in the extraction of artemisinin, which is currently exported to pharmaceutical factories in India and Europe for the manufacture of live-saving ACTs.
References
Abate S, Damtew Z, Mengesha B (2011) Artemisia annua as an alternative potential weed control option. Afr J Food Agri Nutrition Develop 11:1–6
Adaniya S, Shira D (2001) In vitro induction of tetraploid ginger (Zingiber officinalis Roscoe) and its pollen fertility and germinability. Sci Hort 88:277–287
Alin MH, Bjorkman A (1994) Concentration and time dependency of artemisinin efficacy against Plasmodium falciparum in vitro. Am J Trop Med Hyg 50:771–776
Almeida GF, Horsted K, Thamsborg SM, Kyvsgaard NC, Ferreira JFS, Hermansen JE (2012) Use of Artemisia annua as a natural coccidiostat in free-range broilers and its effects on infection dynamics and performance. Vet Parasitol 186:178–187. doi http://dx.doi.org/10.1016/j.vetpar.2011.11.058
Arsenault PR, Wobbe KK, Weathers PJ (2008) Recent advances in artemisinin production through heterologous expression. Curr Med Chem 15:2886–2896
Assured Artemisinin Supply System (A2S2) (2012) Production cycle: from artemisia to ACT, 26 Jan 2012: http://www.a2s2.org/index.php?id=50
Bhakuni RS, Jain DC, Sharma RP, Kumar S (2001) Secondary metabolites of Artemisia annua and their biological activity. Curr Sci 80:35–48
Bowles D, Smallwood M, Graham I (2008) Fast track breeding of A. annua. Ph.D symposium of the Zurich-Basel Plant Science Center, 6 June, Switzerland
Brisibe EA, Daniel JU (2013) Efficacy of fresh whole leaf preparations of Artemisia annua against uncomplicated falciparum malaria based on polymerase chain reaction fingerprinting. Afr J Biotechnol (under review)
Brisibe EA, Udensi O, Chukwurah PN, de Magalhäes PM, Figueira GM, Ferreira JFS (2012) Adaptation and agronomic performance of Artemisia annua L. under lowland humid tropical conditions. Ind Crops Prod 39:190–197. doi:http://dx.doi.org/10.1016/j.indcrop.2012.02.018
Brisibe EA, Brisibe F, Agba D, Abang AE (2011a) Antihyperglycaemic activity and haematological efficacy of Artemisia annua leaves as dietary inclusions in albino rats. Molecules 16: 1-x manuscripts; doi:10.3390/molecules160x000x (Accepted for publication)
Brisibe EA, Adugbo SE, Ekanem U, Brisibe F, Figueira GM (2011b) Controlling bruchid pests of stored cowpea seeds with dried leaves of Artemisia annua and two other common botanicals. Afr J Biotechnol 10:9586–9592
Brisibe EA, Umoren UE, Brisibe F, Magalhaes PM, Ferreira JFS, Luthria D, Wu X, Prior P (2009) Nutritional characterization and antioxidant capacity of different tissues of Artemisia annua L. Food Chem 115:1240–1426
Brisibe EA, Umoren UE, Owai PU, Brisibe F (2008a) Dietary inclusion of dried Artemisia annua leaves for management of coccidiosis and growth enhancement in chicken. Afr J Biotechnol 7:4083–4092
Brisibe EA, Uyoh EA, Brisibe F, Magalhäes PM, Ferreira JFS (2008b) Building a golden triangle for the production and use of artemisinin derivatives against falciparum malaria in Africa. Afr J Biotechnol 7:4884–4896
Brisibe EA (2006) Challenges and opportunities in the local production of artemisinin-based combination therapies against malaria in Nigeria. J Pharm Sci Pharm Pract 8:49–59
Cheeseman IH, Miller BA, Nair S, Nkhoma S, Tan A, Tan JC, Saai SA, Phyo AP, Moo CL, Lwin KM, McGready R, Ashley E, Imwong M, Stepniewska K, Yi P, Dondorp AM, Mayxay M, Newton PN, White NJ, Nosten F, Ferdig MT, Anderson TJC (2012) A major genome region underlying artemisinin resistance in malaria. Science 336:79–82
Chen DH, Ye HC, Li GF (2000) Expression of a chimeric farnesyl diphosphate synthase gene in A. annua L. transgenic plants via Agrobacterium tumefaciens-mediated transformation. Plant Sci 155:179–185
Cherian G, Orr IA, Burke IC, Pan W (2013) Feeding Artemisia annua alters digesta pH and muscle lipid oxidation products in broiler chickens. Poult Sci 92:1085–1090
Debrunner N, Dvorak V, Magalhaes P, Delabays N (1996) Selection of genotypes of A. annua L. for the agricultural production of artemisinin. In: Pank F (ed) international symposium on breeding research on medicinal plants, Quedlinburg, pp 222–225
Delabays N, Darbellay C, Galland N (2002) Variation and heritability of artemisinin content of A. annua L. In: Wright CW (ed) Artemisia. Taylor and Francis, London, pp 197–209
Delabays N, Simonnet X, Gaudin M (2001) The genetics of artemisinin content in A. annua L. and the breeding of high yielding cultivars. Curr Med Chem 8:1795–1801
Delabays N (1994) La Domestication et L’Amelioration Genetique De L’Artemisia annua Dans Le Cadre Du Developpement D’un Nouveau Medicament Contre La Malaria. In: International conference on aromatic and medicinal plants, pp 1–11
Drăgan L, Titilincu A, Dan I, Dunca I, Drăgan M, Mircean V (2010) Effects of A. annua and Pimpinella anisum on Eimeria tenella (Phylum Apicomplexa) low infection in chickens. Sci Parasitol 11:77–82
De Ridder S, van der Kooy F, Verpoorte R (2008) Artemisia annua as a self-reliant treatment for malaria in developing countries. J Ethnopharmacol 120:302–314. doi:10.1016/j.jep.2008.09.017
Efferth T, Herrmann F, Tahrani A, Wink M (2011) Cytotoxic activity of secondary metabolites derived from Artemisia annua L. towards cancer cells in comparison to its designated active constituent artemisinin. Phytomedicine 18:959–969
Efferth T, Romero MR, Wolf DG, Stamminger T, Marin JJG, Marschall M (2008) The antiviral activities of artemisinin and artesunate. Clin Infect Dis 47:804–811
Efferth T, Dunstan H, Sauerberry A, Miyachi H, Chitambar CR (2001) Antimalarial artesunate is also active against cancer. Int J Oncol 18:767–773
Ekanem A, Brisibe EA (2010) Effects of ethanol extract of Artemisia annua L. against monogenean parasites of Heterobranchus longifilis. Parasitol Res 106:1135–1139. doi:10.1007/s00436-010-1787-0
Elfawal MA, Towler MJ, Reich NG, Golenbock D, Weathers PJ, Rich SM (2012) Dried whole plant Artemisia annua as an antimalarial therapy. PLoS ONE 7:e52746. doi:10.1371/journal.pone.0052746
Elford BC, Roberts MF, Phillipson JD, Wilson RJ (1987) Potentiation of the antimalarial activity of qinghaosu by methoxylated flavones. Trans R Soc Trop Med Hyg 81:434–436. doi:10.1016/0035-9203(87)90161-1
Ellman A (2010) Cultivation of Artemisia annua in Africa and Asia. Outlooks Pest Manage 21:84–88
Ferreira JFS, Luthria DL, Sasaki T, Heyerick A (2010) Flavonoids from A. annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 15:3135–3170
Ferreira JFS, Janick J (1995) Floral morphology of A. annua with special reference to trichomes. Int J Plant Sci 156:807–815
Ferreira JFS, Laughlin JC, Delabays N, Magalhães PM (2005) Cultivation and genetics of A. annua L. for increased production of the antimalarial artemisinin. Plant Genet Res 3:206–229
Ferreira JFS (2009) Artemisia species in small ruminant production: their potential antioxidant and anthelmintic effects. In: Morales M (ed) Appalachian workshop and research update: improving small ruminant grazing practices. Mountain State University/USDA, Beaver, WV, pp 53–70
Ferreira JFS (2008) Seasonal and post-harvest accumulation of artemisinin, artemisinic acid, and dihydroartemisinic acid in three accessions of A. annua cultivated in West Virginia, USA. Planta Medica 74:310–311(Abstract only)
Ferreira JFS (2007) Nutrient deficiency in the production of artemisinin, dihydroartemisinic acid, and artemisinic acid in A. annua L. J Agric Food Chem 55:1686–1694
Ferreira JFS, Gonzalez JM (2009) Analysis of underivatized artemisinin and related sesquiterpene lactones by high-performance liquid chromatography with ultraviolet detection. Phytochem Anal 20:91–97
Gilbert B, Alves LF (2003) Synergy in plant medicines. Curr Medicin Chem 10:13–20
Gordi T, Huong DX, Hai TN, Nieu NT, Ashton M (2002) Artemisinin pharmacokinetics and efficacy in uncomplicated-malaria patients treated with two different dosage regimens. Antimicrob Agents Chemother 46:1026–1031
Graham IA, Besser K, Blumer S, Branigan CA, Czechowski T, Elias L, Guterman I, Harvey D, Isaac PG, Khan AM, Larson TR, Li Y, Pawson T, Penfield T, Rae AM, Rathbone DA, Reid S, Ross J, Smallwood MF, Segura V, Townsend T, Vyas D, Winzer T, Bowles D (2010) The genetic map of A. annua L. identifies loci affecting yield of the antimalarial drug artemisinin. Science 327:328–331. doi:10.1126/science.1182612
Griesbach RJ, Kamo KK (1996) The effect of induced polyploidy on the flavonoids of Petunia ‘Mitchell’. Phytochemistry 42:361–363
Hale H, Keasling JD, Renninger N, Diagana TT (2007) Microbially derived artemisinin: A biotechnology solution to the global problem of access to affordable antimalarial drugs. Am J Trop Med Hyg 77:198–202
Hart SP, Ferreira JFS, Wang Z (2007) Efficacy of wormwoods (Artemisia spp.) as an anthelmintic in goats. J An Sci 86:92
Heemskerk W, Schallig H, Piters BD (2006) The world of Artemisia in 44 questions, Royal Tropical Institute (commissioned by the Netherlands Directorate-General for International Cooperation, Ministry of Foreign Affairs), Netherlands, March, p 85 (www.kit.nl/frameset.asp?/development/html/publications_db.asp&frnr=1&itemID=1948)
Hsu E (2006) The history of qing hao in the Chinese materia medica. Trans R Soc Trop Med Hyg 100:505–508
Jones-Brando L, D’Angelo J, Posner GH, Yolken R (2006) In vitro inhibition of Toxoplasma gondii by four new derivatives of artemisinin. Antimicrob Agents Chemother 50:4206–4208
Kappe SHI, Vaughan AM, Boddey JA, Cowman AF (2010) That was then but this is now: malaria research in the time of an eradication agenda. Science 328:862–866. doi:10.1126/science.1184785
Kumar S, Gupta SK, Singh P, Bajpai P, Gupta MM, Singh D, Gupta AK, Ram G, Shasany AK, Sharma S (2004) High yields of artemisinin by multi-harvest of A. annua crops. Ind Crops Prod 19:77–90
Lévesque F, Seeberger PH (2012) Continuous-flow synthesis of the anti-malaria drug artemisinin. Angew Chem Int Ed 51:1706–1709. doi:10.1002/anie.201107446
Lai H, Singh NP (1995) Selective cancer cell cytotoxicity from exposure in dihydroartemisinin and holotransferrin. Cancer Lett 91:41–46
Liu B, Wang H, Du Z, Li G, Ye H (2011) Metabolic engineering of artemisinin biosynthesis in A. annua L. Plant Cell Rep 30:689–694
Liu KCSC, Yang SL, Roberts MF, Elford BC, Phillipson JD (1992) Antimalarial activity of A. annua flavonoids from whole plants and cell-cultures. Plant Cell Rep 11:637–640
Lubbe A, Seibert I, Klimkait T, van der Kooya F (2012) Ethnopharmacology in overdrive: The remarkable anti-HIV activity of Artemisia annua. J Ethnopharmacol 14:854–849. doi: 10.1016/j.jep.2012.03.024
Ma Y, Lu D, Lu X, Liao L, Hu X (2004) Activity of dihydroartemisinin against Leishmania donovani both in vitro and vivo. Chin Med J 117:1271–1273
Mannan A, Ahmed I, Arshad W, Asim MF, Qureshi RA, Hussain I, Mirza B (2010) Survey of artemisinin production by diverse Artemisia species in northern Pakistan. Malaria J 9:310. doi: 10.1186/1475-2875-9-310
Martin VJ, Pitera DJ, Withers ST, Newman JD, Keasling JD (2003) Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol 21:796–802
Merali S, Meshnick SR (1991) Susceptibility of Pneumocystis carinii to artemisinin in vitro. Antimicrob Agents Chemother 35:1225–1227
Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, et al (2013) High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 496:528–532
Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, Brinker A, Moreno DA, Ripoll C, Yakoby N, O’Neal JM, Cornwell T, Pastor I, Fridlender B (2002) Plants and human health in the twenty-first century. Trends Biotechnol 20:522–531
Rath K, Taxis K, Walz G, Gleiter CH, Li S-M, Heide L (2004) Pharmacokinetic study of Artemisinin after oral intake of a traditional preparation of Artemisia annua L. (Annualwormwood). Am J Trop Med Hyg 70:128–132
Rinaldi A (2004) Fighting malaria at the crossroads: the tools to battle the disease exist, but the lack of political will in developed nations jeopardizes their success. EMBO Rep 5:847–851
Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MC, Withers ST, Shiba Y, Sarpong R, Keasling JD (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–943
Romero MR, Serrano MA, Vallejo M, Efferth T, Alvarez M, Marin JJG (2006) Antiviral effect of artemisinin from A. annua against a model member of the flaviviridae family, the bovine viral diarrhoea virus (BVDV). Planta Med 72:1169–1174
Roth RJ, Acton N (1989) A simple conversion of artemisinic acid into artemisinin. J Nat Prod 52:1183–1185
Sachs J, Malaney P (2002) The economic and social burden of malaria. Nature 415:680–685
Sadava D, Philips T, Lin C (2002) Transferrin overcomes drug resistance to artemisinin in human small cell lung carcinoma cells. Cancer Lett 179:151–156
Sangwan NS, Sangwan RS, Kumar S (1998) Isolation of genomic DNA from the antimalarial plant A. annua. Plant Mol Biol Rep 16:1–8
Schmid G, Hofheinz W (1983) Total synthesis of qinghaosu. J Am Chem Soc 105:624–625
Singh ND, Lai H (2001) Selective toxicity of dihydroartemisinin and holotransferrin towards human cancer cells. Life Sci 10:49–56
Tang HQ, Hu J, Yang L, Tan RX (2000) Terpenoids and flavonoids from Artemisia species. Planta Med 66:391–393
Tellez MR, Canel C, Rimando AM, Duke SO (1999) Differential accumulation of isoprenoids in glanded and glandless A. annua L. Photochemistry 52:1035–1040
Teoh KH, Polichuk DR, Reed DW, Nowak G, Covello PS (2006) Artemisia annua L. (Asteraceae) trichome-specific cDNA reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett 580:1411–1416
Townsend T, Segura V, Chigeza G, Penfield T, Rae A, Harvey D, Bowles D, Graham IA (2013) The use of combining ability analysis to identify elite parents for A. annua F1 hybrid production. PLoS ONE 8(4):e61989. doi:10.1371/journal.pone.0061989
Turner KE, Ferreira J F S (2005) Potential use of A. annua in meat goat production systems. In: Cassida K (ed) The conference of the American forage and grassland council, 11–15 June, AFGC, Bloomington, IL, pp 221–225
Utzinger T, Shuhua X, Keiser J, Minggan C, Jiang Z, Tanner M (2001) Current progress in the development and use of artemether for chemoprophylaxis of major human schistosome parasites. Curr Med Chem 8:1841–1859
Van der Kooy F, Verpoorte R (2011) The content of artemisinin in the A. annua tea infusion. Planta Med 77:1754–1756
Van Herpen TWJM, Cankar K, Nogueira M, Bosch D, Bouwmeester HJ, Beekwilder J (2010) Nicotiana benthamiana as a production platform for artemisinin precursors. PLoS ONE 5(12):e14222. doi:10.1371/journal.pone.0014222
Wallaart TE, Bouwmeester HJ, Hille J, Poppinga L, Maijers NC (2001) Amorpha-4,11-diene synthase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta 212:460–465
Wallaart TE, Pras N, Quax WJ (1999) Seasonal variations of artemisinin and its biosynthetic precursors in tetraploid A. annua plants compared with the wild-type. Planta Med 65:723–728
Wang J-X, Tang W, Zhou R, Wan J, Shi L-P, Zhang Y, Yang Y-F, Li Y, Zuo J-P (2008) The new water-soluble artemisinin derivative SM905 ameliorates collagen-induced arthritis by suppression of inflammatory and Th17 responses. Br J Pharmacol 153:1303–1310
Weathers PJ, Arsenault PR, Covello PS, McMickle A, Teoh KH, Reed DR (2011) Artemisinin production in A. annua: studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem Rev 10:173–183. doi:10.1007/s11101-010-9166-0
Willcox M, Falquet J, Ferreira JFS, Gilbert B, Hsu E, Melillo de Magalhães P, Plaizier-Vercammen J, Sharma VP, Wright CW (2007) Artemisia annua as a herbal tea for malaria. Afr J Trad Complement Alternat Med 4:121–123
Willcox M (2009) Artemisia species: from traditional medicines to modern antimalarials–and back again. J Altern Complement Med 15:101–109
World Health Organization (2006) Facts on ACTs, Jan 2006 update. WHO, Geneva, Switzerland
World Health Organization (2002) Meeting on antimalarial drug development. WHO technical report RS/2001/GE/33 (CHN)
Xiao SH, Chollet J, Utzinger J, Matile H, Jinyan M, Tanner M (2001) Artemether administered together with haemin damages schistosomes in vitro. Trans R Soc Trop Med Hyg 95:67–71
Xu XX, Zhu J, Huang DZ, Zhou WS (1986) Total synthesis of arteannuin and deoxyarteannuin. Tetrahedron 42:819–828
Yang DM, Liew FY (1993) Effects of qinghaosu (artemisinin) and its derivatives on experimental cutaneous leishmaniasis. Parasitology 106:7–11
Zhang Y, Sun H (2009) Immunosuppressive effect of ethanol extract of Artemisia annua on specific antibody and cellular responses of mice against ovalbumin. Immunopharmacol Immunotoxicol 31:625–630
Zhu C, Cook SP (2012) A concise synthesis of (+)-artemisinin. J Am Chem Soc 134:13577–13579
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Brisibe, E.A., Chukwurah, P.N. (2014). Production of Artemisinin In Planta and in Microbial Systems Need Not Be Mutually Exclusive. In: Aftab, T., Ferreira, J., Khan, M., Naeem, M. (eds) Artemisia annua - Pharmacology and Biotechnology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-41027-7_15
Download citation
DOI: https://doi.org/10.1007/978-3-642-41027-7_15
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-41026-0
Online ISBN: 978-3-642-41027-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)