Abstract
Speculation surrounds the importance of ecologically cryptic Symbiodinium spp. (dinoflagellates) that occur at low abundances in reef-building corals and in the surrounding environment. Evidence acquired from extensive sampling, long-term monitoring, and experimental manipulation can allow us to deduce the ecology and functional significance of these populations and whether they might contribute to the response of coral-dinoflagellate mutualisms to climate change. Quantitative PCR was used here to diagnose the prevalence, seasonal variation, and abundances of Symbiodinium spp. within and between colonies of the coral, Alveopora japonica. Consistent with broader geographic sampling, only one species comprised 99.9 %, or greater, the population of symbionts in every sample. However, other Symbiodinium including the non-mutualistic species, Symbiodinium voratum, were often detected, but at estimated cell densities thousands-fold less than the dominant symbiont. The temporal variation in prevalence and abundances of these “background” Symbiodinium could not be definitively related to any particular environmental factor including seasonality and water chemistry. The prevalence (proportion detected among host samples), but not abundance, of S. voratum may weakly correspond to increases in environmental inorganic silica (SiO2) and possibly nitrogen (NO3). When multiple background Symbiodinium occurred within an individual polyp, the average cell densities were positively correlated, suggesting non-specific processes of cell sorting and retention by the animal. While these findings substantiate the existence of a broader, yet uncharacterized, diversity of Symbiodinium, we conclude that only those species which can occur in high abundance and are temporally stable are ultimately important to coral-dinoflagellate mutualisms. Many transient Symbiodinium spp., which occur only at trace abundances in the coral’s microbiome, belong to different functional guilds and likely have little, if any, importance to a coral’s physiology. The successful integration between host and symbiont into a stable functional unit should therefore be considered when defining host-symbiont specificity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals constantly interact with microbes in relationships that span a continuum from parasitism to mutualism. Growing awareness of microbial diversity has led to a greater understanding of their ecology and new insights into their importance to the metabolism and development of various hosts especially among marine animals [1]. For example, numerous kinds of microbes (prokaryotic and eukaryotic) are known to occur in close association with reef-building corals and related cnidarians [2–4]. Most significant among these are their symbiotic dinoflagellates (genus Symbiodinium). In a time of rapid climate change, the persistence of the ecosystems that these animals engineer may depend in large part on the physiological diversity of their photosynthetic mutualisms.
One form of resiliency may involve change in resident symbiont populations to species that are stress tolerant [5–9], but see [10–12]. Important questions remain as to whether community-level changes can occur rapidly enough to keep pace with current and projected rates of warming. Ecologically cryptic, or “background,” Symbiodinium spp. are occasionally detected in coral colonies at low background densities via quantitative PCR (qPCR) [8, 13–15] and by next generation DNA sequencing [16, 17] [but see 18]. These observations have fueled speculation about the future ecological importance of these entities by raising the plausibility for large-scale rapid switch to thermally tolerant host-symbiont combinations through replacement or “shuffling” [13].
The capacity for symbiont change in response to environmental shifts is potentially dampened by the high degree of specificity normally exhibited by both partners. Indeed, most hosts appear limited to a small number of Symbiodinium spp. in forming functionally integrated mutualisms. Nonetheless, for many symbiotic cnidarians, partner specificity is influenced in part by irradiance and temperature [e.g., 19]. Thus, many species of host show some flexibility and associate with more than one species of Symbiodinium over their entire ecological and geographical distributions [e.g., 19–25].
While the analyses of background Symbiodinium may detect host-compatible species, not all are well suited or mutualistic with a given animal. Distinct species from within and between evolutionarily divergent “clades” display large differences in ecology. The process of isolating and culturing Symbiodinium from hosts or from the environment (water column and sediment) typically recovers genetic entities (i.e., species) that are not mutualistic species [26–30]. Many of these atypical Symbiodinium appear to exist at low densities and are often undetected by conventional genetic techniques. Moreover, after isolation into culture, they subsequently fail to form stable mutualisms when exposed to aposymbiotic hosts [28, 31]; raising doubts about whether they are ever relevant to a functionally integrated and stable mutualism.
We sought to assess the ecological importance of background Symbiodinium in the temperate symbiotic coral, Alveopora japonica Eguchi, common in coastal benthic communities in China, Korea, Taiwan, and Japan [Fig. 1a, b; 32–35]. Corals living at high latitudes experience large seasonal fluctuations in temperature and light, which may affect stability in their symbioses with Symbiodinium. Extensive sampling over a range of habitats and spatial scales indicates that A. japonica exhibits high specificity for a non-described Clade F Symbiodinium [36–38]. However, S. voratum (= Clade E) was recently isolated into culture from the tissues of A. japonica from Jeju Island, Korea [28], and Lien and co-authors occasionally found traces of Symbiodinium from Clade C in their samples from Japan [39], indicating that other Symbiodinium spp. can occur with this animal.
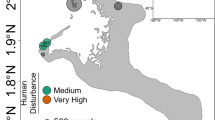
The habitat and geographical distributions of Alveopora japonica. a A. japonica forms small hemispherical colonies that measure 2–10 cm in diameter with fleshy polyps that are extended during the day. b This species can occur in large numbers in protected turbid environments at depths of 5–25 m [34]. c The geographical range of A. japonica occurs in temperate latitudes in the northwestern Pacific (light shading). d Site of sampling is indicated by the red square on the map of Jeju Island. Locations in previously published papers where A. japonica has been collected for analyses of Symbiodinium identity are indicated by diamonds [36], circles [37], and triangles [38]. Light gray arrows show the predominant sea surface currents originating from lower latitudes
Thus, we explored the extent to which multiple Symbiodinium spp. co-occur in the tissues, or microbiome, of A. japonica and whether their prevalence and abundance vary within and between colonies in populations collected each month over a 15-month duration. Individual A. japonica polyps were analyzed using quantitative (q) PCR targeting ribosomal DNA (rDNA) to diagnose the presence and absolute abundances of distantly related Symbiodinium spp. Additional DNA sequencing was employed to identify whether detection of a clade involved one or multiple species. These findings were combined with previously published work to evaluate the importance of ecologically cryptic, or background, diversity. We then reason that host and symbiont specificity should be defined by the stability and functional integration of a partnership.
Materials and Methods
Environmental Measurements, Colony Collection, Transport, and Acclimation
Every month from July 2012 to September 2013, we collected 30 colonies of A. japonica (4–7 cm in diameter, Fig. 1a) from Sindo (33°16′37.65″ N, 126°10′5.51″ E), Jeju Island, The Republic of Korea at depths of 10–13 m using SCUBA (Fig. 1d). Random colonies were obtained from the same A. japonica population in a concentric arc around a fixed center point within an area of 40–50 m2. Water samples were collected at the surface (~300 mL) for chemical analysis and the detection of Symbiodinium in the water column. Bottom water from around colonies of A. japonica was also collected in March 2013. Nutrient and chlorophyll a concentration analyses were conducted on these samples as described by [40].
Colonies were transported to the laboratory at Seoul National University and then individually acclimated in 1-L glass beakers with filtered seawater (FSW) at a temperature range of 18–25 °C under a photon flux density of 50 μmol quanta m−2 s−1 (14 L/10D), simulating the corresponding temperature, salinity, and irradiance at the sampling site. These colonies were maintained for one to several days before further processing (see below).
Environmental water temperatures and salinities were measured at the site of sampling using a YSI Professional Plus instrument (YSI Inc., Yellow Springs, OH, USA). Sea surface temperature and chlorophyll a (as a proxy for phytoplankton abundance) data were acquired from the Giovanni online data system ( http://gdata1.sci.gsfc.nasa.gov/daac-bin/G3/gui.cgi?instance_id=mairs_monthly_hres), maintained by the NASA Goddard Earth Sciences (GES) Data and Information Services Center (DISC). MODIS/Aqua monthly Chl a and SST values averaged over a 4-km grid were acquired from reflectance measurements taken in the vicinity of the collection site at Sindo, Jeju Island (an area between 33.109–33.309 N and 125.982–126.144 E). These values were averaged for each month of the study period and graphed.
Polyp Excision, Maceration, and DNA Extraction
Three polyps (1–1.5 cm in length) were removed from each colony (n = 90 polyps per month) and rinsed thoroughly with FSW to minimize contamination by exogenous Symbiodinium cells. Each polyp was gently blotted using Kimwipes (Kimberly-Clark Co., USA), placed in a 1.5-mL tube, and wet weight determined. To each tube, 1 mL of FSW was then added and the polyp homogenized using a micropestle, and the contents vortexed. To confirm the total cell density of Symbiodinium in A. japonica, 100 μL were transferred from one sample of each colony to 10-mL vial containing 4.9 mL FSW, fixed with 5 % Lugol’s solution. Cells were counted on 1-mL Sedgwick-Rafter chambers (SRCs) and light microscope. The remaining 900-μL aliquot was centrifuged at 13,000 rpm for 1 min at room temperature. The supernatant was discarded and the pellet was resuspended with 200 μL of phosphate-buffered saline (PBS) (Bioneer Corp., Korea). The mixture was immediately subjected to total DNA extraction using the AccuPrep Genomic DNA extraction kit (Bioneer Corp., Korea). The final DNA pellet was rehydrated in 100 μL water.
PCR Amplification, Sequencing, and Phylogenetic Analyses
The specific identity of the dominant and background Symbiodinium were investigated with DNA sequencing of a subset of samples collected during April 2012, July 2013, and August 2013. Amplifications of ITS1-5.8S-ITS2 rDNA and the LSU region D1–D3 were performed using the primer set and conditions developed by [41, 42]. Ribosomal DNA was Sanger sequenced and aligned manually with sequences from other Symbiodinium spp. obtained from GenBank. Maximum Parsimony was assessed using the software PAUP* [43]. Bayesian analyses were conducted using MrBayes v.3.1 [44] using a default GTR + G + I model. For each alignment, four independent Markov Chain Monte Carlo (MCMC) analyses were performed. MP bootstrap values were determined using 1000 replicates.
Design of Dual-Labeled BHQplus Probes and Primer Sets for qPCR
Ribosomal DNA sequences (ITS1, 5.8S, ITS2, and LSU) were used to construct clade-specific primers and probes (Table S1) to detect a particular group of Symbiodinium (Clade) of interest from a mixed population of Symbiodinium comprising more than one clade group (Table S2). These were subsequently compared to published sequences using BLAST homology searches on GenBank. The primers and probes were dual-labeled with the fluorescent dyes FAM and BHQplus (Biosearch Technologies Inc., Novato, CA, USA) at the 5′ and 3′ ends.
The specificity of each of the four Symbiodinium clade-specific primers and probe sets (for clades B, C, E, and F) were also tested using concentrated rDNA extracted from 100,000 cells of each of the 19 Symbiodinium strains representing approximately 10 species (Table S2). The DNA extraction method used on these cultured isolates was the same as the one used for field collected host material (see above).
qPCR Assay Conditions and Standard Curve
Quantitative PCR reactions were performed using 1-μL template DNA combined with 0.2 μM forward and reverse primers, 0.15 μM probe (final concentration), 5 μL HiFast Probe Hi-Rox (Genepole, Gwangmyung, Korea), and PCR-grade water (total final volume = 10 μL). The thermal cycling conditions consisted of 3 min at 95 °C, followed by 40 cycles of 10 s at 95 °C, 20 s at 60 °C, and 20 s at 72 °C. The fluorescence of each reaction tube was quantified in each cycle, and the threshold for a positive reaction was determined using the default settings on the software accompanying the instrument qPCR (Rotor Gene 6000, Qiagen GmBH, Germany), which designated non-specific fluorescence in samples with total changes in fluorescence less than 3 % (relative to the largest change in a sample plate).
Standard curves for TaqMan-based qPCR assays were obtained by using the DNA extracted from known numbers of the cells of the cultured strains CCMP 2459 (Symbiodinium psygmophilum representing Clade B; 500,000 cells), CCMP 2466 (S. goreauii representing Clade C; 500,000 cells), SVIC 3 (S. voratum for Clade E; 500,000 cells), and CCMP 2468 (S. kawagutii representing Clade F; 1,000,000 cells). Genomic DNA from these cells was extracted in the same method described above. Ten-fold serial dilutions of theses DNA extracts spanning five orders of magnitude were used to construct standard curves (R 2 ~ 0.99) for each primer-probe combination. The amplification efficiencies of the specific primers and probe sets of Symbiodinium clade B, C, E, and F were 93, 94, 75, and 94 %, respectively.
Threshold cycle C(t) values for unknown samples were compared against calibration curves based on DNA from known cell quantities of Clades B, C, E, and F, respectively. By comparing the C(t) values of field samples with the C(t) values of clade-specific standard curves, and using the direct cell counts taken before DNA extraction (see above), cell densities detected by each Symbiodinium Clade primer-probe set could be calculated while avoiding the affect of large differences in rDNA copy number between certain Clades [45].
Correlations Between Presence/Abundance Data
To investigate the relationship between the number of polyps with a particular background Symbiodinium at a given time point and its cell abundance detected at that time, we fit a linear model to assess whether presence-abundance were related. This was done by first calculating the percent of polyps that contained a background entity for a given sample time (n = 90 for each month). We then plotted the number of cells detected in all samples that contained the Symbiodinium in question against the number of polyps where its presence was detected for each sampling time point. A linear model was then fit to this data in the statistical software R [46].
The effect of external environmental factors on the cell abundance of background species, including ammonia, nitrate, phosphate, and silica concentrations as well as chlorophyll a and surface temperatures, was assessed using a linear model. Using the statistical software R, we fit a multiple regression linear model to the environmental variables to the abundance data with a 1 month lag time (with the assumption that cell densities are affected by the previous month’s levels). In cases with a high variance in cell number, a square root transformation was applied to these data. Finally, the correlation coefficients between the each background Symbiodinium were calculated using Pearson’s correlations [47, 48].
Results
The average wet weight of each polyp was 0.006 g (±0.003 g). Based on direct cell counts, the standing Symbiodinium population ranged 1.9 × 109 to 7.9 × 109 cells/g ww, and the mean number of Symbiodinium cells per polyp approximated eight million (±3,359,398 SD). Primers and probe sets designed for each Symbiodinium Clade (B, C, E, and F) amplified only those strains representing the target clade of Symbiodinium when tested on 19 Symbiodinium strains (Table S2). For example, the primer/probe set designed for Clade B gave a positive amplification for only CCMP830, CCMP1633, CCMP2459, and CCMP 2462 (all the representative strains of Clade B). Moreover, through these analyses, maximum C(t) cut-off values were established for each set of primers and probe above which further increases in fluorescence were not scored. A maximum C(t) threshold was set at 36 cycles for the positive detection of Clade B and C, whereas C(t) cut-offs of 38 and 31 were set for detecting Clades E and F, respectively.
Sequence analyses of ITS1-5.8S-ITS2 and large sub-unit (LSU) rDNA resolved the specific identities of Symbiodinium being detected with the primers and probes designed for the qPCR of Clades B, E, and F. The phylogenetic analyses of LSU sequences from several samples (n = 4) indicated that the Clade F entity found in A. japonica is a Symbiodinium that is distinct among the known breadth of Clade F diversity described previously from foraminifera around the world (Fig. 2). It is referred to from this point forward as “F Ajap ” and represents a non-described species. The ITS2 sequence for the Clade B Symbiodinium matched with that of S. psygmophilum (= type B2) a cold-water adapted Clade B species known from the north Atlantic and Mediterranean Sea, but the ITS1 differed by a single nucleotide substitution (data not shown). We therefore refer to this entity as B2*. Efforts to sequence rDNA markers for Clade C were not successful, and therefore, it could not be determined whether this represented a single species entity or several. Finally, our analyses of ITS2 rDNA for the Clade E qPCR amplifications verified that it corresponded to S. voratum, a free-living species common to the northwestern Pacific [28].
Ribosomal large sub-unit (LSU) DNA phylogeny identifies the phylogenetic position and uniqueness of the Symbiodinium F Ajap mutualistic with Alveopora japonica. Most Clade F species associate with soritid foraminifera in tropical regions around the world [49-51], whereas others, with enigmatic ecologies, emerge periodically as contaminants when culturing cells from cnidarian host tissues, such as the isolate of S. kawagutii obtained from Montipora capitata in Hawaii. Numbers under each branch-length refer to bootstrap values ≥75 %, based on 1000 iterations (left) and posterior probabilities ≥0.9 (right). LSU sequences of representatives from Clades B and H were used as out-groups
The Symbiodinium F Ajap was the dominant symbiont in all 1260 polyps from 420 colonies of Alveopora japonica examined during the course of this study (Fig. 3). This species on average accounted for ~99.9 % of the total resident Symbiodinium population based on qPCR standard curves generated using DNA extracted from a set number of cells obtained from representative cultures (Table S2). Cell counts where positively correlated with qPCR-based estimations (Fig. S1a, b). Members of Clades B and E were frequently detected at background densities several orders of magnitude lower (Fig. 3). Symbiodinium B2* was detected at every sampling interval, but not always in each polyp nor colony examined (Figs. 3 and 4a), and reached densities approximating 0.013 % of the total population. S. voratum (detected using the “Clade E” primers and probe) was most prevalent during Fall and Winter months reaching densities of about 0.023 % but was rarely detected and often absent from colonies during Spring collections (Figs. 3 and 4a). qPCR detection of “Clade C” occurred in samples from only 2 months, July and September of 2012 (Fig. 3). This entity was the least prevalent of the background Symbiodinium (only detected in a small proportion of colonies and polyps) and occurred at densities no greater than 0.0003 % (Fig. 4a).
Box and whisker plots showing temporal stability and variation in abundances of several Symbiodinium spp. associated with A. japonica whose populations were sampled monthly between 2012 and 2013. Plotting of qPCR analysis of the cell densities of Symbiodinium F Ajap , B2*, Symbiodinium voratum (Clade E), and Clade C (sp. ?) in colonies and polyps shows the median value along with the 25th and 75th percentile as well as the minimum and maximum values (with outliers shown as dots). The calculation of monthly cell densities for a particular Symbiodinium spp. was based only on those samples where it was detected. Estimated cell densities of Symbiodinium F Ajap were at least 1000–10,000 times more abundant than other Symbiodinium spp.
Relationship between background populations, temperature, and primary productivity. a The monthly presence/absences (% prevalence) of “background” populations of B2*, Clade C (sp. ?), and S. voratum detected by qPCR determined for each colony (n = 30) and polyp (n = 90, 3 per colony). b The direct measurements of temperature and chlorophyll a content recorded for each month in surface waters at the monitored site (open symbols) and 5-year means (July 2008 to October 2013) based on remote sensing data on surface waters adjacent to the sampling location. Error bars were drawn to indicate standard deviations (±SD)
The densities of background Symbiodinium B2* and S. voratum were correlated when each co-occurred at cell densities above 50,000 and 500,000/gww, respectively (Fig. S2). However, the presence or absence of these two Symbiodinium spp. did not appear to be significantly affected by the presence of any other species (p > 0.1, linear regression ANOVA). The cell densities of S. voratum corresponded with the proportion of polyps in which it was detected in a given month (Fig. S3).
During the study period, the water temperature measured at Sindo, Jeju Island, ranged between 10.1 and 27.5 °C (Fig. 4b) and the salinity oscillated between 30.3 and 34.7‰ (data not graphed). Chlorophyll a contents in surface waters at the study sight ranged between 0.22 and 2.34 mg/m3 and were consistent with values measured from satellite images, which ranged between 0.3 and 1.0 mg/m3 (Fig. 4b). High primary productivity at the collection site occurred in the late Summer and Spring (Fig. 4b). Nutrient concentrations for ammonium (NH4), nitrate (NO3), phosphate (PO4), and silicon dioxide (SiO2) were graphed and compared against Chlorophyll a concentration and the prevalence of Symbiodinium B2* and S. voratum (Fig. S4 and Table S3). There was a significant positive relationship between average SiO2 and cell counts of S. voratum and a significant negative relationship between average NO3 and cell counts of S. voratum (Table S3a; and only when the nutrient concentrations from the previous month were compared). The abundance of Symbiodinium B2* and S. voratum were highly variable, and when we reapplied, the linear regression model on square root transformed abundance values of S. voratum, significance of both these relationships was not supported (Table S3b).
Discussion
An ever growing body of research has observed various Symbiodinium spp. that exist at low abundances in various habitats including sediment, the water column, and in host microbiomes. Many of these reports surmise that trace cell densities of atypical species are potentially important to coral-dinoflagellate mutualisms, but provide no supporting evidence for the ecological function of these entities. Furthermore, interpretation of qPCR findings is limited because, when used alone, this method currently recognizes only clade-level distinctions and thus coarsely resolves phylogenetically divergent groups, which contain many ecologically distinct species [30]. The investigations conducted here went beyond these preliminary studies by investigating the dynamics of high and low abundance populations of Symbiodinium at monthly intervals for more than a year. Based on our findings and a review of the literature, we contextualize the ecological relevance of low-abundance background Symbiodinium spp. and caution against over interpreting their significance, especially in cases with no additional evidence.
Low-Abundance Transitory Populations of Symbiodinium spp.
Several distinct Symbiodinium occurred among colonies of A. japonica at low abundances (>0.05 %), a finding that is entirely consistent with studies conducted on tropical coral species [13–15]. Shifts in the abundances and prevalence of these entities occurred throughout the 15-month sampling period (Fig. 3). However, while our qPCR primers targeted rapidly evolving rDNA, the technique resolved only distantly related groups (i.e., clades, Table S1). Thus, the power of interpreting ecological function (niche) based on these qPCR data alone was limited until we were able to resolve species identity with additional genetic markers and methods. Moreover, just as our detection of Clade F corresponds explicitly to F Ajap (Fig. 2), we found that our detection of Clade E, and probably Clade B, corresponded to the presence of particular species with ecological attributes distinct from F Ajap .
Stable and transitory populations of Symbiodinium in coral-dinoflagellate mutualisms. (1) Symbiotic Cnidaria circulate large volumes of water (and mucus) through their gastrovascular system for respiration, waste removal, and heterotrophic feeding, a process that introduces numerous small organic particulates as well as bacterial and eukaryotic microbes, which probably includes the cells of Symbiodinium spp. from the environment. Over the course of a normal day, a colony may expel millions of Symbiodinium cells (both viable and necrotic) in the maintenance of the mutualism. At any given time, there may be several distinct Symbiodinium sp. found in the microbiome of the animal. These include dominant, temporally stable, and intracellular symbionts that are important to the growth of the animal (2), low “background” and potentially non-mutualistic species (3), and free-living non-symbiotic species (4)
Unlike other background species, the prevalence of S. voratum (but not abundance) appears to sharply increase one month after a large pulse of inorganic nitrogen (NH4, NO3) and silica (SiO2) into the environment (Fig. S1). Its occurrence in colonies of A. japonica was transitory and ultimately dropped in prevalence and became absent, only to reemerge a few months later (Figs. 3 and 4a). Symbiodinium voratum appears to be primarily free-living; it is common in coastal waters of the temperate northwest Pacific Ocean where our research was conducted and yet does not occur in corals at high abundances, in contrast to mutualistic Symbiodinium. Furthermore, it will not form symbioses during controlled experiments with aposymbiotic hosts [28]. This species is cultivable from water samples, the surfaces of macroalgae, as well as from Alveopora japonica. It may actually undergo occasional planktonic blooms and gain additional nutrients by feeding on bacteria and a wide range of microalgal prey species [reviewed in 28]. When these ecological attributes are considered together, its occurrence in A. japonica could simply reflect its relative abundance in the environment at the time of sampling. Its increasing cell abundances are related with the frequency of occurrence among polyps, which further supports this interpretation (Fig. S3).
Corals constantly sort and concentrate particles [52]. Thus, the presence/absence of S. voratum in these animals may simply relate to the interplay of external biotic or abiotic factors that govern the relative abundance of this species in the external environment. There was no strong relationship between the abundance of Symbiodinium B2* or S. voratum and the nutrient concentrations we measured. A multiple linear regression analyses identified the potential importance of silica in explaining the prevalence and abundance of S. voratum (Table S3ab). Because dinoflagellates are not known to require silica [53], this relationship may indirectly correspond to intermediate factors such as the availability of prey. Alternatively, the pattern may be due to the high variance in abundance data; indeed, the pattern was not significant after a square root transform was performed (Table S3b). We failed to detect the presence S. voratum in water samples obtained near colonies of A. japonica during the March 2013 sampling (M. J. Lee unpubl. data) indicating its rarity or absence in the environment at that time. This was consistent with the measurements of S. voratum in animal tissues during that same month which found a very low prevalence (~1 % of polyps examined) and low cell density (Fig. 3). This suggests that rarity or absence in the environment might relate to rarity or absence in the host microbiome. Future studies of this kind should consider routine sampling the environment to relate the abundances of internal background vs. external Symbiodinium populations.
Most unexpected was our detection of a Symbiodinium in Clade B, which occurred commonly as a background entity over the course of our study (Fig. 4a). Very few Clade B Symbiodinium spp. are known in symbioses with animals from the Indo-Pacific. Several exceptions are reliably found in specific hosts collected off the coasts of Australia, at tropical and temperate latitudes [12, 54, 55]. Another species, S. minutum (Clade B), occurs wherever the anemone Exaiptasia (=Aiptasia) pallida has been introduced [56]. The particular Clade B entity we detected appears to be closely related to that of Symbiodinium psygmophilum (= type B2), a cold-water adapted species common to animals in the temperate North Atlantic Ocean and Mediterranean Sea [57]. Thus, this appears to be a western Pacific version of this high latitude lineage, designated here as B2*. However, despite reports from several regional surveys of Symbiodinium diversity, Clade B species are not known to occur in corals from this region, at least in abundances that would support the nutritional requirements of an obligate host [58]. Consequently, these findings exemplify how sensitive detection measures can discover rare Symbiodinium whose ecological niche is unknown [30], but appear to be incapable of achieving high stable abundances in host tissues [29].
Most other symbiotic cnidarians that co-occur with A. japonica in coastal waters around Jeju Island associate with Clade C Symbiodinium spp. [37, 38]. It is well documented that several types of Clade C exist in the region, but each exhibits high host specificity [38, 39]. On the few occasions when Clade C was detected in A. japonica, we were unable to determine the specific identity of this Symbiodinium. These background populations may have comprised a single entity or multiple phylogenetically distinct lineages (requiring species descriptions) in Clade C. Because there is no evidence for an entirely free-living, or non-mutualistic, species in Clade C, the low prevalence and extremely low abundances of Clade C in A. japonica may reflect temporary fluctuations in environmental concentrations of various Clade C spp. from the normal discharge of excess symbiont cells by nearby colonies of other coral taxa [59–62]. In this situation, the sensitivity of next generation sequencing could resolve questions about the identity of these Symbiodinium and their probable source [17].
Stress experiments that were designed to induce an increase in background Symbiodinium to densities similar to F Ajap ultimately failed when colonies of A. japonica died as temperatures were slowly increased by only a few degrees above the mean summer high (~27 °C) for Jeju Island (M. J. Lee unpubl. data). Therefore, this particular coral, like many others, may not be open to hosting a second species of Symbiodinium, even under conditions of ecological opportunity created by stress [8].
In Hospite Stability in the Identity of High-Abundance Symbiodinium sp.
The dominance of Symbiodinium F Ajap in A. japonica throughout the duration of our study [>99.9 % of the resident symbiont population] is consistent with previous findings of stability in the identity of the dominant Symbiodinium in colonies monitored over time [63–69]. The vertical transmission of symbionts from generation to generation appears to initiate greater selection pressure, which is likely to facilitate ecological specialization and hence speciation [70]. Thus, like most corals that brood larvae, A. japonica harbors a host-specific Symbiodinium [Fig. 2; 71].
Seasonal oscillations in irradiance and temperature can affect symbiont cell densities [Fig. S1b; 72] but appear to have little effect on the species composition of a symbiont population within a coral [64, 69]. Collectively, these and many other observations (not cited) indicate that temporal stability and partner specificity are the norm for most coral-dinoflagellate symbioses. Even so, many reef-building corals exhibit some flexibility with more than one Symbiodinium sp. over environmental gradients related to changes in water depth, reef habitat, and geographical location. However, despite the large diversity of Symbiodinium spp. present in many of these environments, each coral species ultimately depends on a very restricted subset of Symbiodinium spp. for their survival and growth [19, 22]. Thus, the existence of background Symbiodinium spp. within individual colonies is potentially important to the mutualism only if they are able to attain dominance under particular environmental conditions [6, 20, 73].
Partner stability is important for many reasons in the maintenance of a mutualism [74, 75]. Processes that govern most mutualisms have probably evolved mechanisms to limit intra and inter-specific competition, thus minimizing the negative effects of cheating and competition [75]. Using conventional genetic approaches, Symbiodinium F Ajap is essentially the only Symbiodinium sp. found in A. japonica throughout the coral’s distribution (Fig. 1c, d). Its high abundance, relative to background Symbiodinium (measured by several orders of magnitude, Fig. 3), means that it provides photosynthate, which contributes to host metabolism. The in hospite abundance, temporal stability, and ecological prevalence of this particular Symbiodinium are attributes critical to the physiological performance of a sustained mutualism and should be considered when defining host-symbiont specificity [76]. Similar arguments of stability and functionality are made when evaluating the relative significance of bacterial consortia associated with humans [77].
Functional and Ecological Significance of Background Symbiodinium
Microbes of many different kinds associate in various ways with reef corals [2–4]. Moreover, corals are heterotrophic and constantly consume a variety of small particles including eukaryotic microalgae [52, 78] as well as organic and inorganic material [79]. The combined microbial diversity of commensals and prey particles is thus high. The use of qPCR primers specific for certain groups of microalgae sporadically detect the presence of diatoms, haptophytes, cryptophytes, non-Symbiodinium dinoflagellates, etc., and is dependent on their abundances [80]. The qPCR detection of free-living microalgae would not be interpreted as entities viable to an animal’s symbiosis (nor would their presence be used to argue against the existence of host-symbiont specificity). The recognition that Symbiodinium belong to different functional groups [30], some of which are not mutualistic as is S. voratum, combined with the reality that corals are continually cycling particles into (and out of) their gastrovascular systems and possess a mucosal layer that contains a rich diversity of microbes [2], questions the validity of extrapolating a significance to background Symbiodinium without additional confirmatory evidence.
Trace densities of a Symbiodinium sp. capable of achieving high densities in a host are potentially important. Changes in the dominance among host-compatible Symbiodinium are influenced by acute abiotic (e.g., high light/low light conditions) and biotic (i.e., competition) factors [6, 81, 82]. Thus, the vast majority symbiont “shuffling” [sensu 83], if or when it occurs (usually during experimental manipulations), involves those species of Symbiodinium that otherwise dominate host individuals found living in different habitats (e.g., shallow vs. deep; inshore vs. offshore). Thus, instances of change in the dominant symbiont occur in experimentally manipulated hosts and typically involve a few host-compatible species.
There is one notable exception where an atypical species of Symbiodinium increased from background levels to dominate numerous host taxa from several reef habitats. The invasive species, Symbiodinium trenchii, increased in prevalence and abundance during and after the 2005 mass bleaching event in the eastern Caribbean [8]. It occurred at low densities in many reef coral taxa several months before the peak of thermal stress [84] but was later found at high densities in many unbleached colonies of several important reef-building species, which gave them the appearance of being unaffected by the severe stress. Thus, as temperatures increased, S. trenchii appeared to have proliferated opportunistically from background cells in the symbiont population to dominate many colonies and prevented some corals from turning white. The ecological nature of this Symbiodinium is potentially significant to the symbiosis ecology of corals when exposed to increasingly stressful environmental conditions [85]. However, the physiological and ecological importance of background S. trenchii (Clade D) is more the exception because no other atypical Symbiodinium sp. appear to exhibit patterns of cell proliferation and spreading, which can compensate for losses in a coral’s normal symbiont population [29, 86].
We now recognize that there are Symbiodinium spp. in close association with animals whose ecological niche differs from habitually mutualistic species [28, 29]. They occur in many different habitats and environments including the sediment, water column, and on the surfaces of macroalgae and other organisms [87–92]. These Symbiodinium spp. (e.g., S. voratum, S. necroappetens, S. pilosum) occur in tropical, sub-tropical, and temperate environments where they exhibit diverse ecological roles and may contribute in various ways to marine food webs [93]. Therefore, while it is one piece of evidence to detect unusual Symbiodinium at low abundances in hospite, or in the environment, inferring significance to their functionality for dinoflagellate-animal symbioses is another matter entirely and requires significantly more follow-up examination.
A case in point involves Symbiodinium Clade A, which contains formally recognized species that illustrate this range of ecological diversity [94]. In addition to the mutualistic species S. microadriaticum and S. tridacnidorum [95], this group contains S. pilosum, a species cultured from cnidarians, but has yet to be identified in field collected host tissues. Given its inability to infect aposymbiotic experimental animals, it probably exists as a free-living non-mutualistic species similar to S. voratum (Clade E) and S. kawagutii (Clade F). Another species in Clade A, S. necroappetens, attains densities that are detected as the dominant Symbiodinium in coral tissues that are diseased or severely bleached [29]. It has been cultured from healthy symbiotic cnidarians at separate Caribbean locations, suggesting that cells of this species normally exist at low abundances on an animal’s surface or in the gastrovascular system. Evidence for the detection of Symbiodinium Clade A (in a sample) through the application of qPCR, which currently resolves to the clade level, could be interpreted several ways depending on which species is actually present. For those species with no known benefit to the animal, similar to our observations of S. voratum (= Clade E) in Alveopora japonica (Fig. 3), their presence is likely transient and contingent on multiple factors.
A summary diagram of our interpretations, based the findings presented here and observations from the published literature, is illustrated in Fig. 5. It shows the presence of several species of Symbiodinium; one of which is dominant while others are present that are opportunistic or incompatible with the host but living in the animal’s microbiome along with other microbes (prokaryotes not shown). It is intended to promote contemplation about the different potential interactions between symbiotic cnidarians and various Symbiodinium spp.
In conclusion, the existence of non-mutualistic Symbiodinium, which may cycle into and out of a coral’s gastrovascular system, and/or are a part of the rich diversity of microbes associated with an animal’s mucous, cautions against inferring that background Symbiodinium are functionally significant to the host without additional confirmatory evidence. Thus, concepts of host-symbiont specificity should not be predicated on the detection of low abundant Symbiodinium species. With the increasing use of qPCR screening or next generation DNA sequencing, our knowledge on the diversity and distribution of ecologically rare or cryptic Symbiodinium spp. will continue to grow. However, speculation about whether Symbiodinium found at low abundances in an animal, or in the environment, are important to photosynthetic corals and their response to climate change requires more in-depth evidence and consideration.
References
McFall-Ngai M, Hadfield MG, Bosch TC, Carey HV, Domazet-Loso T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF, Hentschel U, King N, Kjelleberg S, Knoll AH, Kremer N, Mazmanian SK, Metcalf JL, Nealson K, Pierce NE, Rawls JF, Reid A, Ruby EG, Rumpho M, Sanders JG, Tautz D, Wernegreen JJ (2013) Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci U S A 110:3229–3236. doi:10.1073/pnas.1218525110
Knowlton N, Rohwer F (2003) Multispecies microbial mutualisms on coral reefs: the host as a habitat. Am Nat 162:S51–62. doi:10.1086/378684
Kirk NL, Thornhill DJ, Kemp DW, Fitt WK, Santos SR (2013) Ubiquitous associations and a peak fall prevalence between apicomplexan symbionts and reef corals in Florida and the Bahamas. Coral Reefs 32:847–858. doi:10.1007/s00338-013-1038-9
Amend AS, Barshis DJ, Oliver TA (2012) Coral-associated marine fungi form novel lineages and heterogeneous assemblages. ISME J 6:1291–1301. doi:10.1038/ismej.2011.193
Berkelmans R, van Oppen MJ (2006) The role of zooxanthellae in the thermal tolerance of corals: a 'nugget of hope' for coral reefs in an era of climate change. Proc Biol Sci 273:2305–2312. doi:10.1098/rspb.2006.3567
Rowan R, Knowlton N, Baker A, Jara J (1997) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388:265–269. doi:10.1038/40843
Jones AM, Berkelmans R, van Oppen MJ, Mieog JC, Sinclair W (2008) A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc Biol Sci 275:1359–1365. doi:10.1098/rspb.2008.0069
LaJeunesse TC, Smith RT, Finney J, Oxenford H (2009) Outbreak and persistence of opportunistic symbiotic dinoflagellates during the 2005 Caribbean mass coral 'bleaching' event. Proc Biol Sci 276:4139–4148. doi:10.1098/rspb.2009.1405
Kemp DW, Hernandez-Pech X, Iglesias-Prieto R, Fitt WK, Schmidt GW (2014) Community dynamics and physiology of Symbiodinium spp. before, during, and after a coral bleaching event. Limnol Oceanogr 59:788–797. doi:10.4319/lo.2014.59.3.0788
McGinley MP, Aschaffenburg MD, Pettay DT, Smith RT, LaJeunesse TC, Warner ME (2012) Symbiodinium spp. in colonies of eastern Pacific Pocillopora spp. are highly stable despite the prevalence of low-abundance background populations. Mar Ecol Prog Ser 462:1–7. doi:10.3354/meps09914
Stat M, Loh WKW, LaJeunesse TC, Hoegh-Guldberg O, Carter DA (2009) Stability of coral–endosymbiont associations during and after a thermal stress event in the southern Great Barrier Reef. Coral Reefs 28:709–713. doi:10.1007/s00338-009-0509-5
Goulet TL, LaJeunesse TC, Fabricius KE (2008) Symbiont specificity and bleaching susceptibility among soft corals in the 1998 Great Barrier Reef mass coral bleaching event. Mar Biol 154:795–804. doi:10.1007/s00227-008-0972-5
Mieog JC, van Oppen MJH, Cantin NE, Stam WT, Olsen JL (2007) Real-time PCR reveals a high incidence of Symbiodinium clade D at low levels in four scleractinian corals across the Great Barrier Reef: implications for symbiont shuffling. Coral Reefs 26:449–457. doi:10.1007/s00338-007-0244-8
Silverstein RN, Correa AM, Baker AC (2012) Specificity is rarely absolute in coral-algal symbiosis: implications for coral response to climate change. Proc Biol Sci 279:2609–2618. doi:10.1098/rspb.2012.0055
Kennedy EV, Foster NL, Mumby PJ, Stevens JR (2015) Widespread prevalence of cryptic Symbiodinium D in the key Caribbean reef builder, Orbicella annularis. Coral Reefs 34:519–531. doi:10.1007/s00338-015-1264-4
Thomas L, Kendrick GA, Kennington WJ, Richards ZT, Stat M (2014) Exploring Symbiodinium diversity and host specificity in Acropora corals from geographical extremes of Western Australia with 454 amplicon pyrosequencing. Mol Ecol 23:3113–3126. doi:10.1111/mec.12801
Quigley KM, Davies SW, Kenkel CD, Willis BL, Matz MV, Bay LK (2014) Deep-sequencing method for quantifying background abundances of symbiodinium types: exploring the rare symbiodinium biosphere in reef-building corals. PLoS One 9:e94297. doi:10.1371/journal.pone.0094297
Arif C, Daniels C, Bayer T, Banguera-Hinestroza E, Barbrook A, Howe CJ, LaJeunesse TC, Voolstra CR (2014) Assessing Symbiodinium diversity in scleractinian corals via next-generation sequencing-based genotyping of the ITS2 rDNA region. Mol Ecol 23:4418–4433
Finney JC, Pettay DT, Sampayo EM, Warner ME, Oxenford HA, LaJeunesse TC (2010) The relative significance of host-habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodinium. Microb Ecol 60:250–263. doi:10.1007/s00248-010-9681-y
Ulstrup KE, Van Oppen MJH (2003) Geographic and habitat partitioning of genetically distinct zooxanthellae (Symbiodinium) in Acropora corals on the Great Barrier Reef. Mol Ecol 12:3477–3484. doi:10.1046/j.1365-294X.2003.01988.x
LaJeunesse TC, Bhagooli R, Hidaka M, DeVantier L, Done T, Schmidt GW, Fitt WK, Hoegh-Guldberg O (2004) Closely related Symbiodinium spp. differ in relative dominance in coral reef host communities across environmental, latitudinal and biogeographic gradients. Mar Ecol Prog Ser 284:147–161. doi:10.3354/meps284147
LaJeunesse TC, Pettay DT, Sampayo EM, Phongsuwan N, Brown B, Obura DO, Hoegh-Guldberg O, Fitt WK (2010) Long-standing environmental conditions, geographic isolation and host-symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium. J Biogeogr 37:785–800. doi:10.1111/j.1365-2699.2010.02273.x
Tonk L, Sampayo EM, LaJeunesse TC, Schrameyer V, Hoegh-Guldberg O (2014) Symbiodinium (Dinophyceae) diversity in reef-invertebrates along an offshore to inshore reef gradient near Lizard Island, Great Barrier Reef. J Phycol 50:552–563. doi:10.1111/jpy.12185
LaJeunesse TC, Wham DC, Pettay DT, Parkinson JE, Keshavmurthy S, Chen CA (2014) Ecologically differentiated stress-tolerant endosymbionts in the dinoflagellate genus Symbiodinium (Dinophyceae) Clade D are different species. Phycologia 53:305–319. doi:10.2216/13-186.1
Baker AC, Romanski AM (2007) Multiple symbiotic partnerships are common in scleractinian corals, but not in octocorals: comment on Goulet (2006). Mar Ecol Prog Ser 335:237–242. doi:10.3354/meps335237
Santos SR, Taylor DJ, Coffroth MA (2001) Genetic comparisons of freshly isolated versus cultured symbiotic dinoflagellates: implications for extrapolating to the intact symbiosis. J Phycol 37:900–912. doi:10.1046/j.1529-8817.2001.00194.x
LaJeunesse TC (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141:387–400. doi:10.1007/s00227-002-0829-2
Jeong HJ, Lee SY, Kang NS, Yoo YD, Lim AS, Lee MJ, Kim HS, Yih W, Yamashita H, LaJeunesse TC (2014) Genetics and morphology characterize the dinoflagellate Symbiodinium voratum, n. sp., (Dinophyceae) as the sole representative of Symbiodinium Clade E. J Eukaryot Microbiol 61:75–94. doi:10.1111/jeu.12088
LaJeunesse TC, Lee SY, Gil-Agudelo DL, Knowlton N, Jeong HJ (2015) Symbiodinium necroappetens sp nov (Dinophyceae): an opportunist 'zooxanthella' found in bleached and diseased tissues of Caribbean reef corals. Eur J Phycol 50:223–238. doi:10.1080/09670262.2015.1025857
Parkinson JE, Coffroth MA, LaJeunesse TC (2015) New species of Clade B Symbiodinium (Dinophyceae) from the greater Caribbean belong to different functional guilds: S. aenigmaticum sp. nov., S. antillogorgium sp. nov., S. endomadracis sp. nov., and S. pseudominutum sp. nov. J Phycol 51:850–858. doi:10.1111/jpy.12340
LaJeunesse TC (2001) Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: in search of a "species" level marker. J Phycol 37:866–880. doi:10.1046/j.1529-8817.2001.01031.x
Song JI (1991) A systematic study on the Korean Anthozoa. 12. Order Scleractinia. Korean J Syst Zool 7:127–150
Denis V, Chen CA, Song JI, Woo S (2013) Alveopora japonica beds thriving under kelp. Coral Reefs 32:503–503. doi:10.1007/s00338-013-1019-z
Sugihara K, Yamano H, Choi K-S, Hyeong K (2014) Zooxanthellate scleractinian corals of Jeju Island, Republic of Korea: 111-130. doi: 10.1007/978-4-431-54783-9_6
Sheppard A, Fenner D, Edwards A, Abrar M, Ochavillo D (2008) Alveopora japonica. The IUCN Red List of Threatened Species 2008
Rodriguez-Lanetty M, Chang S-J, Song J-I (2003) Specificity of two temperate dinoflagellate-anthozoan associations from the north-western Pacific Ocean. Mar Biol 143:1193–1199. doi:10.1007/s00227-003-1165-x
Chang S-J, Rodriguez-Lanetty M, Yanagi K, Nojima S, Song J-I (2011) Two anthozoans, Entacmaea quadricolor(order Actiniaria) and Alveopora japonica(order Scleractinia), host consistent genotypes of Symbiodinium spp. across geographic ranges in the northwestern Pacific Ocean. Anim Cells Syst 15:315–324. doi:10.1080/19768354.2011.611174
De Palmas S, Denis V, Ribas-Deulofeu L, Loubeyres M, Woo S, Hwang SJ, Song JI, Chen CA (2015) Symbiodinium spp. associated with high-latitude scleractinian corals from Jeju Island, South Korea. Coral Reefs 34:919–925. doi:10.1007/s00338-015-1286-y
Lien YT, Fukami H, Yamashita Y (2012) Symbiodinium clade C dominates zooxanthellate corals (Scleractinia) in the temperate region of Japan. Zool Sci 29:173–180. doi:10.2108/zsj.29.173
APHA (1995) American Public Health Association. Standard methods for the examination of water and wastewater. APHA, Washington D. C
Litaker RW, Vandersea MW, Kibler SR, Reece KS, Stokes NA, Steidinger KA, Millie DF, Bendis J, Pigg RJ, Tester PA (2003) Identification of Pfiesteria piscicida(Dinophyceae) and Pfiesteria-like organisms using internal transcribed spacer-specific PCR assays. J Phycol 39:754–761
Kang NS, Jeong HJ, Moestrup O, Park TG (2011) Gyrodiniellum shiwhaense n. gen., n. sp., a new planktonic heterotrophic dinoflagellate from the coastal waters of western Korea: morphology and ribosomal DNA gene sequence. J Eukaryot Microbiol 58:284–309. doi:10.1111/j.1550-7408.2011.00544.x
Swofford DL (2014) PAUP* phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland, Massachusetts
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi: 10.1093/bioinformatics/btg180
LaJeunesse TC, Reyes-Bonilla H, Warner ME, Wills M, Schmidt GW, Fitt WK (2008) Specificity and stability in high latitude eastern Pacific coral-algal symbioses. Limnol Oceanogr 53:719–727. doi:10.4319/lo.2008.53.2.0719
Team R-C (2015) R: a language and environment for statistical computing. https://www.R-project.org
Conover WJ (1999) Practical nonparametric statistics. Wiley, New York
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River
Pochon X, Pawlowski J, Zaninetti L, Rowan R (2001) High genetic diversity and relative specificity among Symbiodinium-like endosymbiotic dinoflagellates in soritid foraminiferans. Mar Biol 139:1069–1078. doi: 10.1007/s002270100674
Pochon X, Garcia-Cuetos L, Baker AC, Castella E, Pawlowski J (2007) One-year survey of a single Micronesian reef reveals extraordinarily rich diversity of Symbiodinium types in soritid foraminifera. Coral Reefs 26:867–882. doi: 10.1007/s00338-007-0279-x
Garcia-Cuetos L, Pochon X, Pawlowski J (2005) Molecular evidence for host–symbiont specificity in soritid foraminifera. Protist 156:399–412. doi: 10.1016/j.protis.2005.08.003
Muscatine L (1973) Nutrition of corals Biology and Geology of Coral Reefs, pp. 77-115
Smayda TJ (2002) Adaptive ecology, growth strategies and the global bloom expansion of dinoflagellates. J Oceanogr 58:281–294. doi:10.1023/A:1015861725470
Rodriguez-Lanetty M, Loh W, Carter D, Hoegh-Guldberg O (2001) Latitudinal variability in symbiont specificity within the widespread scleractinian coral Plesiastrea versipora. Mar Biol 138:1175–1181. doi:10.1007/s002270100536
Silverstein RN, Correa AMS, LaJeunesse TC, Baker AC (2011) Novel algal symbiont (Symbiodinium spp.) diversity in reef corals of Western Australia. Mar Ecol Prog Ser 422:63–75
Thornhill DJ, Xiang Y, Pettay DT, Zhong M, Santos SR (2013) Population genetic data of a model symbiotic cnidarian system reveal remarkable symbiotic specificity and vectored introductions across ocean basins. Mol Ecol 22:4499–4515. doi:10.1111/mec.12416
LaJeunesse TC, Parkinson JE, Reimer JD (2012) A genetics-based description of Symbiodinium minutum sp. nov. and S. psygmophilum sp. nov. (dinophyceae), two dinoflagellates symbiotic with cnidaria. J Phycol 48:1380–1391. doi:10.1111/j.1529-8817.2012.01217.x
Muscatine L (1990) The role of symbiotic algae in carbon and energy flux in reef corals. In: Dubinski Z (ed) Coral reefs: ecosystems of the world. Elsevier, New York, pp 75–87
Stimson J, Kinzie RA III (1991) The temporal pattern and rate of release of zooxanthellae. J Exp Mar Biol Ecol 153:63–74. doi:10.1016/S0022-0981(05)80006-1
Hoegh-Guldberg O, McCloskey LR, Muscatine L (1987) Expulsion of zooxanthellae by symbiotic cnidarians from the Red Sea. Coral Reefs 5:201–204. doi:10.1007/BF00300964
Jones RJ, Yellowlees D (1997) Regulation and control of intracellular algae (= zooxanthellae) in hard corals. Philos Trans R Soc B Biol Sci 352:457–468. doi:10.1098/rstb.1997.0033
Baghdasarian G, Muscatine L (2000) Preferential expulsion of dividing algal cells as a mechanism for regulating algal-cnidarian symbiosis. Biol Bull (Woods Hole) 199:278–286
Goulet TL, Coffroth MA (2003) Stability of an octocoral-algal symbiosis over time and space. Mar Ecol Prog Ser 250:117–124
Thornhill DJ, LaJeunesse TC, Kemp DW, Fitt WK, Schmidt GW (2006) Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Mar Biol 148:711–722. doi:10.1007/s00227-005-0114-2
Thornhill DJ, Xiang Y, Fitt WK, Santos SR (2009) Reef endemism, host specificity and temporal stability in populations of symbiotic dinoflagellates from two ecologically dominant Caribbean corals. PLoS One 4:e6262. doi:10.1371/journal.pone.0006262
Suwa R, Hirose M, Hidaka M (2008) Seasonal fluctuation in zooxanthellar genotype composition and photophysiology in the corals Pavona divaricata and P. decussata. Mar Ecol Prog Ser 361:129–137. doi:10.3354/meps07372
Pettay DT, Wham DC, Pinzón JH, LaJeunesse TC (2011) Genotypic diversity and spatial-temporal distribution of Symbiodinium clones in an abundant reef coral. Mol Ecol 20:5197–5212. doi:10.1111/j.1365-294X.2011.05357.x
Baums IB, Devlin-Durante MK, LaJeunesse TC (2014) New insights into the dynamics between reef corals and their associated dinoflagellate endosymbionts from population genetic studies. Mol Ecol 23:4203–4215. doi:10.1111/mec.12788
Thornhill DJ, Fitt WK, Schmidt GW (2006) Highly stable symbioses among western Atlantic brooding corals. Coral Reefs 25:515–519. doi:10.1007/s00338-006-0157-y
LaJeunesse TC (2005) "Species" radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene-Pliocene transition. Mol Biol Evol 22:570–581. doi:10.1093/molbev/msi042
Harii S, Omori M, Yamakawa H, Koike Y (2001) Sexual reproduction and larval settlement of the zooxanthellate coral Alveopora japonica Eguchi at high latitudes. Coral Reefs 20:19–23. doi:10.1007/s003380000134
Fitt WK, McFarland FK, Warner ME, Chilcoat GC (2000) Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol Oceanogr 45:677–685
Hsu C-M, Keshavmurthy S, Denis V, Kuo C-Y, Wang J-T, Meng P-J, Chen CA (2012) Temporal and spatial variations in symbiont communities of catch bowl coral Isopora palifera (Scleractinia: Acroporidae) on reefs in Kenting National Park, Taiwan. Zool Stud 51:1343–1353
Sachs JL, Simms EL (2006) Pathways to mutualism breakdown. Trends Ecol Evol 21:585–592. doi:10.1016/j.tree.2006.06.018
Douglas AE (2008) Conflict, cheats and the persistence of symbioses. New Phytol 177:849–858. doi:10.1111/j.1469-8137.2007.02326.x
Schoenberg DA, Trench RK (1980) Genetic variation in Symbiodinium (= Gymnodinium) microadriaticum Freudenthal, and specificity in its symbiosis with marine invertebrates. III. specificity and infectivity of Symbiodinium microadriaticum. Proc R Soc Lond, Ser B: Biol Sci 207. doi:10.1098/rspb.1980.0031
Eloe-Fadrosh EA, Rasko DA (2013) The human microbiome: from symbiosis to pathogenesis. Annu Rev Med 64:145–163. doi:10.1146/annurev-med-010312-133513
Houlbreque F, Ferrier-Pagès C (2009) Heterotrophy in tropical scleractinian corals. Biol Rev Camb Philos Soc 84:1–17. doi:10.1111/j.1469-185X.2008.00058.x
Hall NM, Berry KLE, Rintoul L, Hoogenboom MO (2015) Microplastic ingestion by scleractinian corals. Mar Biol 162:725–732. doi:10.1007/s00227-015-2619-7
Leal MC, Ferrier-Pages C, Calado R, Thompson ME, Frischer ME, Nejstgaard JC (2013) Coral feeding on microalgae assessed with molecular trophic markers. Mol Ecol 23:3870–3876. doi:10.1111/mec.12486
Toller W, Rowan R, Knowlton N (2001) Repopulation of zooxanthellae in the caribbean corals Montastrea annularis and M. faveolata following experimental and disease-associated bleaching. Biol Bull 2001:360–373
Grottoli AG, Warner ME, Levas SJ, Aschaffenburg MD, Schoepf V, McGinley M, Baumann J, Matsui Y (2014) The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Glob Chang Biol 20:3823–3833. doi:10.1111/gcb.12658
Baker AC (2003) Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu Rev Ecol Evol Syst 34:661–689. doi:10.1146/annurev.ecolsys.34.011802.132417
Smith RT (2008) Specificity, stability and comparative physiology of coral-Symbiodinium mutualisms: evaluating the potential for acclimation and/or adaptation in reef corals. Florida International University
Pettay DT, Wham DC, Smith RT, Iglesias-Prieto R, LaJeunesse TC (2015) Microbial invasion of the Caribbean by an Indo-Pacific coral zooxanthella. Proc Natl Acad Sci U S A 112:7513–7518. doi:10.1073/pnas.1502283112
LaJeunesse TC, Smith R, Walther M, Pinzón J, Pettay DT, McGinley M, Aschaffenburg M, Medina-Rosas P, Cupul-Magana AL, Perez AL, Reyes-Bonilla H, Warner ME (2010) Host-symbiont recombination versus natural selection in the response of coral-dinoflagellate symbioses to environmental disturbance. Proc Biol Sci 277:2925–2934. doi:10.1098/rspb.2010.0385
Hirose M, Reimer JD, Hidaka M, Suda S (2008) Phylogenetic analyses of potentially free-living Symbiodinium spp. isolated from coral reef sand in Okinawa, Japan. Mar Biol 155:105–112. doi:10.1007/s00227-008-1011-2
Porto I, Granados C, Restrepo JC, Sanchez JA (2008) Macroalgal-associated dinoflagellates belonging to the genus Symbiodinium in Caribbean reefs. PLoS One 3:e2160. doi:10.1371/journal.pone.0002160
Littman RA, van Oppen MJH, Willis BL (2008) Methods for sampling free-living Symbiodinium (zooxanthellae) and their distribution and abundance at Lizard Island (Great Barrier Reef). J Exp Mar Biol Ecol 364:48–53. doi:10.1016/j.jembe.2008.06.034
Takabayashi M, Adams LM, Pochon X, Gates RD (2011) Genetic diversity of free-living Symbiodinium in surface water and sediment of Hawai'i and Florida. Coral Reefs 31:157–167. doi:10.1007/s00338-011-0832-5
Sweet MJ (2013) Symbiodinium diversity within Acropora muricata and the surrounding environment. Mar Ecol 35:343–353. doi:10.1111/maec.12092
Kohli GS, Neilan BA, Brown MV, Hoppenrath M, Murray SA (2014) Cob gene pyrosequencing enables characterization of benthic dinoflagellate diversity and biogeography. Environ Microbiol 16:467–485. doi:10.1111/1462-2920.12275
Jeong HJ, Yoo YD, Kang NS, Lim AS, Seong KA, Lee SY, Lee MJ, Lee KH, Kim HS, Shin W, Nam SW (2012) Heterotrophic feeding as a newly identified survival strategy of the dinoflagellate Symbiodinium. Proc Nat Acad Sci 109:12604–12609. doi: 10.1073/pnas.1204302109
LaJeunesse TC, Loh W, Trench RK (2009) Do introduced endosymbiotic dinoflagellates ‘take’ to new hosts? Biol Invasions 11:995–1003. doi:10.1007/s10530-008-9311-5
Lee SY, Jeong HJ, Kang NS, Jang TY, Jang SH, Lajeunesse TC (2015) Symbiodinium tridacnidorum sp nov., a dinoflagellate common to Indo-Pacific giant clams, and a revised morphological description of Symbiodinium microadriaticum Freudenthal, emended Trench & Blank. Eur J Phycol 50:155–172 doi:10.1080/09670262.2015.1018336
Acknowledgments
We thank Young Duk Koh for collecting samples and Tae Young Jang, An Suk Lim, and Éric Potvin for technical support. This paper was supported by the National Research Foundation of Korea Grant funded by the Korea Government/MSIP (NRF-2015-M1A5A1041806) and Management of marine organisms causing ecological disturbance and harmful effect Program of Korea Institute of Marine Science and Technology Promotion (KIMST) award to HJJ. Contributions provided by T.C.L. were funded in part by the Pennsylvania State University and a grant from the National Science Foundation (IOS-1258058).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Figure S1
The correspondence between estimated cell densities based on qPCR fluorescence measurements and hemocytometer counts. (GIF 107 kb)
Figure S2
Positive relationship between cell densities of B2* and S. voratum graphed by water temperatures at the time samples when collected. Pairwise comparisons where considered only when concentrations of B2* occurred above of 50,000 cells/gww, a range where qPCR estimates of cell numbers are more accurate. (GIF 131 kb)
Figure S3
Correspondence between estimated cell concentrations of S. voratum (per gram wet weight of host tissue) and its prevalence (percentage) in polyps of A. japonica. (GIF 81 kb)
Figure S4
Concentration of ammonium (NH4), nitrate (NO3), phosphate (PO4), and silica dioxide (SiO2) in surface waters at Jeju Island, Korea every month over the 15-month survey period. These values are graphed against (A) measures of Chlorophyll a content, as well as the prevalence (percent) of (B) Symbiodinium sp. Clade B, and (C) S. voratum (Clade E) detected in the polyps (n = 90) of Alveopora japonica using qPCR. (GIF 123 kb)
Table S1
(DOCX 68 kb)
Table S2
(DOCX 72 kb)
Table S3
(DOCX 80 kb)
Rights and permissions
About this article
Cite this article
Lee, M.J., Jeong, H.J., Jang, S.H. et al. Most Low-Abundance “Background” Symbiodinium spp. Are Transitory and Have Minimal Functional Significance for Symbiotic Corals. Microb Ecol 71, 771–783 (2016). https://doi.org/10.1007/s00248-015-0724-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-015-0724-2