Abstract
The use of feathers to line bird’s nests has traditionally been interpreted as having a thermoregulatory function. Feather-degrading bacteria growing on feathers lining nests may have antimicrobial properties, which may provide an additional benefit to lining nests with feathers. We test the hypothesis that the production of antimicrobial substances by feather bacteria affects the microbiological environment of the nest, and therefore the bacterial density on eggshells and, indirectly, hatching success. These effects would be expected to differ between nests lined with pigmented and white feathers, because bacteria grow differently on feathers of different colors. We experimentally manipulated the composition of pigmented and unpigmented feathers in nests of the barn swallow (Hirundo rustica) and studied the antimicrobial properties against the keratin-degrading bacterium Bacillus licheniformis of bacteria isolated from feathers of each color. Analyzed feathers were collected at the end of the incubation period, and antimicrobial activity was defined as the proportion of bacteria from the feathers that produce antibacterial substances effective against B. licheniformis. Our experimental manipulation affected antimicrobial activity, which was higher in nests with only white feathers at the beginning of incubation. Moreover, white feathers showed higher antimicrobial activity than black ones. Interestingly, antimicrobial activity in feathers of one of the colors correlated negatively with bacterial density on feather of the opposite color. Finally, antimicrobial activity of white feathers was negatively related to eggshell bacterial load. These results suggest that antimicrobial properties of feathers in general and of white feathers in particular affect the bacterial environment in nests. This environment in turn affects the bacterial load on eggshells, which may affect hatching success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial communities on feathers have recently received attention by evolutionary ecologists and ornithologists [1, 2] due to their possible effects on efficiency of avian flight and because their use as nest lining material may influence bacterial environments within nests [3, 4]. Some bacteria growing on feathers have keratinolytic activity that degrades feathers [5–9], affecting flight capacity, thermoregulation, and therefore, survival [2, 6, 10]. These feather-degrading bacteria (FDB; [1]) may also affect sexually selected feather coloration, as shown by relationships between brightness of feather colors and the density of keratinolytic bacteria on feathers [11, 12]. Bacteria may also differentially degrade white and black patches in flight feathers that differ between males and females, affecting the overall color pattern and perhaps sexual selection [13]. Moreover, feather coloration may affect the bacterial community because white and black feathers are degraded differentially by Bacillus licheniformis [14, 15]. This bacterium grows better on unmelanized white feathers than on black ones, suggesting that melanin reduces degradation [14, 16].
B. licheniformis, like other keratinolytic bacteria, lives in soil and is a common feather-degrading bacterium [6, 17, 18]. This bacterium produces many antimicrobial substances [19–27] that are used against other microbes [28, 29]. Thus, antimicrobial substances from FDB including B. licheniformis growing on feathers in nest lining might affect the rest of the nest bacterial community. Reducing eggshell bacterial loads with antibiotics from FDB has been suggested as an additional function of feathers lining nests [3, 4]. This effect may have important fitness consequences, because higher bacterial loads on eggshells can decrease hatching success [30–34]. The use of feathers to line nests [35, 36] has typically been interpreted to stem from their thermal or insulation properties [37], as a barrier against parasites [38, 39] or as sexual signals [40, 41]. However, beneficial effects of feathers on hatching success might also result from antibiotic-producing bacteria that grow on them.
The intensity of bacterial competition could change the effectiveness of antimicrobials [42, 43]. Because B. licheniformis grows better on white feathers, which lack melanin, than on melanized feathers [14, 16], competition and interference with other bacteria might be greater in nests with white feathers than in nests lined with melanic feathers. We might therefore expect antimicrobials from bacteria recovered from high-density communities on white feathers to be more effective than antimicrobials from bacteria recovered from lower-density communities on dark feathers [3]. The use of white feathers to line nests might, in turn, reduce the probability of bacterial infection of eggs and nestlings, perhaps explaining the observed preference of barn swallows (Hirundo rustica) for white feathers as nest lining materials [33]. Previous studies showed that nests with lining feathers experimentally replaced by white feathers had lower bacterial densities and higher hatching success than those with feathers of other colors [4, 33]. These effects could be mediated by higher initial bacterial densities on white feathers and/or by antimicrobials from white feathers being more effective than those from bacteria on pigmented feathers.
By manipulating the color composition of feathers lining the nests of barn swallows, we experimentally produced nests lined with only white or pigmented feathers. The hypothesis that antimicrobials from keratinolytic bacteria affect the bacterial environment of nests (i.e., eggshell bacterial load) and consequently hatching success therefore predicts that bacteria in white feathers from nest linings will be more active against other feather-degrading bacteria than those from pigmented feathers. The antimicrobial activity of feathers, defined as the proportion of bacteria from the feathers that produce antibacterial substances effective against B. licheniformis, should therefore be higher in experimentally manipulated white-feather nests, and for white feathers in mixed nests. The hypothesis also posits that the predicted increase in interference and competition would result in antimicrobials should affect the bacterial environment of nests and therefore decrease eggshell bacterial load. Thus, we predicted a negative relationship between antimicrobial activity of nest lining feathers and eggshell bacterial loads. Here, we test these predictions of the hypothetical antimicrobial properties of bacteria isolated from nest lining feathers of different colors.
Materials and Methods
Study Area and Experimental Procedure
Fieldwork was performed at Kraghede, Denmark (57° 12′ N, 10° 00′ E). For a detailed description of the study area, see Møller [44]. Barn swallows usually breed in dairy farms in the area. Starting with the arrival of the birds, breeding areas were visited twice a week to check old nests and determine breeding activity, laying date, and clutch size. After clutch completion, we performed the experimental manipulation of feathers.
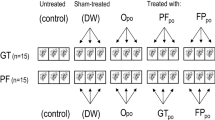
Barn swallows line their nests with feathers from other birds, and incorporate both white and pigmented feathers [4, 33]. Our experiment consisted of randomly removing all the white feathers or all the black feathers from each finished nest after the clutch was completed. We first removed and counted all pigmented and unpigmented feathers in the nest’s cup. For nests randomly assigned to the “white” treatment, we removed all colored feathers and added all the white feathers from a previously manipulated nest. For nests randomly assigned to the “pigmented” treatment, we removed all the white feathers and added all the colored feathers from the previous “white” nest. This experimental procedure has been described and discussed elsewhere in detail [4, 33].
The ongoing nest-building activity of barn swallows during the incubation stage partially disrupted the experiment, because at the end of incubation most nests were lined with both pigmented and white feathers [4, 33]. This behavior allows us to collect two feathers of each color per experimental nest (white nests, n = 21; pigmented nests, N = 13) at the end of incubation, and these feathers were individually kept in sterile 15-mL Falcon tubes until laboratory analyses were performed. At the end of incubation, we also collected bacterial samples from eggshells. In an attempt to prevent contamination among nests while handling eggs and nests, we wore latex gloves cleaned with ethanol between nests. Bacterial samples were collected by cleaning eggshells with a sterile RAYON swab (Nuova APTACA, s.r.l.) slightly moistened with sterile sodium phosphate buffer (0.2 M; pH 7.2). The whole clutch was cleaned with the same swab, spending about 10 s per egg, and the swab was subsequently preserved in an Eppendorf tube containing the sterile buffer and stored at 4 °C until laboratory analyses were performed. Estimates of bacterial load were standardized to the total eggshell surface by normalizing by the number and surface area of the eggs in each nest. The length and width of each egg was measured with calipers (accuracy, 0.02 mm), and eggshell surface was estimated according to Narushin [45]. Further details of the sampling procedures and bacterial load estimation can be found in Peralta-Sánchez et al. [4].
Differences in the sample size of nests of different experimental treatments occurred for several reasons: Some nests were lined with feathers of only one color (white or pigmented) (two); hatching sometimes occurred before the second sampling (five); predation (one); abandonment (one); or the nest fell down (one). The experimental groups did not vary significantly with respect to clutch size (ANOVA; F = 1.41, P = 0.243), laying date (ANOVA; F = 0.73, P = 0.399), the number of white (regression; F = 3.81, P = 0.060) or pigmented feathers at the end of incubation (regression; F = 0.49, P = 0.491) or the arcsine-transformed hatching success (regression; F = 1.46, P = 0.235), so we are confident that our results are not biased by differences in sample size between treatments.
Laboratory Work
Eggshell Samples
In the laboratory, bacterial samples from eggshells were collected from Eppendorf tubes after vigorous vortexing for at least three periods of 5 s each. Serial tenfold dilutions to 10−6 were cultured by spreading 100 μl of sample (measured with a micropipette) homogeneously on plates containing four different sterile solid growth media (Scharlau Chemie S.A. Barcelona). We used tryptic soy agar (TSA), a broadly used general medium for growing mesophilic bacteria, and three specific media: Kenner Fecal Agar for growing bacteria from the genus Enterococcus; Vogel-Johnsson Agar for bacteria from the genus Staphylococcus; and Hecktoen Enteric Agar for Gram-negative bacteria from the family Enterobacteriaceae. Plates were incubated at 37 °C for 72 h, and then the number of colonies on each plate was counted. Bacterial density was estimated as colony-forming units (CFU) per square centimeter. For more details of the media, culturing, within-clutch repeatability and bacterial characteristics, see Peralta-Sánchez et al. [4].
All collected feathers and samples from eggshells were stored at 4 °C and analyzed within a month of collection. Although sample storage has traditionally been considered a major problem in environmental microbiology, Lauber et al. [46] have shown that the relative abundance of most bacterial communities from soil samples was largely unaffected by temperature (i.e., 20 °C, 4 °C, −20 °C, and −80 °C) even after 14 days of storage. Moreover, we have demonstrated with a large dataset of 525 nests, two study years, and 19 species of birds that storage time does not explain variation in bacterial counts. Similarly, the rank position of different bird species as shown by a comparison of ranked values of mesophilic bacterial loads from samples collected in 2006, either stored for less than 3 days or for up to 1 month, did not differ (for more details, see [47]). Consequently, we are confident that variation in storage duration did not affect our results.
In 2011, we performed a series of field and laboratory controls to detect possible environmental and/or laboratory contamination. In the field, while visiting and collecting eggshell samples from 42 nests of magpies (Pica pica), house sparrows (Passer domesticus), hoopoes (Upupa epops), and spotless starlings (Sturnus unicolor) at the egg-laying stage, we exposed swabs to the air for 10 s and then stored them in microcentrifuge tubes. Within the next 24 h, samples were cultured in the laboratory. No bacteria were detected in 32 of the samples; one colony was detected in six samples, and two colonies were detected in four samples. In the laboratory, we also performed two different negative controls at the time we cultured the collected samples. The first control consisted of spreading phosphate buffer directly onto TSA plates (phosphate control). As a second negative control, we opened a TSA plate in our flow chamber and then incubated it (chamber control). No bacteria grew on any TSA control plates. Thus, our estimates of eggshell bacterial loads were not affected by external contamination (for more details, see Peralta-Sánchez et al. [47]).
Feather Samples: Bacterial Densities and Antimicrobial Properties
Feathers were collected from the Falcon tubes under sterile conditions (Bunsen burner and sterile Petri dishes) with sterile forceps. For every feather, the 0.5 mm tip was discarded, and 1 cm2 was cut from the next piece of the feather using sterilized scissors. Each piece of feather was introduced in a 1.5 ml Eppendorf tube with 1 ml sterile phosphate buffer (0.2 M; pH 7.2). After three shaking periods of 5 s each in vortex, we performed serial tenfold dilutions to 10−4. We plated each serial dilution on six TSA replicates by spreading 100 μl of supernatant with a sterile Drigalski loop. Plates were incubated at 28 °C for 24 h, and then CFUs were counted from the dilution replicates that contained 30–300 colonies. Variation in CFUs was significantly lower within feathers than between feathers (ANOVA, log-transformed CFUs as dependent variable; nest as factor, F 135,646 = 4.98, P < 0.001), and we used mean values in subsequent analyses. Bacterial density was calculated by multiplying the average number of CFU by the dilution factor and by the original sample volume.
After counting, antimicrobial properties of each bacterial colony were assessed by covering a random replicate with a single agar slide of phosphate buffered Brain Heart Infusion-agar with an inoculum of B. licheniformis D-13. This indicator strain was obtained from the reference strains in the Lactic Bacteria Research Group, Department of Microbiology, University of Granada. B. licheniformis are known to be feather-degrading and antimicrobial-producing bacteria and therefore are appropriate for testing the antimicrobial activity of bacteria collected from nest lining feathers under different levels of interference competition.
The indicator bacterium was prepared by picking one single colony from a previously cultivated Petri dish in buffered TSA, dropping it in 6 mL phosphate buffered Brain-Heart Infusion, and incubating it at 28 °C overnight. The agar slide consisted of 6 mL of phosphate buffered Brain Heart Infusion-agar, in which 100 μL of the overnight indicator bacteria were added after melting and agar temperature stabilizes at 50 °C. Once the upper agar slide was solid, plates were incubated for 12 h at 28 °C, and colonies that did or did not produce an inhibition halo were counted. The antimicrobial activity of each community was estimated as the percentage of CFU in the plate that inhibited the growth of B. licheniformis.
Statistics
We performed General Linear Mixed Model analyses, where the dependent variables were the log-transformed number of colony forming units and the arcsine-transformed B. licheniformis antimicrobial activity estimated for each collected feather. Arcsine transformations are appropriate for proportions, and log transformations for bacterial abundance were required because of the large dynamic range [48]. Feather color and treatment were considered fixed factors, and treatment nested within nest identity was considered to be a random factor. We included in the analyses interactions between treatment and feather color, and between feather color and treatment nested within nest identity.
By general linear models (GLM), we explored whether bacterial densities on feathers and their antimicrobial activity covaried with hatching success (the proportion of eggs that hatched successfully) and bacterial load on eggshells. Second, we augmented the models with information about white and pigmented feathers within each nest (number of feathers in the nests, feather bacterial densities, and antimicrobial activity). We used mean values per experimental nest and feather coloration.
Residuals of all statistical models did not differ from normality. All analyses were two-tailed and conducted with STATISTICA 8.0 (StatSoft, Inc.). SE represents the standard error of the mean.
Results
Among Feathers Variation in Antimicrobial Properties and Bacterial Density
Variation in B. licheniformis antimicrobial activity per feather was significantly explained by nest identity, feather color, and the interaction between feather color and treatment (Table 1). White feathers showed a significantly higher antimicrobial activity than pigmented feathers. This effect is especially larger in experimental pigmented nests (Fig. 1).
Weighted means of B. licheniformis-activity for pigmented (filled circles) and white feathers (empty circles) at the end of the incubation period in experimental nests with pigmented and white feathers. Values on the Y axis were transformed back into proportions. Bars show standard error of the mean
Bacterial densities on white and pigmented feathers within the same nest did not differ significantly: Among-nest variation was larger than within-nest variation (Table 1), and consequently mean values of bacterial density per nest were used in subsequent analyses.
Relationships Between Antimicrobial Activity and Bacterial Density on the Feathers
Antimicrobial activity of pigmented (linear regression, r 2 adjusted = 0.14; Beta (SE) = −0.42 (0.16); F 1,32 = 6.78, P = 0.014) and white feathers (linear regression, r 2 adjusted = 0.15; Beta (SE) = −0.41 (0.16); F 1,32 = 6.55, P = 0.015) was negatively related to bacterial density of feathers of opposite color within the same nest. These results did not change when the experimental treatment was included as an additional factor in the analyses (ESM, Table 1). Because antimicrobial properties and bacterial density of feathers of the same color were not related (ESM, Table 1), these results suggest that antimicrobial properties of bacteria from feathers of one color regulated bacterial densities of feathers of the other color. Moreover, antimicrobial activity of white and pigmented feathers from the same nest was positively related (r 2 adjusted = 0.51, n = 34, P = 0.002) suggesting the existence of factors (i.e., level of interference competition) acting at the level of nests. Detected associations did not depend of the number of white or black feathers in the nest at the end of incubation, since it did not explain mean bacterial density or antimicrobial activity on either white or black feathers (ESM, Table 2).
Relationships Between Antimicrobial Properties of Feathers, Eggshell Bacterial Loads, and Hatching Success
Consistent with previous results, experimental treatment explained a significant proportion of variance of density of mesophilic bacteria and Enterobacteriaceae. Eggshells of white nests harbored a lower load of mesophilic bacteria and Enterobacteriaceae than eggshells from pigmented nests (Table 2). In addition, antimicrobial activity of white feathers was negatively related to density of mesophilic bacteria and Enterococcus on the eggshells, while antimicrobial activity of pigmented feathers was positively related to density of mesophilic bacteria, Enterococcus and Enterobacteriaceae (Table 2). None of these results depended on excluding non-significant factors from the statistical models.
Finally, neither antimicrobial activity of white nor pigmented feathers explained significant variation in hatching success (GLM for white feathers: arcsine transformed hatching success as dependent variable; experimental treatment as factor, F 1,29 = 0.94, P = 0.341; log-transformed CFUs on feathers, F 1,29 = 0.29, P = 0.594; arcsine transformed antimicrobial activity, F 1,29 = 0.02, P = 0.891; GLM for pigmented feathers: arcsine transformed hatching success as dependent variable; experimental treatment as factor, F 1,29 = 0.73, P = 0.400; log-transformed CFUs on feathers, F 1,29 = 1.51, P = 0.229; arcsine transformed antimicrobial activity, F 1,29 = 0.21, P = 0.652).
Discussion
Our main findings are that antimicrobial properties of bacteria growing on feathers lining nests are correlated with the bacterial loads of eggshell of barn swallows and that these effects depend on feather pigmentation, likely because feather pigments affect growth of feather-degrading bacteria that produce antimicrobial compounds. Specifically, bacteria that produce antibiotics that inhibit the growth of B. licheniformis were more abundant on white than on pigmented feathers, and the antimicrobial activity of white lining feathers was negatively correlated with the density of bacteria on the eggshells. These results suggest a role for feathers lining nests in determining microbial communities of avian nests, and perhaps the effects of these communities on eggshell microbial loads and host fitness.
Feathers are largely composed of keratin, a recalcitrant molecule that few organisms can degrade [49, 50]. B. licheniformis produces several antimicrobial substances (see “Introduction”), and bacterial strains isolated from feathers of different colors likely also differ in their antimicrobial properties. Accordingly, we found that antimicrobial activity against B. licheniformis was higher for colonies from white than from pigmented feathers. Antimicrobial substances reduce bacterial competition for resources [51]. Thus, antimicrobials should primarily affect bacterial strains that use similar resources affecting co-existing strains that are closely related [29]. Our study detected the predicted higher antimicrobial activity of colonies from white feathers and also demonstrated that antimicrobial effects can be detected on objects in contact with such feathers (in this case, feathers of different colors, but perhaps also eggshells and nestlings).
If benefits associated with using feathers to line nests are partially mediated by antimicrobial substances from bacteria growing on them, there should be a direct link between eggshell bacterial loads and antimicrobial properties of feathers of different colors. Our results partially supported this prediction. The loads of bacteria on the eggshells of swallows reduced antimicrobial activity of isolates from white feathers as expected if bacteria from white feathers have a beneficial effect. However, antimicrobial activity of colonies from pigmented feathers had the opposite effects on eggshell bacterial load after the effect of antimicrobial activity of white feathers was considered. Antimicrobial activities of white and pigmented feathers from the same nest were in any case positively correlated, and colonies from white feathers reduced eggshell bacterial loads more than did colonies from pigmented feathers. Consequently, the results are consistent with the expectation that white feathers have greater antimicrobial activity, which explains the preference of barn swallows for unpigmented nest lining feathers.
Bacterial loads on eggshells are associated with risk of trans-shell infection and decreased embryo viability [30, 32, 34, 52]. We have shown in previous work that experimental manipulation of the color composition of feathers lining nests affects eggshell bacterial loads [4]. Additionally, experimental nests with more white feathers added at the beginning of incubation had a lower probability of hatching failure [33]. These two previous results suggested two different beneficial effects of lining nests with white feathers. Although we did not detect an effect of antimicrobial properties on hatching success, the results presented here suggest that the previously detected experimental effects [4, 33] might be mediated by differential antimicrobial properties of bacteria from white and pigmented feathers lining the nests. The bacterial load on eggshells has been positively correlated with the probability of trans-shell infection in a tropical bird [31, 52], although this relationship seems weaker in Mediterranean environments [53, 54]. This lack of a link between hatching success, antimicrobial substances, and bacterial load on eggshells may be related to incubation behavior by females [30] or maternal effects such as antimicrobial substances on eggs [55, 56]. The use of feathers, especially white ones, may provide an additional defense against egg infection, and hence a larger sample size might be necessary to rigorously test for an effect on hatching success.
Traditionally, the use of feathers as nest lining material has been proposed to be a behavioral trait that improves the insulation and thermoregulation of avian nests [38, 57, 58]. Barn swallows with an experimentally reduced number of feathers in the nests increased their incubation effort, and nestlings were in poorer condition (lower body mass) and experienced a longer nestling period than did nestlings in control nests [59]. These effects were interpreted in terms of reduced thermoregulation but might also stem from increased risk of trans-shell bacterial infection from higher eggshell bacterial loads. The increase in incubation effort of swallows could also be related to its effects in reducing eggshell bacterial loads and therefore reducing the probability of hatching failure [30, 52]. The effect on developing nestlings could be a consequence of increased incubation effort reducing the energy budget of females for investing in nestling care, which could result in a longer nestling period.
Barn swallows prefer white feathers for lining their nests [4, 33], and our results show the beneficial effects of this preference and suggest a new mechanism underlying it. Our results also suggest that antimicrobial properties of white feathers may affect those of pigmented feathers, and vice versa. An increased density of FDB from feathers lining nests may also be costly because they might colonize feathers of parents during incubation. This possibility together with our results allows us to speculate that swallows and other birds may attempt to establish an intermediate proportion of white and pigmented feathers relative to both thermal and bacterial environmental conditions. This study thus opens new directions for future investigations of the role of bacterial communities on feathers and of their production of antimicrobial substances in determining the quality of the overall bacterial nest environment and fitness.
References
Gunderson AR (2008) Feather degrading bacteria: a new frontier in avian and host–parasite research? Auk 125:972–979
Møller AP, Peralta-Sánchez JM, Nielsen JT, López-Hernández E, Soler JJ (2012) Goshawk prey have more bacteria than non-prey. J Anim Ecol 81:403–410
Soler JJ, Martín-Vivaldi M, Peralta-Sánchez JM, Ruiz-Rodríguez M (2010) Antibiotic-producing bacteria as a possible defence of birds against pathogenic microorganisms. Open Ornithol 2:29–36
Peralta-Sánchez JM, Møller AP, Martín-Platero AM, Soler JJ (2010) Number and colour composition of nest lining feathers predict eggshell bacterial community in barn swallow nests: an experimental study. Funct Ecol 24:426–433
Williams CM, Richter CS, Mackenzie JM, Shih JCH (1990) Isolation, identification, and characterization of a feather degrading bacterium. Appl Environ Microbiol 56:1509–1515
Burtt EHJ, Ichida JM (1999) Occurrence of feather-degrading bacilli in the plumage of birds. Auk 116:364–372
Shawkey MD, Mills KL, Dale C, Hill GE (2005) Microbial diversity of wild bird feathers revealed through culture-based and culture-independent techniques. Microb Ecol 50:40–47
Bisson IA, Marra PP, Burtt EH, Sikaroodi M, Gillevet PM (2007) A molecular comparison of plumage and soil bacteria across biogeographic, ecological, and taxonomic scales. Microb Ecol 54:65–81
Bisson IA, Marra PP, Burtt EH, Sikaroodi M, Gillevet PM (2009) Variation in plumage microbiota depends on season and migration. Microb Ecol 58:212–220
Møller AP (1991) The effect of feather nest lining on reproduction in the swallow Hirundo rustica. Ornis Scand 22:396–400
Shawkey MD, Pillai SR, Hill GE, Siefferman LM, Roberts SR (2007) Bacteria as an agent for change in structural plumage color: correlational and experimental evidence. Am Nat 169:S112–S121
Shawkey MD, Pillai SR, Hill GE (2009) Do feather-degrading bacteria affect sexually selected plumage color? Naturwissenschaften 96:123–128
Ruiz-de-Castañeda R, Burtt EH, Gonzalez-Braojos S, Moreno J (2012) Bacterial degradability of an intrafeather unmelanized ornament: a role for feather-degrading bacteria in sexual selection? Biol J Linn Soc 105:409–419
Gunderson AR, Frame AM, Swaddle JP, Forsyth MH (2008) Resistance of melanized feathers to bacterial degradation: is it really so black and white? J Avian Biol 39:539–545
Grande JM, Negro JJ, Torres MJ (2004) The evolution of bird plumage colouration: a role for feather-degrading bacteria? Ardeola 51:375–383
Goldstein G, Flory KR, Browne BA, Majid S, Ichida JM, Burtt EH (2004) Bacterial degradation of black and white feathers. Auk 121:656–659
Lucas FS, Broennimann O, Febbraro I, Heeb P (2003) High diversity among feather-degrading bacteria from a dry meadow soil. Microb Ecol 45:282–290
Whitaker JM, Cristol DA, Forsyth MH (2005) Prevalence and genetic diversity of Bacillus licheniformis in avian plumage. J Field Ornithol 76:264–270
Cladera-Olivera F, Caron GR, Brandelli A (2004) Bacteriocin-like substance production by Bacillus licheniformis strain P40. Lett Appl Microbiol 38:251–256
Galvez A, Maqueda M, Martínez-Bueno M, Lebbadi M, Valdivia E (1993) Isolation and physicochemical characterization of an antifungal and antibacterial peptide produced by Bacillus licheniformis A12. Appl Microb Biot 39:438–442
Galvez A, Valdivia E, Gonzalezsegura A, Lebbadi M, Martínez-Bueno M, Maqueda M (1993) Purification, characterization, and lytic activity against Naegleria fowleri of 2 amoebicins produced by Bacillus licheniformis A12. Appl Environ Microbiol 59:1480–1486
Galvez A, Maqueda M, Cordovilla P, Martínez-Bueno M, Lebbadi M, Valdivia E (1994) Characterization and biological activity against Naegleria fowleri of amoebicins produced by Bacillus licheniformis D-13. Antimicrob Agents Chemother 38:1314–1319
Lebbadi M, Galvez A, Valdivia E, Martínez-Bueno M, Maqueda M (1994) Biological activity of amoebicin M4-A from Bacillus licheniformis M-4. Antimicrob Agents Chemother 38:1820–1823
Kakudo S, Kikuchi N, Kitadokoro K, Fujiwara T, Nakamura E, Okamoto H, Shin M, Tamaki M, Teraoka H, Tsuzuki H, Yoshida N (1992) Purification, characterization, cloning, and expression of a glutamic acid-specific protease from Bacillus licheniformis Atcc-14580. J Biol Chem 267:23782–23788
Manachini PL, Fortina MG (1998) Production in sea-water of thermostable alkaline proteases by a halotolerant strain of Bacillus licheniformis. Biotechnol Lett 20:565–568
Nthangeni MB, Patterton HG, van Tonder A, Vergeer WP, Litthauer D (2001) Over-expression and properties of a purified recombinant Bacillus licheniformis lipase: a comparative report on Bacillus lipases. Enzyme Microb Tech 28:705–712
Pattnaik P, Kaushik JK, Grover S, Batish VK (2001) Purification and characterization of a bacteriocin-like compound (Lichenin) produced anaerobically by Bacillus licheniformis isolated from water buffalo. J Appl Microbiol 91:636–645
Atlas RM, Bartha R (1998) Microbial ecology: fundamentals and applications. Benjamin-Cummings Pub Co, Redgood City
Madigan MT, Martinko JM, Parker J (2004) Brock biology of microorganisms. Pearson Prentice Hall, Madrid
Cook MI, Beissinger SR, Toranzos GA, Arendt WJ (2005) Incubation reduces microbial growth on eggshells and the opportunity for trans-shell infection. Ecol Lett 8:532–537
Cook MI, Beissinger SR, Toranzos GA, Rodriguez RA, Arendt WJ (2005) Microbial infection affects egg viability and incubation behavior in a tropical passerine. Behav Ecol 16:30–36
Board RG, Fuller R (1994) Microbiology of the avian egg. Chapman & Hall, London
Peralta-Sánchez JM, Møller AP, Soler JJ (2011) Colour composition of nest lining feathers affects hatching success of barn swallows, Hirundo rustica (Passeriformes: Hirundinidae). Biol J Linn Soc 102:67–74
Soler JJ, Peralta-Sanchez JM, Martín-Platero AM, Martín-Vivaldi M, Martínez-Bueno M, Møller AP (2012) The evolution of size of the uropygial gland: mutualistic feather mites and uropygial secretion reduce bacterial loads of eggshells and hatching failures of European birds. J Evol Biol 25:1779–1791
Cramp S (1998) The complete birds of the Western Palearctic on CD-ROM Software Optimedia. Oxford University Press, Oxford
Hansell MH (2000) Bird nests and construction behaviour. Cambridge University Press, Cambridge
Hansell MH (1995) The demand for feathers as building material by woodland nesting birds. Bird Study 42:240–245
Winkler DW (1993) Use and importance of feathers as nest lining in tree swallows (Tachycineta bicolor). Auk 110:29–36
Stephenson S, Hannon S, Proctor H (2009) The function of feathers in tree swallow nests: insulation or ectoparasite barrier? Condor 111:479–487
Veiga JP, Polo V (2005) Feathers at nests are potential female signals in the spotless starling. Biol Lett 1:334–337
Sanz JJ, Garcia-Navas V (2011) Nest ornamentation in blue tits: is feather carrying ability a male status signal? Behav Ecol 22:240–247
Brook I (1999) Bacterial Interference. Crit Rev Microbiol 25:155–172
Becker J, Eisenhauer N, Scheu S, Jousset A (2012) Increasing antagonistic interactions cause bacterial communities to collapse at high diversity. Ecol Lett 15:468–474
Møller AP (1987) Egg predation as a selective factor for nest design: an experiment. Oikos 50:91–94
Narushin VG (1997) The avian egg: geometrical description and calculation parameters. J Agr Eng Res 68:201–205
Lauber CL, Zhou N, Gordon JI, Knight R, Fierer N (2010) Effect of storage conditions on the assessment of bacterial community structure in soil and human-associated samples. FEMS Microbiol Lett 307:80–86
Peralta-Sánchez JM, Martín-Platero AM, Martínez-Bueno M, Oñate M, Ruiz-Rodríguez M, Soler JJ (2012) Avian life history traits influence eggshell bacterial loads: a comparative study. Ibis 154:725–737
Sokal RR, Rohlf FJ (1995) Biometry. W. H. Freeman, San Francisco, USA
Gupta R, Ramnani P (2006) Microbial keratinases and their prospective applications: an overview. Appl Microbiol Biot 70:21–33
McKittrick J, Chen PY, Bodde SG, Yang W, Novitskaya EE, Meyers MA (2012) The structure, functions, and mechanical properties of keratin. JOM-US 64:449–468
Brook I, Gober AE (1998) Bacterial interference in the nasopharynx following antimicrobial therapy of acute otitis media. J Antimicrob Chemother 41:489–492
Cook MI, Beissinger SR, Toranzos GA, Rodriguez RA, Arendt WJ (2003) Trans-shell infection by pathogenic micro-organisms reduces the shelf life of non-incubated bird’s eggs: a constraint on the onset of incubation? Proc R Soc Lond B 270:2233–2240
Ruiz-de-Castañeda R, Vela AI, Lobato E, Briones V, Moreno J (2011) Bacterial loads on eggshells of the pied flycatcher: environmental and maternal factors. Condor 113:200–208
Wang JM, Firestone MK, Beissinger SR (2011) Microbial and environmental effects on avian egg viability: do tropical mechanisms act in a temperate environment? Ecology 92:1137–1145
Wellman-Labadie O, Picman J, Hincke MT (2007) Avian antimicrobial proteins: structure, distribution and activity. World Poult Sci J 63:421–438
Wellman-Labadie O, Picman J, Hincke MT (2008) Antimicrobial activity of cuticle and outer eggshell protein extracts from three species of domestic birds. Br Poult Sci 49:133–143
Palmgren P, Palmgren M (1939) Über die Wärmeisolierungskapazität verschiedener Kleinvogelnester. Ornis Fenn 16:1–6
Møller AP (1984) On the use of feathers in bird’s nests: predictions and tests. Ornis Scand 15:38–42
Møller AP (1987) Nest lining in relation to the nesting cycle in the swallow Hirundo rustica. Ornis Scand 18:148–149
Acknowledgments
We thank E. Lopez-Hernández for technical assistance and Einar Flensten-Jenssen for providing accommodation and interesting discussions during fieldwork. We appreciate the comments by three anonymous referees that improved the quality of the manuscript. This study was funded by Ministerio de Ciencia e Innovación/FEDER (project CGL2007-61251/BOS and CGL2010-19233-C03-01) and Junta de Andalucía (project P09-RNM-4557). JMPS was funded by Ministerio de Educación and Consejería de Innovación, Ciencia y Empresa under International Excellence Campus Program (CEI Granada). RK was supported in part by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM Table 1
(DOCX 18 kb)
ESM Table 2
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Peralta-Sánchez, J.M., Soler, J.J., Martín-Platero, A.M. et al. Eggshell Bacterial Load Is Related to Antimicrobial Properties of Feathers Lining Barn Swallow Nests. Microb Ecol 67, 480–487 (2014). https://doi.org/10.1007/s00248-013-0338-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-013-0338-5