Abstract
Microorganisms have shaped the evolution of a variety of defense mechanisms against pathogenic infections. Radioactivity modifies bacterial communities and, therefore, bird hosts breeding in contaminated areas are expected to adapt to the new bacterial environment. We tested this hypothesis in populations of barn swallows (Hirundo rustica) from a gradient of background radiation levels at Chernobyl and uncontaminated controls from Denmark. Investment in defenses against keratinolytic bacteria was measured from feather structure (i.e., susceptibility to degradation) and uropygial secretions. We studied degradability of tail feathers from areas varying in contamination in laboratory experiments using incubation of feathers with a feather-degrading bacterium, Bacillus licheniformis, followed by measurement of the amount of keratin digested. The size of uropygial glands and secretion amounts were quantified, followed by antimicrobial tests against B. licheniformis and quantification of wear of feathers. Feathers of males, but not of females, from highly contaminated areas degraded at a lower rate than those from medium and low contamination areas. However, feathers of both sexes from the Danish populations showed little evidence of degradation. Individual barn swallows from the more contaminated areas of Ukraine produced the largest uropygial secretions with higher antimicrobial activity, although wear of feathers did not differ among males from different populations. In Denmark, swallows produced smaller quantities of uropygial secretion with lower antimicrobial activity, which was similar to swallow populations from uncontaminated areas in Ukraine. Therefore, barn swallows breeding in contaminated areas invested more in all defenses against keratinolytic bacteria than in uncontaminated areas of Ukraine and Denmark, although they had similar levels of feather wear. Strong natural selection exerted by radioactivity may have selected for individuals with higher defense capacity against bacterial infections during the 30 years since the Chernobyl disaster.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nuclear accidents are among the most devastating phenomena that occur in modern societies because significant amounts of radioactivity are released to the environment, and due to a continuous chronic exposure to radiation, their detrimental effects are accumulated over time (Møller and Mousseau 2013). The worst nuclear accident to date occurred at the Chernobyl Nuclear Power Plant in 1986. After 30 years, DNA damage in cells of free-living animals continues as a consequence of the accident (Bonisoli-Alquati et al. 2010). There are considerable evolutionary and ecological effects in terms of species richness, abundance of animals (Møller and Mousseau 2007), and abnormalities (Møller et al. 2007) linked to levels of radiation. Such effects have also been found in feathers of birds such as the barn swallow (Hirundo rustica). Individuals breeding in contaminated areas around Chernobyl show a high frequency of partial albinism and paler melanin-based colors (Møller and Mousseau 2006), abnormal barbs that prevent them from fusing normally, and different color pattern, morphology, and shape (Møller 1993; Møller et al. 2013) than in uncontaminated areas. It is therefore possible that radioactivity affects feather functionality through, for instance, feather degradation.
Feathers can be degraded by the action of physical agents such as abrasion (Burtt 1986) or by microorganisms such as bacteria or fungi, which, through the secretion of keratinases, are able to digest keratin (Burtt and Ichida 1999), the main component of feathers (Sangali and Brandelli 2000). Keratinolytic bacteria inflict heavy costs on their bird hosts by making holes in feathers (Ruiz-Rodríguez et al. 2009), which reduce the efficiency of flight performance, especially significant in long-distance migratory birds such as swallows (Barbosa et al. 2003). The bacterial environment may affect the probability of infection of feathers (Bisson et al. 2007), and ionizing radiation alters microbial community composition (Jones et al. 2004; McNamara et al. 2007). The microbial diversity in the soil is lower in contaminated areas around Chernobyl probably due to radiation exposure (Romanovskaya et al. 1998), but also the amount of mesophylic bacteria isolated from swallow feathers is reduced compared to uncontaminated areas (Czirjak et al. 2010). In addition, some microorganisms have been shown to experience higher mutation rates in Chernobyl (Ragon et al. 2011), while highly resistant and genetically adapted species may be favored in radioactive areas (Mironenko et al. 2000; Zhdanova et al. 2000). Bacteria originating from sites exposed to different background radiation in Chernobyl (after 30 years of evolution), and subsequently experimentally subject to different radiation levels in the laboratory, differed in their level of radio-resistance depending on the level of radiation at the sites of origin (Ruiz-González et al. 2016).
Particular feather composition (i.e., pigments, Burtt et al. 2011) and antimicrobial chemicals from uropygial secretion (Shawkey et al. 2003; Ruiz-Rodríguez et al. 2009) are the main lines of defense of birds against keratinolytic bacteria. Thus, since bacterial communities in contaminated areas changed, we could expect differences in investment in defenses to be adjusted to the new microbial environment. The intensity of selection exerted by bacteria differs between sexes for feathers subject to sexual selection (Ruiz de Castañeda et al. 2012; Ruiz-Rodríguez et al. 2015). Hence, defenses against bacterial degradation of ornaments may also differ between males and females.
Briefly, we here studied investment in defenses against keratinolytic bacteria in populations of barn swallows (H. rustica) breeding under different levels of ionizing radiation around Chernobyl and in control populations from Denmark. We know that keratinolytic bacterial load differs between male and female barn swallows (Czirjak et al. 2010) and, thus, we explore possible sexual differences in antibacterial defenses. First, we studied susceptibility of feathers to degradation by keratinolytic bacteria by means of a laboratory experiment. This experiment was performed with the outermost feathers of the tail, a sexually selected character in male barn swallows (e.g., Møller et al. 1998) to assess differences in degradation among sexes. Second, we estimated the quantity of uropygial secretion produced by individual swallows in the field, and the antimicrobial properties in laboratory experiments. Third, we evaluated the level of wear of tail feathers from all populations, which will depend on environmental conditions and on bird’s investment in defenses. Because birds from highly contaminated areas in Chernobyl are more stressed than those from uncontaminated areas (Møller and Mousseau 2007), we hypothesized that antimicrobial defenses would be less developed in the former than in the latter populations. Alternatively, birds breeding in contaminated areas may have developed adaptations to radiation (Møller and Mousseau 2016), for which it could also be expected that they invest more against microbial infections. In addition, since the two sexes may invest in feather composition differentially in ornamental feathers to show their ability to cope against parasites (Ruiz de Castañeda et al. 2012; Ruiz-Rodríguez et al. 2015), sex-linked differences in degradation susceptibility should also be expected. However, since selective pressures may also differ among areas and/or countries (e.g., sexual selection, ionizing radiation, or bacterial loads), the expected sexual differences may only be detected in some but not all populations. In addition, in the case of ionizing radiation being an important force shaping investment in feather structure and antimicrobial defenses, it could be expected that Danish and uncontaminated populations from Ukraine would be similar.
Material and methods
Sampling procedures
Field work was performed in Ukraine from June 3 to 7 in 2014 and from May 29 to June 9 in 2015, and in Denmark from June 9 to July 4 in 2014, June 12 to July 6 in 2015. Barn swallows were captured by placing mist nets at the entrance of farm buildings where they breed. Populations from Chernobyl used in this study were Rudnia and Vesniane with high levels of contamination, within the Chernobyl Exclusion Zone (<30 km from the nuclear power plant); Dytiatki with intermediate contamination outside but close to the exclusion zone (<1 km), and Voronkov with a low background contamination located at >100 km of the exclusion zone. The radiation levels were measured at ground level using a hand-held dosimeter (Model: Inspector, SE International, Inc., Summertown, TN, USA), and independently of the study year, it was 1.5, 1.5–2, 0.2–0.5, and 0.02 μSv/h in each of the four locations, respectively. We considered three levels of environmental contamination according to environmental radiation, which was used as a categorical variable: high (Rudnia and Vesniane), medium (Dytiatki), and low (Voronkov). For more details on study areas, see Czirjak et al. (2010). In addition, an uncontaminated population of barn swallows breeding in Denmark was also sampled. In 2014, an external long tail feather was removed and used for evaluation of susceptibility to degradation and estimation of wear. In 2015, the height of the uropygial gland was measured (always by the same person) as a proxy of its size, which was found to have a high repeatability in hoopoes (Upupa epops) (Martín-Vivaldi et al. 2009). Although we do not have data on the total volume of glands from swallows used in the present work, we know that uropygial height of spotless starlings is strongly positively correlated with gland volume (Ruiz-Rodríguez et al., unpublished results). The secretion was extracted by placing a sterile micro-capillary (32 mm 9 μl−1) in the gland opening and slightly pressing the gland to completely remove its secretion. Extraction of the secretion was always performed by the same person. The length of the capillary filled with the secretion was considered as a proxy of the volume of secretion. Feathers were kept at 4 °C and secretions at −20 °C until laboratory analysis.
The age of individuals was estimated following Balbontín and Møller (2015), which mainly consists in unbanded birds being considered yearlings at first capture. Because of the low dispersal rates of barn swallows and the continuous monitoring of the populations since 2000, we were able to accurately estimate age.
Susceptibility to bacterial degradation
One centimeter of the distal part of each feather was cut and put in sterile Eppendorf tubes previously weighed on a precision balance (AB135-5/FACT (±0.00001 g), Mettler Toledo, Spain). Then, the tubes were weighed again to calculate the mass of each piece of feather, and feathers were autoclaved before the experiment (Gunderson et al. 2008). All these procedures were performed in a laminar flow cabinet under sterile conditions.
A buffer was prepared by mixing 9.34 mM NH4Cl, 8.55 mM NaCl, 1.72 mM K2HPO4, 2.92 mM KH2PO4, 0.49 mM MgCl2-6H2O, and 0.01 % yeast extract in 100 ml distilled water (following Goldstein et al. 2004). From this mix, 5 ml was deposited in each experimental glass tube, and then sterilized. A colony of Bacillus licheniformis previously isolated on TSA plates was introduced with a sterile loop in each experimental tube along with the sterilized feather piece. We selected this bacterium because it is very common in birds of different species and different populations (Burtt and Ichida 1999; Lucas et al. 2003; Shawkey et al. 2005; Bisson et al. 2007). Moreover, Bacillus spp. are also very common in soils (Watanabe and Hayano 1995), including areas subject to ionizing radiation around Chernobyl (Romanovskaya et al. 1998, 2002), from which birds may acquire plumage bacteria (Lucas et al. 2003). Thus, it is highly probable that barn swallows encounter Bacillus spp. during their annual cycle, even those breeding in radioactively contaminated areas.
After vortexing, we collected 1 ml from each tube as a basal measurement (i.e., 0 h of incubation) and kept it separately at 4 °C until measurement in the spectrophotometer (see below). Experimental tubes were incubated at 37 °C in constant agitation at 120 rpm, in an orbital agitator (VWR, Spain), and 1 ml was collected after 14 days (408 h). Samples were centrifuged to remove bacterial cells, and absorbance of the supernatant was estimated using a spectrophotometer (Helios Zeta UV–vis, Thermo Scientific, UK) at 230 nm (Goldstein et al. 2004). The oligopeptide concentration in the supernatant originates from keratin degradation, and thus is directly proportional to the amount of feather degraded. Calibration curves of absorbance and oligopeptide concentration (from 0 to 300 μg/ml) were obtained by using bovine serum albumin (BSA) (before experiment: F 1,6 = 618.36, p < 0.0001, R 2 = 0.99; after experiment: F 1,6 = 497.78, p < 0.0001, R 2 = 0.98), which allowed us to extrapolate the absorbance to values of protein concentration.
To assure that oligopeptide measurements were due to the degradation of feathers by Bacillus exclusively, we also prepared three control treatments (five tubes of each) containing buffer only, buffer and the feather, and buffer and bacteria. There were no significant differences in degradation among the three control groups (F 2,13 = 1.12, p = 0.35), and they had significantly less oligopeptide concentration at the end of the experiment than treatment tubes (control vs. experimental tubes, repeated-measures ANOVA, F 1,261 = 4.63, p = 0.03; effect size: d = 0.71, r = 0.33, which correspond to a medium effect). Therefore, we can assume that oligopeptides measured in experimental samples were significantly influenced by the action of Bacillus degrading the feather.
We analyzed feathers of 247 individuals in total, 136 from Ukraine (levels of contamination: high, 19 ♀♀ and 27 ♂♂; medium, 19 ♀♀ and 24 ♂♂; low, 24 ♀♀ and 23 ♂♂) and 111 from Denmark (55 ♂♂ and 56 ♀♀).
Antimicrobial activity of uropygial secretion
The antimicrobial activity of the uropygial secretion was measured by means of antagonistic plates. Those plates were prepared as follows: 15 ml of a culture medium previously prepared and sterilized (1.8 % of brain–heart infusion (BHI) and 0.8 % agar in 0.1 M pH 7 phosphate buffer) was melted and then maintained at 50 °C for 10 min. Then, 100 μl of a 12-h culture of B. licheniformis D13 was added to the medium, vigorously vortexed, and spread onto a Petri dish. After solidifying about 30 min later, a drop of each uropygial secretion was placed on the plates and later incubated for 12 h at 28 °C. We attempted to homogenize the quantity of secretion deposited in the plates by using just the two first millimeter of secretion from each capillary. This activity was always performed by the same person. After incubation, plates were checked for inhibition halos; that is, transparent zones around the secretion drop in which the indicator bacterium growth was inhibited by the secretion. Those halos were measured (in mm) from the limit of the drop to the end of the halo (i.e., where the indicator bacteria growth begins).
We analyzed secretions from 151 individuals in total, 63 from Ukraine (levels of contamination: high, 5 ♀♀ and 12 ♂♂; medium, 3 ♀♀ and 6 ♂♂; low, 21 ♀♀ and 16 ♂♂) and 88 from Denmark (41 ♂♂ and 47 ♀♀).
Wear of feathers
Feathers were photographed under a binocular lens (Nikon SMZ1500, Melville, NY, USA) connected to a camera (Nikon Digital Sight DS Fi1). Three pictures of each tail feather were taken from different parts (distal, white patch and proximal) at ×2 magnification. Degradation levels were ranked from 0 to 3 following Ruiz-Rodríguez et al. (2015), in which 0 implied no visual degradation, 1 that less of 1/3 of the feather was degraded, 2 if the wear was between 1/3 and 2/3 of the feather, and 3 if the degradation was extended to more than 2/3 of the feather. Two different estimations of degradation were made per picture (repeated-measures ANOVA, F 1,743 = 4285.86, p < 0.0001, repeatability = 96 %), and the mean of both estimates was used in the analyses. The degradation level of each feather was considered to be the sum of degradation of the three different parts.
Statistical analyses
Differences in susceptibility to degradation were measured by using repeated-measures ANOVA. Two models were developed: one to compare differences among the two countries, and another to assess differences among populations subject to different radiation levels in Ukraine. The two dependent variables (oligopeptide concentration at the beginning and at the end of the experiment) were included as within individual effects, sex and country or level of contamination were used as the categorical predictors, and feather mass was a covariable. Since the mortality rate of Chernobyl barn swallows, and in particular females, is higher than in other populations (Møller et al. 2012a), we also checked the possible effects of age on investment in defenses against keratinolytic bacteria when comparing Denmark and Chernobyl, and thus age was also included as additional covariable.
The degradation status of the feathers was evaluated by means of a General Linear Model (GLM) in which the estimation of degradation was the dependent variable, while sex and country or level of contamination were used as the categorical predictors.
Finally, differences in size of the uropygial gland were performed with the log-transformed variable to adjust to a normal distribution, and analyzed by using a GLM in which the categorical predictors were the sex and the country or radiation level in Chernobyl. However, the volume and antimicrobial activity of the secretion were not possible to normalize, and for these, non-parametric tests (Kruskal-Wallis) were performed.
Results
Degradability experiments
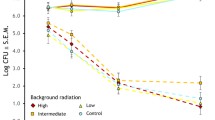
At the beginning of the experiment, there were no significant differences in oligopeptide concentration among sexes and localities (Fisher post-hoc tests, all p > 0.24). However, degradation rate of keratin at the end of the experiment differed between Ukraine and Denmark (RM-A, F 1,241 = 5.31, p = 0.02, Fig. 1). Fisher post-hoc analyses indicated that these differences were due to Ukrainian male feathers degrading at a higher rate than feathers from Danish individuals (♂♂ Chernobyl vs. (1) ♂♂ Denmark: p = 0.02, and (2) ♂♂ Chernobyl vs. ♀♀ Denmark: p = 0.003). In Chernobyl, feathers of males tended to degrade at a higher rate than in females, although not significantly so (p = 0.07). However, degradation in Ukrainian females did not differ significantly from that of Danish individuals of both sexes (both p > 0.22). There was no significant effect of age in degradability of feathers from Ukraine (F 1,133 = 0.44, p = 0.50) and Denmark (F 1,107 = 0.24, p = 0.61).
In Chernobyl, we found differences in degradability rate among populations subject to different radiation levels (RM-A, F 2,129 = 3.26, p = 0.04, Fig. 1, Table 1). Individuals less susceptible to degradation were from highly contaminated areas (Fisher post-hoc test, (1) high vs. low: p = 0.01, (2) high vs. medium: p = 0.04, (3) low vs. medium: p = 0.73), and this negative association was similar for males and females (interaction between sex and contamination, p = 0.92). Although the sex did not explain differences in susceptibility to degradation (Table 1), we could see in the post-hoc tests that male feathers degraded at a higher rate than those of females (Fisher post-hoc test, p = 0.02) in the two populations with low and medium background radiation, while feathers of both sexes in contaminated areas differed significantly from those in the other populations (Fisher post-hoc test, all p < 0.03).
Uropygial glands and secretions
The volume of uropygial wax extracted differed between countries, but not between sexes or ages (GLM, country: F 1,208 = 56.65, p < 0.001; sex: F 1,208 = 0.08, p = 0.77; age: F 1,208 = 1.65, p = 0.20), with individuals from Ukraine producing more secretions. Interaction among country and sex was not significant (F 1,210 = 0.89, p = 0.34). There were also differences among the Ukrainian populations (GLM, radiation level: F 2,80 = 11.17, p < 0.001; sex: F 1,80 = 0.10, p = 0.74), with barn swallows from contaminated areas producing larger amounts of secretion (Fisher post-hoc tests, high vs. low: p < 0.001, and high vs. medium (p < 0.001)); interaction between radiation level and sex was not significant (F 2,81 = 0.91, p = 0.40). The size of the uropygial gland was larger in Danish individuals (χ 2 1 = 129.08, p < 0.001), while no significant differences were found among the different Chernobyl sites (χ 2 1 = 1.96, p = 0.37).
Ukrainian individuals had higher capacity to inhibit Bacillus growth than Danish ones (χ 2 1 = 68.93, p < 0.001). In Ukraine, no significant differences were detected among the populations (χ 2 1 = 2.03, p = 0.36). Although there was no a main effect of contamination level, it is worth mentioning that post-hoc tests revealed differences between individuals in low compared to medium and high contamination levels (high: p = 0.008; medium: 0.07). The highest antimicrobial activity against B. licheniformis was found in populations under high and medium background radiation.
The sexes did not differ significantly in uropygial height or antimicrobial activity of secretions (all p > 0.25).
Wear of feathers
We found no significant differences in the wear of feathers between sexes or countries (GLM, country: F 1,244 = 2.23, p = 0.13; sex: F 1,244 = 0.18, p = 0.67). However, the interaction between sex and country was significant (F 1,245 = 3.97, p = 0.04). Inspection of the interaction plots revealed that although males from both countries had feathers with the same level of wear, females from Denmark had feathers that were more damaged than those from Ukrainian females (Fisher post-hoc test, p = 0.01).
Within the Chernobyl sites, there were no significant differences between sexes (GLM, F 1,132 = 1.18, p = 0.27), while there was a marginally significant difference among populations (F 2,132 = 2.73, p = 0.06). Interaction among both factors was not significant (F 2,133 = 2.33, p = 0.10). However in the post-hoc tests, we could see differences in feathers of females from different populations: feathers from more contaminated areas were significantly less damaged than those from populations with medium and low radiation level (both p = 0.01).
Discussion
Susceptibility to bacterial degradation
Feathers from Ukrainian barn swallows degraded at a higher rate than those from Denmark, although differences were mainly due to higher susceptibility to degradation in males. However in Ukraine, feathers of male barn swallows from populations subject to medium and low levels of contamination were more susceptible to degradation. Different selective pressures due to diverse life-history traits could be shaping investment in feather structure of birds from different populations. Geographical differences, migration routes and distances to winter quarters, or different environmental microbiota, may explain why Danish populations have similar degradation susceptibility as in the contaminated areas in Chernobyl.
In Danish populations, degradation was similar among sexes. However in Ukraine, males degraded at a higher rate (Fig. 1). We are aware of ostensible geographic variation in tail length of male barn swallows probably due to geographic differences in the intensity of natural and sexual selection (Møller 1994; Hasegawa et al. 2010). The outermost tail feather is a sexually selected trait in male and female barn swallows, in particular the tip (Cuervo and De Ayala 2014), and bacteria are known to influence the development of these traits (Shawkey et al. 2009). The structure of such feather ornaments may have evolved to signal how individuals cope with bacterial infections as shown by Ruiz de Castañeda et al. (2012) and Ruiz-Rodríguez et al. (2015) for pied flycatchers and spotless starlings, respectively. In both cases, ornamental feathers degraded at higher rates than non-ornamentals and, at least for flycatchers, ornament degradation is related to breeding date and parental effort (Ruiz-de-Castañeda et al. 2015). Thus, it is possible that the detected geographic variation in feather degradability among populations was due to variation in the strength of selection pressures.
Swallows are migratory birds, and selective pressures on tail feathers would likely purge individuals with degraded or abnormal feathers from the population, which are more abundant in contaminated areas (see “Introduction”). Thus, even if these feathers function as sexually selected characters in highly contaminated areas, males that develop feathers more resistant to microbial degradation may have been selectively favored during the 30 years since the nuclear accident.
Uropygial secretion
Production of uropygial secretion and its antimicrobial activity was similar in populations from Denmark and uncontaminated areas in Ukraine. We failed in detect a main effect of contamination in the antimicrobial activity against B. licheniformis of populations from Ukraine. However, there was a stronger activity in swallows from more contaminated areas, which also produced more uropygial secretion. These findings are consistent with stronger selection for birds with higher defenses against bacterial infections. Since no differences in abundance of cultivable keratinolytic bacteria in barn swallows from areas differing in radiation level were found (Czirjak et al. 2010), it is reasonable to think that bacteria in more contaminated areas have more capacity to degrade the feathers, which would exert higher pressures on birds. This is in accordance with our findings in the secretion and also in the susceptibility to degradation of feathers from different areas.
Although the antimicrobial activity of uropygial secretions is an ongoing topic of discussion in the literature, several studies have found antimicrobial components in uropygial secretion (Martín-Platero et al. 2006; Martín-Vivaldi et al. 2010; Ruiz-Rodríguez et al. 2013) and direct evidence of inhibition of bacterial growth (Shawkey et al. 2003; Ruiz-Rodríguez et al. 2015), including feather-degrading bacteria (Møller et al. 2009). However, other studies have failed to detect antimicrobial properties of secretion (Giraudau et al. 2013; Reneerkens et al. 2008). The present study adds evidence to the antimicrobial activity of secretion by performing antagonistic tests against B. licheniformis.
Previous studies of other birds found a positive relationship between uropygial gland size and amount of secretion produced (Martín-Vivaldi et al. 2009; Ruiz-Rodríguez et al. 2015). We also found a positive association between gland height and volume of secretion, but only for swallows captured in contaminated areas in Ukraine, which might be related to the relatively higher selection pressures at these locations (see above). In contaminated areas, the use of the secretion could be more continuous due to the highest selective pressure exerted on birds. However in Denmark, if the use of the secretion is lower, it could be more difficult to find a relationship between the volume of secretion and gland size, because it would depend on the time since the last preening.
Wear of feathers
Feathers of female barn swallows from highly contaminated areas were less worn than in medium and low contaminated areas in Ukraine. Barn swallows experience reduced reproductive success in areas with high background radiation and, thus, invest less effort in reproduction than in less contaminated areas; particularly females (Møller et al. 2005). Thus, such females could allocate more time to preening and self-maintenance, increasing investment in antimicrobial defenses. However, feather wear is similar in males from different areas. Since mortality increases with level of radiation (Møller et al. 2012a), and the abundance of birds in contaminated areas decreased by around two thirds in Chernobyl (Møller and Mousseau 2007), natural selection in contaminated areas could have resulted in only males that invest the most in defenses against keratinolytic bacteria and can maintain a viable level of feather wear.
Alternative explanations could be that swallows captured in highly contaminated areas were immigrants from other populations (Møller et al. 2006). In that case, barn swallows from uncontaminated populations might invest more in defenses against keratinolytic bacteria. However, we found the opposite trend. Another possibility is that due to their higher mortality rate (Møller et al. 2005, 2012a), individual barn swallows from Chernobyl are younger, which could affect the level of defenses against feather-degrading bacteria. However, this explanation can be rejected because we did not find an effect of age on degradation rate of feathers or characteristics of the uropygial gland. Thus, natural selection purging individuals with more worn feathers is the more likely explanation.
Microorganisms are considered an important selective force shaping the evolution of eukaryotes (McFall-Ngai et al. 2013). Sudden changes in environmental bacterial communities and thus in selective pressures exerted on hosts may affect the capacity of hosts to protect themselves in the long term. In addition, exposure to high levels of radiation has profound effects on organisms causing severe health problems or even death (Møller and Mousseau 2006; Møller et al. 2012a). This may have selected for males with more resistant phenotypes against microorganisms in contaminated areas, while sexual selection may be stronger in less contaminated areas. Despite differences in susceptibility to degradation and differences in amounts of uropygial secretions, barn swallow feathers from Denmark and Ukraine had similar levels of damage, i.e., they may have invested more in defenses in Ukraine to maintain the same level of wear.
Human impact on the environment may have important consequences for birds and other organisms. Nuclear accidents constitute a strong selective force, and thus it is necessary to study the long-term consequences for the performance of organisms and their interactions. Changes in populations of some species may indirectly affect entire communities, thus altering ecosystem functioning (Møller et al. 2012b). Therefore, it is important to study the consequences of radiation on host-pathogen dynamics. Finally, defense mechanisms of hosts against new microbial environments in contaminated habitats will contribute to a better understanding of the consequences of contemporary problems caused by humans and may help to identify possible solutions.
References
Balbontín J, Møller AP (2015) Environmental conditions during early life accelerate the rate of senescence in a short-lived passerine bird. Ecology 96:948–959
Barbosa A, Merino S, Cuervo JJ, De Lope F, Møller AP (2003) Feather damage of long tails in barn swallows Hirundo rustica. Ardea 91:85–90
Bisson IA, Marra PP, Burtt EH, Sikaroodi M, Gillevet PM (2007) A molecular comparison of plumage and soil bacteria across biogeographic, ecological, and taxonomic scales. Microb Ecol 54:65–81
Bonisoli-Alquati A, Voris A, Mousseau TA, Møller AP, Saino N, Wyatt MD (2010) DNA damage in barn swallows (Hirundo rustica) from the Chernobyl region detected by use of the comet assay. Comp Biochem Phys C 151:271–277
Burtt EH (1986) An analysis of physical, physiological, and optical aspects of avian coloration with emphasis on wood-warblers. Ornithol Monographs 38
Burtt EH, Ichida JM (1999) Occurrence of feather-degrading bacilli in the plumage of birds. Auk 116:364–372
Burtt EH, Schroeder MR, Smith LA, Sroka JE, Mcgraw KJ (2011) Colourful parrot feathers resist bacterial degradation. Biol Lett 7:214–216
Cuervo JJ, De Ayala RM (2014) Effects of experimental tail shortening on the phenotypic condition of barn swallows Hirundo rustica: implications for tail-length evolution. J Avian Biol 45:345–353
Czirjak GA, Møller AP, Mousseau TA, Heeb P (2010) Microorganisms associated with feathers of barn swallows in radioactively contaminated areas around Chernobyl. Microb Ecol 60:373–380
Giraudau M, Czirjak GA, Duval C, Bretagnolle V, Gutierrez C, Guillon N, Heeb P (2013) Effect of preen oil on plumage bacteria: an experimental test with the mallard. Behav Process 92:1–5
Goldstein G, Flory KR, Browne BA, Majid S, Ichida JM, Burtt EH (2004) Bacterial degradation of black and white feathers. Auk 121:656–659
Gunderson AR, Frame AM, Swaddle JP, Forsyth MH (2008) Resistance of melanized feathers to bacterial degradation: is it really so black and white? J Avian Biol 39:539–545
Hasegawa M, Arai E, Watanabe M, Nakamura M (2010) Mating advantage of multiple male ornaments in the barn swallow Hirundo rustica gutturalis. Ornithol Sci 9:141–148
Jones HE, West HM, Chamberlain PM, Parekh NR, Beresford NA, Crout NMJ (2004) Effects of gamma irradiation on Holcus lanatus (Yorkshire fog grass) and associated soil microorganisms. J Environ Radioactiv 74:57–71
Lucas FS, Broennimann O, Febbraro I, Heeb P (2003) High diversity among feather-degrading bacteria from a dry meadow soil. Microb Ecol 45:282–290
Martín-Platero AM, Valdivia E, Ruiz-Rodríguez M, Soler JJ, Martín-Vivaldi M, Maqueda M, Martínez-Bueno M (2006) Characterization of antimicrobial substances produced by Enterococcus faecalis MRR 10–3, isolated from the uropygial gland of the hoopoe (Upupa epops). Appl Environ Microbiol 72:4245–4249
Martín-Vivaldi M, Peña A, Peralta-Sánchez JM, Sánchez L, Ananou S, Ruiz-Rodríguez M, Soler JJ (2010) Antimicrobial chemicals in hoopoe preen secretions are produced by symbiotic bacteria. Proc R Soc B-Biol Sci 277:123–130
Martín-Vivaldi M, Ruiz-Rodríguez M, Soler JJ, Peralta-Sánchez JM, Méndez M, Valdivia E, Martín-Platero AM, Martínez-Bueno M (2009) Seasonal, sexual and developmental differences in hoopoe Upupa epops preen gland morphology and secretions: evidence for a role of bacteria. J Avian Biol 40:191–205
McFall-Ngai M, Hadfield MG, Bosch TCG, Carey HV, Domazet-Loso T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF, Hentschel U, King N, Kjelleberg S, Knoll AH, Kremer N, Mazmanian SK, Metcalf JL, Nealson K, Pierce NE, Rawls JE, Reid A, Ruby EG, Rumpho M, Sanders JG, Tautz D, Wernegreen JJ (2013) Animals in a bacterial world, a new imperative for the life sciences. PNAS 110:3229–3236
McNamara NP, Griffiths RI, Tabouret A, Beresford NA, Bailey MJ, Whiteley AS (2007) Appl Soil Ecol 37:1–9
Mironenko NV, Alekhina IA, Zhdanova NN, Bulat SA (2000) Intraspecific variation in gamma-radiation resistance and genomic structure in the filamentous fungus Alternaria alternate: a case study of strains inhabiting Chernobyl reactor nr 4. Ecotoxicol Environ Saf 45:177–187
Møller AP (1993) Morphology and sexual selection in the barn swallow Hirundo rustica in Chernobyl, Ukraine. Proc R Soc B-Biol Sci 252:51–57
Møller AP (1994) Sexual selection and the barn swallow. Oxford University Press, Oxford
Møller AP, Barbosa A, Cuervo JJ, de Lope F, Merino S, Saino N (1998) Sexual selection and tail streamers in the barn swallow. Proc R Soc B-Biol Sci 265:409–414
Møller AP, Barnier F, Mousseau TA (2012a) Ecosystems effects 25 years after Chernobyl: pollinators, fruit set and recruitment. Oecologia 170:1155–1165
Møller AP, Bonisoli-Alquati A, Rudolfsen G, Mousseau TA (2012b) Elevated mortality among birds in Chernobyl as judged from skewed age and sex ratios. PLoS ONE 7, e35223
Møller AP, Bonisoli-Alquati A, Mousseau TA (2013) High frequency of albinism and tumours in free-living birds around Chernobyl. Mutat Res-Gen Toxicol Environ 757:52–59
Møller AP, Czirjak GA, Heeb P (2009) Feather micro-organisms and uropygial antimicrobial defences in a colonial passerine bird. Funct Ecol 23:1097–1102
Møller AP, Hobson KA, Mousseau TA, Peklo AM (2006) Chernobyl as a population sink for barn swallows: tracking dispersal using stable isotope profiles. Ecol Appl 16:1696–1705
Møller AP, Mousseau TA (2006) Biological consequences of Chernobyl: 20 years on. Trends Ecol Evol 21:200–207
Møller AP, Mousseau TA (2007) Species richness and abundance of forest birds in relation to radiation at Chernobyl. Biol Lett 3:483–486
Møller AP, Mousseau TA (2013) Assessing effects of radiation on abundance of mammals and predator–prey interactions in Chernobyl using tracks in the snow. Ecol Indic 26:112–116
Møller AP, Mousseau TA (2016) Are organisms adapting to ionizing radiation at Chernobyl? Trends Ecol Evol 31:281–289
Møller AP, Mousseau TA, de Lope F, Saino N (2007) Elevated frequency of abnormalities in barn swallows from Chernobyl. Biol Lett 3:414–417
Møller AP, Mousseau TA, Milinevsky G, Peklo A, Pysanets E, Szép T (2005) Condition, reproduction and survival of barn swallows from Chernobyl. J Anim Ecol 74:1102–1111
Ragon M, Restoux G, Moreira D, Møller AP, Lopez-Garcia P (2011) Sunlight exposed biofilm microbial communities are naturally resistant to Chernobyl ionizing-radiation levels. PLoS ONE 6, e21764
Reneerkens J, Versteegh MA, Schneider AM, Piersma T, Burtt EH (2008) Seasonally changing preen-wax composition: Red Knots’ (Calidris canutus) flexible defense against feather-degrading bacteria? Auk 125:285–290
Romanovskaya VA, Sokolov IG, Rokitko PV, Chernaya NA (1998) Effect of radioactive contamination on soil bacteria in the 10-km zone around the Chernobyl nuclear power plant. Microbiol 67:226–231
Romanovskaya VA, Rokitko PV, Mikheev AN, Gushcha NI, Malashenko YR, Chernaya NA (2002) The effect of γ-radiation and desiccation on the viability of the soil bacteria isolated from the alienated zone around the Chernobyl Nuclear Power Plant. Microbiol 71:608–613
Ruiz de Castañeda R, Burtt EH, González-Braojos S, Moreno J (2012) Bacterial degradability of an intrafeather unmelanized ornament: a role for feather-degrading bacteria in sexual selection? Biol J Linn Soc 105:409–419
Ruiz-de-Castañeda R, Burtt EH, González-Braojos S, Moreno J (2015) Bacterial degradability of white patches on primary feathers is associated with breeding date and parental effort in a migratory bird. Ibis 157:871–876
Ruiz-González MX, Czirják GA, Genevaux P, Møller AP, Mousseau TA, Heeb P (2016) Resistance of feather-associated bacteria to intermediate levels of ionizing radiation near Chernobyl. Sci Rep 6:22969
Ruiz-Rodríguez M, Valdivia E, Soler JJ, Martín-Vivaldi M, Martin-Platero AM, Martinez-Bueno M (2009) Symbiotic bacteria living in the hoopoe’s uropygial gland prevent feather degradation. J Exp Biol 212:3621–3626
Ruiz-Rodríguez M, Martínez-Bueno M, Martín-Vivaldi M, Valdivia E, Soler JJ (2013) Bacteriocins with a broader antimicrobial spectrum prevail in enterococcal symbionts isolated from the hoopoe’s uropygial gland. FEMS Microbiol Ecol 85:495–502
Ruiz-Rodríguez M, Tomás G, Martin-Gálvez D, Ruiz-Castellano C, Soler JJ (2015) Bacteria and the evolution of honest signals. The case of ornamental throat feathers in spotless starlings. Funct Ecol 29:701–709
Sangali S, Brandelli A (2000) Feather keratin hydrolysis by a Vibrio sp strain kr2. J Appl Microbiol 89:735–743
Shawkey MD, Pillai SR, Hill GE (2003) Chemical warfare? Effects of uropygial oil on feather-degrading bacteria. J Avian Biol 34:345–349
Shawkey MD, Mills KL, Dale C, Hill GE (2005) Microbial diversity of wild bird feathers revealed through culture-based and culture-independent techniques. Microb Ecol 50:40–47
Shawkey MD, Pillai SR, Hill GE (2009) Do feather-degrading bacteria affect sexually selected plumage color? Naturwissenschaften 96:123–128
Watanabe K, Hayano K (1995) Seasonal variation of soil protease activities and their relation to proteolytic bacteria and Bacillus spp. in paddy field soil. Soil Biol Biochem 27:197–203
Zhdanova NN, Zakharchenko VA, Vember VV, Nakonechnaya LT (2000) Fungi from Chernobyl: mycobiota of the inner regions of the containment structures of the damaged nuclear reactor. Mycol Res 104:1421–1426
Acknowledgments
M. Ruiz-Rodríguez had a Postdoc from the program “Andalucía Talent Hub” (Agencia Andaluza del Conocimiento, Junta de Andalucía). Funding was provided in part from the Samuel Freeman Charitable Trust, the US Fulbright Program, the CNRS (France), the American Council of Learned Societies, the University of South Carolina College of Arts and Sciences, and the Spanish Ministerio de Economía y Competitividad (European funds (FEDER)) (CGL2013-48193-C3-1-P). We also thank two anonymous referees for comments that have improved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Alexandre Roulin
Rights and permissions
About this article
Cite this article
Ruiz-Rodríguez, M., Møller, A.P., Mousseau, T.A. et al. Defenses against keratinolytic bacteria in birds living in radioactively contaminated areas. Sci Nat 103, 71 (2016). https://doi.org/10.1007/s00114-016-1397-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-016-1397-5