Abstract
Whether or not treatment with antibiotics or probiotics for bacterial vaginosis (BV) is associated with a change in the diversity of vaginal microbiota in women was investigated. One hundred fifteen women, consisting of 30 healthy subjects, 30 BV-positive control subjects, 30 subjects with BV treated with a 7-day metronidazole regimen, and 25 subjects with BV treated with a 10-day probiotics regimen, were analyzed to determine the efficacy and disparity of diversity and richness of vaginal microbiota using 454 pyrosequencing. Follow-up visits at days 5 and 30 showed a greater BV cure rate in the probiotics-treated subjects (88.0 and 96 %, respectively) compared to the metronidazole-treated subjects (83.3 and 70 %, respectively [p = 0.625 at day 5 and p = 0.013 at day 30]). Treatment with metronidazole reduced the taxa diversity and eradicated most of the BV-associated phylotypes, while probiotics only suppressed the overgrowth and re-established vaginal homeostasis gradually and steadily. Despite significant interindividual variation, the microbiota of the actively treated groups or participants constituted a unique profile. Along with the decrease in pathogenic bacteria, such as Gardnerella, Atopobium, Prevotella, Megasphaera, Coriobacteriaceae, Lachnospiraceae, Mycoplasma, and Sneathia, a Lactobacillus-dominated vaginal microbiota was recovered. Acting as vaginal sentinels and biomarkers, the relative abundance of Lactobacillus and pathogenic bacteria determined the consistency of the BV clinical and microbiologic cure rates, as well as recurrent BV. Both 7-day intravaginal metronidazole and 10-day intravaginal probiotics have good efficacy against BV, while probiotics maintained normal vaginal microbiota longer due to effective and steady vaginal microbiota restoration, which provide new insights into BV treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial vaginosis (BV) is the most common disorder in women of reproductive age. BV can be microbiologically characterized by replacement of the lactobacilli-predominant vaginal microbiota by potential pathogenic vaginal bacteria [1]. The change from a healthy, H2O2- and lactic acid-producing lactobacilli-dominated microbiota to a complex multispecies microbiota can occur relatively quickly and result in BV [2, 3]. Our previous studies have profiled the overall structure of vaginal communities and clearly demonstrated that BV is associated with a dramatic increase in the taxonomic richness and diversity of vaginal microbiota [1, 4]. The dramatic shifts in vaginal microbiota contribute to pH elevation and sialidase and amine production, which eventually lead to the observed signs and symptoms [1, 5].
The current recommended treatment of BV includes metronidazole (oral or vaginal) or clindamycin (vaginal) [6, 7]; however, the short-term (30 days) cure rate is often poor and recurrences are common [5, 8–10]. Recently, probiotic therapy has been shown to be effective against BV and confers a range of other health benefits [11, 12]. The increased dominance of lactobacilli after active treatment has been shown to be effective in assessing the normality or “health” of the vaginal microbiota [5]. The diversity and richness of the vaginal microbiota after short-term treatment might also affect the long-term efficacy against BV. Fredricks et al. used quantitative polymerase chain reaction (qPCR) assays to assess the vaginal-predominant microbiota in BV patients after metronidazole treatment and showed that BV-associated bacteria decreased dramatically [15]. Of note, the details of the overall structure of the vaginal microbiota after treatment with metronidazole or probiotics have not been thoroughly investigated.
In the present clinical cohort study, living preparations of Lactobacillus delbrueckii subsp. lactis DM8909 were used to treat women with symptomatic BV vaginally, while metronidazole was used as a control. To characterize the vaginal microbiota after treatment, we characterized the overall structure of the vaginal microbiota using 454 pyrosequencing. These results were helpful in clarifying the efficacy of probiotics on BV and the effects of probiotics on the diversity of vaginal microbiota, thus providing new insights into BV treatment.
Materials and Methods
Probiotics
L. delbrueckii subsp. lactis DM8909, derived from a healthy gut, was identified by the Institute of Microbiology of the Chinese Academy of Science. L. delbrueckii subsp. lactis DM8909, which was used in the current study, does not contain a plasmid and has been used and approved as a probiotics by the Food and Drug Administration of China since 2001. Each suppository contained at least 109 colony-forming units of live Lactobacillus, can produce various anti-infective agents, including lactic acid and H2O2, and is able to co-aggregate efficiently with vaginal pathogens. It is also able to adhere to human epithelial cells at high levels and exert colonization resistance to displace vaginal pathogens.
Study Population
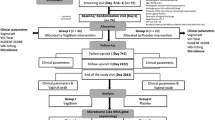
A clinical cohort trial was conducted to compare intravaginal metronidazole (500 mg once daily at bedtime for 7 days [BV-M group]) and an intravaginal probiotic Lactobacillus capsule (once daily at bedtime for 10 days [BV-L group]) in the treatment of BV. One hundred ninety-one women, consisting of 121 women actively treated for BV, 30 BV-positive controls before treatment, and 40 healthy controls (CN group) who presented to our hospital for routine gynecologic examinations between July 2010 and June 2011, were recruited in the treatment trial. Informed written consent was obtained from all participants prior to enrollment, with approval of the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (Zhejiang Province, China). Individuals who participated in this study were examined by two gynecologists. BV status was assessed using the Amsel clinical criteria for all subjects [16] and confirmed using Gram stain criteria (Nugent scores) [17]. The inclusion and exclusion criteria for these patients were shown in detail in Supplementary Information Materials and Methods as previously described [1, 18].
Therapy was initiated after the enrollment visit or immediately after the end of menstruation if menses were expected within a 7-day period. One hundred twenty-one BV-positive women were randomized to treatment with intravaginal metronidazole (n = 68) and intravaginal probiotics (n = 53, Fig. 1), while 40 healthy controls were not treated. All participants were asked to avoid sexual intercourse and vaginal douching during treatment. Among the participants, 55 BV-positive women (BV-M [n = 30] and BV-L women [n = 25]) and 30 CN women completed two menstrual cycles and had vaginal swabs for Nugent scoring and bacterial diversity analysis. Samples were obtained 5 and 30 days after the last treatment in the three groups, and vaginal swabs were collected from 30 BV-positive women (BV group) during the same time period, which were used as positive controls. The BV participants with clinical cures were evaluated according to Amsel criteria in combination with Nugent scores (<4). The clinical data for each participant are shown in Table S1.
Diversity and Richness of Vaginal Microbiota Analysis
When women underwent genital examinations before and after treatment, vaginal swabs were obtained near the mid-vagina using a sterile swab from each woman, packaged, and placed in ice packs. BV status was assessed with the Amsel clinical criteria and Nugent scoring system; the vaginal swabs used for bacterial genomic DNA extraction were transferred to the laboratory in an ice box immediately and stored at −80 °C after preparation within 15 min for further analysis. One hundred fifteen samples obtained from the first follow-up after treatment were used to determine the bacterial diversity of vaginal microbiota. The methods for total bacterial genomic DNA extraction, PCR amplification, qPCR, 454 pyrosequencing, and statistical analysis were performed as described in our previous studies [1, 19] (see Supplementary Information Materials and Methods).
Accession Numbers
The sequence data have been deposited in the GenBank Sequence Read Archive with accession number SRP007938.
Results
Clinical Outcome
Of the 121 BV patients enrolled in the current study, 55 who completed the two follow-up visits could be evaluated for efficacy. Of the 55 participants, 22 of 25 patients (88.0 %) were cured with Lactobacillus, compared with 25 of 30 patients (83.3 %) who were cured with metronidazole (p = 0.625) at the first follow-up visit based on the Amsel criteria and Nugent scoring criteria. The Lactobacillus and metronidazole treatment groups were similar with respect to overall clinical outcomes. Both metronidazole and probiotics rapidly relieved the clinical signs and symptoms of BV, such as vaginal discomfort, elevated pH, and homogeneous malodorous vaginal discharge, and the Nugent scores were <4 (Table S1). At the second follow-up visit during the next menstrual cycle, only four women in the BV-M group and one woman in the BV-L group had clinically persistent BV; however, 5 of 25 clinically cured women in the BV-M group relapsed, while no relapses occurred in the BV-L group (p < 0.05; Table 1). Our result indicated that a 10-day regimen of Lactobacillus DM8909, administered as an intravaginal suppository, was as effective as a 7-day regimen of vaginal metronidazole (500 mg) in the treatment of BV, with no recorded relapses.
Different Diversity of Vaginal Microbiota After Treatment
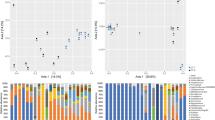
Table S2 summarizes the indices of vaginal bacterial diversity based on the operational taxonomic units (OTUs) estimated and clearly showed that bacterial diversity in the BV-M group and BV-L group was much more complex than the CN group, while less complex than the BV-positive control group (p < 0.05). Our data also showed that the vaginal bacterial diversity in the BV-L group was significantly different from women in the BV-M group (p < 0.05). By rarefaction analysis estimates, the trend for species richness was as follows: BV-L > BV > BV-M > CN (Fig. 2a). In the four groups, a long tail in the rank abundance curves was observed, indicating that the majority of OTUs was present at low abundance (Fig. 2b). The vaginal bacterial communities in each group were grouped according to community composition using UniFrac metrics (Fig. 2c). The clustering was complemented by an analysis of bacterial richness using the number of shared and unique OTUs in the four groups (Fig. 2d), indicating that a core microbiome existed in healthy vaginas. Principal coordinates analysis (PCA) showed that two BV actively treated samples and healthy control samples clustered more tightly than the BV-positive control, although several clinically persistent BV actively treated samples clustered into the BV-positive control group (Fig. 3c).
Rarefaction curves were used to estimate richness (in this case the number of taxa at a 3 % dissimilarity level) among CN, BV, and BV treated with metronidazole and Lactobacillus groups at the first follow-up visit (at day 5) (a). The vertical axis shows the number of OTUs that would be expected to be found after sampling the number of tags or sequences shown on the horizontal axis. Rank abundance curve of bacterial OTUs derived from four groups (b). Differentiation in vaginal bacterial communities from CN, BV, and BV treated with metronidazole and Lactobacillus groups at the first follow-up visit (at day 5) and 115 individual samples. Community differentiation was measured by using the unweighted UniFrac algorithm; the scale bar indicates the distance between clusters in UniFrac units. All of the branch nodes shown here were found to be significant (p < 0.001), indicating that BV, CN, and two BV-treated groups harbored distinct bacterial communities, while BV-treated groups showed significant microbiota restoration (c). Venn diagram representation of the OTU richness shared among bacterial 16S rRNA gene libraries from the four groups. Total observed richness was 6,143 OTUs at 97 % similarity. Percentages reflect those OTUs unique to that group (d)
The relative abundance of vaginal bacterial V3 tags obtained by pyrosequencing from CN, BV, and BV treated with metronidazole and Lactobacillus at the first follow-up visit (at day 5), by genus (a) and by phylum (b). Phylogenetic classification for the pyrosequencing analysis obtained from Ribosomal Database Project Classifier analyses. PCA of vaginal bacterial communities among four groups obtained by pyrosequencing and performed using R program (c)
Lactobacilli-Dominant Vaginal Microbiota After Treatment
The total number of unique sequences from the vagina was 33,722, which was assigned into seven phyla, while Actinobacteria, Bacteroidetes, Firmicutes, and Fusobacteria constituted the dominant phyla (Fig. 3b). In the development of BV, the vaginal bacterial profiles could change quite rapidly and extensively from a profile in which members of the Firmicutes, especially lactobacilli, dominate to a profile with a high abundance of Bacteroidetes, Fusobacteria, and Actinobacteria members, while reversal of the composition of vaginal microbiota indicated the recovery of BV after treatment (Fig. S1A). At the genus level, sequences from the four groups represented 161 different genera (Table S3). Figure 3a shows that 28 genera constituted >99 % of the vaginal microbiota, while other rare minor genera accounted for <1 % of the vaginal microbiota, which confirmed the rank abundance curve in Fig. 2b. Specifically, the nearly equivalent change in trends in the BV-M and BV-L women were an increase in Lactobacillus and a decrease in Gardnerella, Atopobium, Coriobacteriaceae, Megasphaera, Gemella, Lachnospiraceae, Sneathia, Prevotella, and Mycoplasma (Fig. S1B) [1]. In agreement with the clinical outcome, the higher relative abundance of Lactobacillus after active treatment was one of the most important parameters for restoration of normal vaginal homeostasis; however, there were also dramatic interindividual variations among these samples, especially for BV-positive controls and BV-L participants (Fig. S2A, B). The predominant bacteria of the vaginal microbiota in women who were clinically cured of BV and those with clinically persistent BV following one short-term treatment are presented in Fig. 4. The relative abundance of predominant bacteria in the BV-M group was more concentrated and much more dispersed in the BV-L group. Verifying the results of pyrosequencing by qPCR, our data showed more abundant total bacteria and Lactobacillus crispatus subgroups colonized in the BV-L group, while Lactobacillus iners was the most predominant bacteria in the BV-M group at the first follow-up visit (Table S4).
Discussion
The results of the current clinical cohort trial suggest that metronidazole and the probiotics, L. delbrueckii subsp. lactis DM8909, can cure BV by >80 % after short-term treatment. Both agents can be used to treat BV. The vaginal microbiota plays an important role in determining the efficacy of agents for BV. With the next generation of high-throughput sequencing techniques, most of the clinically cured subjects could restore lactobacilli-dominant vaginal microbiota and maintain vaginal homeostasis over a relative long period. However, the overall structure of vaginal microbiota was different after treated with metronidazole and probiotics, including the diversity, composition, and richness, which might be ascribed to the different antimicrobial mechanisms. For the first time, we have characterized the disparity of vaginal microbiota after BV treatment in depth.
As an antimicrobial agent, metronidazole has been used extensively for the management and prophylaxis of anaerobic infections, including symptomatic BV [7]. Notably, the relative abundance of lactobacilli increased with recovery, coinciding with a decrease in the members of Actinobacteria, Bacteroidetes, and Fusobacteria, indicating a tendency for lactobacilli-dominant vaginal microbiota restoration. Endogenous lactobacilli, such as L. iners, become the predominant bacteria of the vaginal microbiota after treatment; however, our data indicated that metronidazole could not re-establish healthy vaginal homeostasis after eradicating BV-associated pathogens, as the total number of vaginal bacteria was reduced significantly. The vulnerable vagina could re-establish vaginal eubiosis after killing those pathogens thoroughly in one side and could also be easily re-colonized by residual pathogenic microorganisms in the other side. An equilibrium in the vaginal microbiota must be established for a relatively long time. Patients with a higher BV recurrence rate (16 %) should be followed more closely for residual pathogenic microorganisms. A previous study has also highlighted the persistent problem of BV, resorting to long-term antibiotic therapy to prevent recurrences [10]. However, Bradshaw et al. reported that prolonged metronidazole treatment does not prevent the recurrence of BV or abnormal vaginal microbiota in the majority of women [8]. In addition, the decrease in susceptibility to metronidazole for those pathogenic microorganisms could lead to final BV treatment failure, especially for the recently described metronidazole-resistant Atopobium vaginae [20]. For those clinical persistent infections, a higher relative abundance of pathogenic bacteria, such as Gardnerella and Atopobium, were also found in the present study. The taxonomic distribution of these predominant bacteria in the vagina also confirmed the relationship between the clinical cure rate and residual pathogens, thus the higher BV recurrence rate in this group.
The use of L. delbrueckii subsp. lactis DM8909 for BV treatment, like other probiotic strains, such as Lactobacillus GR-1, RC-14, Lactobacillus casei rhamnosus, Lactobacillus brevis, and Lactobacillus plantarum, can improve urogenital health through immune modulation, pathogen displacement, and creation of a niche less conducive to proliferation of pathogens and virulence factors [11, 13, 14, 21]. In addition, L. delbrueckii subsp. lactis DM8909 could colonize in the vagina for a relatively long time and facilitate re-establishment of the physiologic vaginal microbiota. Other than metronidazole, probiotics could not eradicate most of the pathogenic microorganisms rapidly, but only inhibit the overgrowth of those pathogenic bacteria gradually. Thus, the diversity of vaginal microbiota was much more complex than that of the BV-M group. Although the relative abundance of lactobacilli was slightly lower, the total number of lactobacilli was more than that in metronidazole-treated group. The replenishment of these exogenous Lactobacillus intravaginally restores vaginal homeostasis with different mechanisms, including producing hydrogen peroxide, lactic acid, and bacteriocins, which could suppress the overgrowth of those pathogenic microorganisms mentioned above and encourage the recovery of endogenous lactobacilli [22–24]. A previous study has reported that the loss of endogenous vaginal lactobacilli is important in the acquisition of BV [25]. The recovery of endogenous lactobacilli is one of the most important markers or sentinels for BV cure. Indeed, the restoration of vaginal endogenous lactobacilli is a slow process. The restoration of endogenous lactobacilli steadily after probiotics treatment, which confers strong colonization resistance, would replace pathogens and re-establish vaginal homeostasis. This could explain why women treated with probiotics have a lower recurrence rate in the long term. For those participants with antibiotic-resistant microorganisms and recurrences, it is necessary to treat them with other antibiotics based on antimicrobial susceptibility profiles alone or in combination with probiotics, or with complete replacement by probiotics. Research has shown that the intravaginal use of Lactobacillus, or antibiotics plus probiotics, yields a cure rate of approximately 88 % [11, 26]. The alternative natural treatment could be effective in the microbiologic and clinical resolution of BV without side effects [13, 27, 28].
There were several limitations to the current study. First, the present study was not a double-blind, placebo-controlled clinical trial, which presented an opportunity for bias of the clinical outcome after treatment. Second, there was no long-term follow-up analysis of the vaginal microbiota after active treatment. For such an analysis, it would be helpful to guide the clinical treatment of BV and evaluate its prognosis. Third, recurrent BV treatment was not included in the present study, which might be more useful for elucidating BV treatment failure with antibiotic administration. Fourth, clinically persistent BV should undergo antibiotic-resistant gene detection in the vaginal microbiota after metronidazole treatment, which suggests that treatment failed with the recommended therapy directly and that an alternative therapy must be chosen, such as probiotics.
Conclusions
The present study indicated that Lactobacillus DM8909, administered as an intravaginal suppository, is as effective as vaginal metronidazole in the treatment of bacterial vaginosis, with a lower relapse rate. Our results provide convincing evidence that the overall structure of vaginal microbiota differed significantly between women with BV treated with metronidazole and probiotics, at least in the short term. Specifically, multiple analyses indicated that the richness was greater in the Lactobacillus-treated group, while evenness tended to be greater in the metronidazole-treated group. However, despite dramatic interpersonal variations, both actively treated groups had an apparent increase in the lactobacilli-dominant vaginal microbiota and a dramatic reduction in pathogenic microorganisms. The changing pattern of vaginal microbiota after active treatment showed an obvious trend of vaginal microbiota restoration and vaginal homeostasis re-establishment. Along with antibiotic administration, probiotics offered an alternative means to treat and prevent BV and help to maintain a healthy vaginal ecosystem.
References
Ling Z, Kong J, Liu F, Zhu H, Chen X, Wang Y, Li L, Nelson KE, Xia Y, Xiang C (2010) Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genomics 11:488
Fredricks DN, Fiedler TL, Marrazzo JM (2005) Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 353:1899–1911
Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA (1993) The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis 16(Suppl 4):S273–281
Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ (2011) Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108(Suppl 1):4680–4687
Hummelen R, Fernandes AD, Macklaim JM, Dickson RJ, Changalucha J, Gloor GB, Reid G (2010) Deep sequencing of the vaginal microbiota of women with HIV. PLoS One 5:e12078, LID - e12078 [pii]
Carey JC, Klebanoff MA, Hauth JC, Hillier SL, Thom EA, Ernest JM, Heine RP, Nugent RP, Fischer ML, Leveno KJ, Wapner R, Varner M (2000) Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med 342:534–540
Workowski KA, Berman S (2010) Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep 59:1–110
Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, Horvath LB, Kuzevska I, Fairley CK (2006) High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 193:1478–1486
Sobel JD, Schmitt C, Meriwether C (1993) Long-term follow-up of patients with bacterial vaginosis treated with oral metronidazole and topical clindamycin. J Infect Dis 167:783–784
Sobel JD, Ferris D, Schwebke J, Nyirjesy P, Wiesenfeld HC, Peipert J, Soper D, Ohmit SE, Hillier SL (2006) Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol 194:1283–1289
Anukam K, Osazuwa E, Ahonkhai I, Ngwu M, Osemene G, Bruce AW, Reid G (2006) Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect 8:1450–1454
Uehara S, Monden K, Nomoto K, Seno Y, Kariyama R, Kumon H (2006) A pilot study evaluating the safety and effectiveness of Lactobacillus vaginal suppositories in patients with recurrent urinary tract infection. Int J Antimicrob Agents 28(Suppl 1):S30–34
Mastromarino P, Macchia S, Meggiorini L, Trinchieri V, Mosca L, Perluigi M, Midulla C (2009) Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin Microbiol Infect 15:67–74
Petricevic L, Witt A (2008) The role of Lactobacillus casei rhamnosus Lcr35 in restoring the normal vaginal flora after antibiotic treatment of bacterial vaginosis. BJOG 115:1369–1374
Fredricks DN, Fiedler TL, Thomas KK, Mitchell CM, Marrazzo JM (2009) Changes in vaginal bacterial concentrations with intravaginal metronidazole therapy for bacterial vaginosis as assessed by quantitative PCR. J Clin Microbiol 47:721–726
Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK (1983) Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74:14–22
Nugent RP, Krohn MA, Hillier SL (1991) Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29:297–301
Ling Z, Liu X, Chen X, Zhu H, Li L, Nelson KE, Xia Y, Xiang C (2011) Diversity of cervicovaginal microbiota associated with female lower genital tract infections. Microb Ecol 61:704–714
Ling Z, Kong J, Jia P, Wei C, Wang Y, Pan Z, Huang W, Li L, Chen H, Xiang C (2010) Analysis of oral microbiota in children with dental caries by PCR-DGGE and barcoded pyrosequencing. Microb Ecol 60:677–690
Ferris MJ, Masztal A, Aldridge KE, Fortenberry JD, Fidel PL Jr, Martin DH (2004) Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC Infect Dis 4:5
Martinez RC, Franceschini SA, Patta MC, Quintana SM, Gomes BC, De Martinis EC, Reid G (2009) Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol 55:133–138
Reid G, Bruce AW, Fraser N, Heinemann C, Owen J, Henning B (2001) Oral probiotics can resolve urogenital infections. FEMS Immunol Med Microbiol 30:49–52
Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher HJ (2011) Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 9:27–38
Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, Gurguis A, Faro S (2001) Defense factors of vaginal lactobacilli. Am J Obstet Gynecol 185:375–379
Hawes SE, Hillier SL, Benedetti J, Stevens CE, Koutsky LA, Wolner-Hanssen P, Holmes KK (1996) Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 174:1058–1063
Anukam K, Osazuwa E, Ahonkhai I, Ngwu M, Osemene G, Bruce AW, Reid G (2006) Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect 8:1450–1454
Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, Yarova-Yarovaya Y, Fiedler T, Cox M, Stamm WE (2011) Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis 52:1212–1217
Antonio MA, Meyn LA, Murray PJ, Busse B, Hillier SL (2009) Vaginal colonization by probiotic Lactobacillus crispatus CTV-05 is decreased by sexual activity and endogenous Lactobacilli. J Infect Dis 199:1506–1513
Acknowledgments
This work was supported by the National Basic Research Program of China (973 program) Grant 2013CB531404 and a Qiu-Shi Scholarship from Zhejiang University. We would like to thank all the participants recruited in this study.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Zongxin Ling, Xia Liu, and Weiguang Chen contributed equally to this work.
Clinical Trial Registration: ChiCTR-TRC-11001484 (www.chictr.org)
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 139 kb)
Table S1
The clinical data for each participant in our study (XLS 48 kb)
Table S2
Comparison of phylotype coverage and diversity estimation of the 16S rRNA gene libraries at the 3 % dissimilarity from the pyrosequencing analysis (DOC 39 kb)
Table S3
All genera assigned using the RDP classifier with at least 50 % as bootstrap support (XLS 299 kb)
Table S4
The abundance of vaginal bacteria relative to total Bacteria gene copy number by species-specific qPCR for clinical cured participants in the 5-day follow-up after treatment (DOC 47 kb)
Table S5
List of the 112 8-bp barcodes used to tag each PCR product analyzed as part of the study (DOC 42 kb)
Table S6
Species-specific primer sets for detection of vaginal bacteria by qPCR (DOC 84 kb)
Fig. S1
Differences of phylum (A) and predominant genera (B) detected in vagina from CN, BV, and BV treated with metronidazole and Lactobacillus individuals (*p < 0.05, **p < 0.01) (JPEG 42 kb)
Fig. S2
Interindividual variations in the proportion of major phyla (A) and genera (B) in 115 vaginal samples from four groups. Only the seven largest phyla and 23 largest genera are shown in the figure. These data illustrate the dramatic interindividual variation (JPEG 120 kb)
Rights and permissions
About this article
Cite this article
Ling, Z., Liu, X., Chen, W. et al. The Restoration of the Vaginal Microbiota After Treatment for Bacterial Vaginosis with Metronidazole or Probiotics. Microb Ecol 65, 773–780 (2013). https://doi.org/10.1007/s00248-012-0154-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-012-0154-3