Abstract
In this report, four Bacillus strains were tested for effects on plant fitness and disease protection of oilseed rape (Brassica napus). The strains belonged to newly discovered plant-associated Bacillus amyloliquefaciens and a recently proposed species, Bacillus endophyticus. The fungal pathogens tested represented different infection strategies and included Alternaria brassicae, Botrytis cinerea, Leptosphaeria maculans, and Verticillium longisporum. The B. amyloliquefaciens strains showed no or a weak plant growth promoting activity, whereas the B. endophyticus strain had negative effects on the plant as revealed by phenological analysis. On the other hand, two of the B. amyloliquefaciens strains conferred protection of oilseed rape toward all pathogens tested. In vitro experiments studying the effects of Bacillus exudates on fungal growth showed clear growth inhibition in several but not all cases. The protective effects of Bacillus can therefore, at least in part, be explained by production of antibiotic substances, but other mechanisms must also be involved probably as a result of intricate plant–bacteria interaction. The protective effects observed for certain Bacillus strains make them highly interesting for further studies as biocontrol agents in Brassica cultivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants exist in a complex ecological community where, e.g., inorganic nutrients, water, temperature, and light are parameters that can be measured and analyzed to describe the system. A more complex growth effect is the interaction of various organisms with plants where especially the soil is a rich source of various microorganisms [8]. Whereas most microorganisms do not interact with a given plant, some microorganisms may be beneficial or detrimental to the host plant. Of vital importance for many plants are, e.g., nitrogen fixation symbionts [6] in intimate association with plant tissues. Another habitat colonized by various microorganisms is the surface of plant tissues or the phyllosphere [15]. Certain bacteria function as plant growth promoting rhizobacteria (PGPR), i.e., they support the growth of plants [23]. Whereas such positive growth effects to plants originally was observed for Rhizobacteria, several other kinds of bacteria such as Bacillus, Pseudomonas, and Serratia strains [16] have also been found to be beneficial to plants. The PGPR effect can be due to, e.g., production of plant hormones like cytokinins and gibberellins or by increasing the amount of minerals and nitrogen available for the plant [22].
Microorganisms isolated from soil or plant tissue mediates protection of various plants toward different pathogens and insect pests in several different studies [1, 18, 19, 20, 24, 33, 36]. Bacteria and fungi that have been shown to protect plants include several genera where Streptomyces, Pseudomonas, and Bacillus are some representatives [10]. A wide spectrum of pest control by beneficial bacteria has been reported including protection against oomycetes as Peronospora parasitica [28], bacteria as Pseudomonas syringae [12], viruses as Cucumber mosaic virus [24], and insects as Cucumber beetles [35]. This biocontrol is effective in several unrelated plant species where cucumber, bean, oilseed rape, and tomato are examples of crops that have been protected by bacteria that are experimentally applied [18, 28]. Accordingly, the use of beneficial bacteria as environmental friendly pest control to increase yield and quality of crop plants has great potential.
Protection based on biocontrol agents can be mediated in many ways. Bacteria living in vascular tissue of plants or in the rhizosphere are in an appropriate position to protect the plant from deleterious organisms. Competition for growth space and nutrients by the beneficial bacteria can indirectly protect the plant from harmful microorganisms. Siderophores and phytic acid [13] have been shown to be important for some bacteria to protect plants through competition for nutrients. Bacteria can produce a wide array of antibiotics that can be effective against many different fungi and bacteria. Peptide antibiotics represent the predominant class of bacterially produced antibiotics [10]. Alteration of the plant cell wall that causes an increased protection to pathogens has been found to occur with both Bacillus subtilis and Pseudomonas aeruginosa [2, 3, 30]. Production of salicylic acid by bacteria can make the plant more tolerant to pests and pathogens by stimulating systemic acquired resistance (SAR), a common defense program induced in plants to combat pathogens [5]. Some bacteria have been shown to be able to induce a plant defense system called induced resistance (IR) or induced systemic resistance (ISR) [5, 28]. This defense is activated by jasmonate- and ethylene-dependent but salicylate-independent signaling and has been shown to be effective against insects, viruses, bacteria, and fungi [28].
The genus Bacillus is characterized by rod-shaped, facultative aerobe, endospore-forming bacteria that live in soil and often colonize the plant rhizosphere [20]. There is great genetic diversity in this genus, which include strains that have been found to live as endophytes inside plant vascular tissues but also as human pathogens like Bacillus anthracis. Several Bacillus spp. produce different kinds of antibiotics [14, 22, 29, 30, 32, 34] and some Bacillus strains, mainly B. subtilis, Bacillus cereus, and Bacillus amyloliquefaciens, are known to mediate protection against pathogens on plants [1, 19, 30, 33, 34].
The aim of this study was to evaluate the effects of treatment of oilseed rape (Brassica napus) with certain strains of Bacillus, which were shown in our earlier studies to belong to the genetically separated taxa of plant-associated bacteria referred to as Bacillus endophyticus [21] or B. amyloliquefaciens [20]. One goal was to evaluate the strains as potential biocontrol agents against four serious fungal pathogens to Brassica species, Alternaria brassicae, Botrytis cinerea, Leptosphaeria maculans, and Verticillium longisporum [11, 17, 26]. Another goal was to investigate if and how these strains might differ in the effect they have on the plants to provide a better understanding of how the bacteria–plant interaction operates. Is plant protection provided by all related bacterial strains? How is this protection mediated? How specific is this protection, and does it respond the same way toward all the pathogens or in a pathogen species-specific way? To approach these questions, we used three closely related strains of B. amyloliquefaciens originally isolated by Reva [20]. Bacillus UCMB-5033 and UCMB-5036 were isolated from inner tissues of cotton plants (Gossypium barbadense) in Tadjikistan, whereas UCMB-5113 is a red pigmented bacteria isolated from the soil of Karpaty mountains, Ukraine. Their plant colonization abilities on B. napus differ from high, for UCMB-5113, to very low for UCMB-5033, and none, for UCMB-5036 [20], which prompted further studies of protection abilities. The bacterial strain UCMB-5715T is the type culture of a newly proposed species, B. endophyticus [21]. Although this Bacillus strain was also isolated from the inner tissue of cotton plants together with B. amyloliquefaciens strains, it represents a phylogenetic lineage that is quite distant from other Bacillus species. Comparative analysis of sequences of 16S rRNA genes showed that this species is closely related to Bacillus sporothermodurans and Bacillus gibsonii and that they all are close to Jeotgalibacillus alimentarius (data not shown). This strain was included in our investigation to serve as a reference strain for B. endophyticus. To investigate how protection was mediated, production of antifungal compounds by the bacteria was investigated. A study of plant phenology was also performed to investigate effects of bacterial treatment on plant growth and development.
Materials and Methods
Bacillus Spore Solution
Bacillus strains: UCMB-5715T, UCMB-5033, UCMB-5036, and UCMB-5113, were all grown in Luria–Bertani (LB) media at 28°C with agitation for three days to allow for production of spores. The bacterial cultures were then heat treated at 75°C for 10 min to select for Bacillus spores and to kill possible contaminants. The spore concentration was determined by viable count analysis of an aliquot on LB plates and the stock spore solution was kept refrigerated until use.
Seed Treatment
Oilseed rape (B. napus, cv. Westar) seeds were surface sterilized for 20 min in 20% sodium hypochlorite followed by a brief rinse with 50% methanol. The seeds were mixed with each Bacillus spore solutions with a concentration of 107 spores ml-1. LB or water served as control but were not different from each other. The seeds were then left on agitation for two h until planting in soil. Standard soil (K-jord Weibulls) was sterilized in an autoclave for 30 min, and after allowing the soil to cool, the process was repeated.
Effects of Bacillus Inoculation on Plant Development
Sterile soil was put into 200 11 × 11 × 5 cm pots. One seed from separate treatments was put into each pot with a forceps. The pots were then arranged into five trays for every treatment, eight pots on each tray, and grown in a greenhouse using a 16/8 h photoperiod with minimum temperature settings at 22/18°C. All pots were given a similar amount of water to exclude differences between treatments depending on water status. The plants were observed continuously to identify any phenotypic differences between treatments. The characteristics that were measured included germination efficiency, number of true leaves developed at week two, three, and four, how many plants that were flowering at week three and four, and how many plants that survived seed development. Plants were harvested and the seeds were pooled for each treatment and were weighted. The germination rate of the harvested seeds was tested by planting 40 seeds from each treatment into sterile soil.
Plant Growth for Pathogen Studies
One seed was put into each pot (8 × 8 × 5 cm pots with sterile soil) with forceps. The pots were then arranged into different trays depending on treatment. The plants were grown in a growth chamber under 18/6 h photoperiod at 22/18°C using fluorescent light of approximately 200 μmol m-2 s-1. All pots received similar amounts of water to exclude differences between treatments depending on water availability.
Preparation of Fungal Spores
The fungal strains, A. brassicae (980:3), V. longisporum (Vd11), B. cinerea (30158), and L. maculans (1245) were grown on potato dextrose agar (PDA) plates (Sigma Chemical Co., St Louis, MI) in a growth chamber (16/8 h photoperiod at 21/16°C) for a month or until spores had been produced. The plates were harvested by addition of sterile water to the plates followed by scraping the agar surface with a pipette tip to release the spores. This procedure was repeated once, and the spores were pooled and filtered through miracloth. The concentration of spores was measured with a Bürkner chamber, and the final concentration was adjusted to 107 spores ml-1. The spore solution was stored at 4°C until use.
Inoculation of Plants with Pathogens
Plants that had a similar size (five true leaves) were chosen from each treatment. The plants were coded and mixed into trays in a random design. The inoculation of pathogen was done differently according to the fungus studied. A. brassicae and B. cinerea spores were sprayed on three-week-old plants. The A. brassicae infection was performed with 15 plants from each treatment and was replicated. B. cinerea infection experiments were replicated thrice with 8, 7, and 10 plants in each experiment. In the case of V. longisporum, 400 ml of spore solution was put into the tray containing the protruding roots of two-week-old plants as disease development is slower. This experiment was first done with 10 plants from each treatment, and then, was repeated with varying amounts of plants from each treatment. L. maculans was applied by puncturing two leaves on each plant twice with a pipette tip without damaging the mid-vein. Then, 10 μl spore solution was applied on each puncture. This experiment was first performed with 15 plants and then repeated twice with 10 plants from each treatment. Control plants were always treated the same way as other samples with the exception that sterile water was applied. Plants were kept in mini greenhouses or wrapped in plastic, 12 h before and 12 h after inoculation to maintain high humidity and facilitate infection.
Scoring
All plants were scored before decoding the material. A. brassicae- and B. cinerea-infected plants were divided into four groups. The groups were; uninfected, (1); <1/2 of the leaves infected, (2); >1/2 of the leaves infected, (3); and the last step, dead plants, (4). The scoring took place one week after inoculation. V. longisporum were scored after two weeks by two parameters; growth retarded compared to negative control or unaffected plants. L. maculans infection was scored after one week. Presence of necrotic lesions at the punctures was used to score plants as either infected or uninfected.
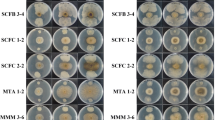
Antifungal Test
Bacillus strains, UCMB-5715T, UCMB-5033, UCMB-5036, UCMB-5113, were all grown in LB media at 28°C with agitation for 24 h when stationary growth had been achieved for all bacteria. The cultures were diluted to a concentration of 107 spores ml-1. The cultures were then sterile filtered through 0.45-μm filters to remove the bacteria.
Multiwell plates with PDA media were used. Each well was filled with 100 μl spore solution from the different fungi. Six rows encompassing four wells were filled with 200 μl of water, 190 μl water and 10 μl culture filtrate, 180 μl water and 20 μl culture filtrate, 150 μl water and 50 μl culture filtrate, 100 μl water and 100 μl culture filtrate, or 0 μl water and 200 μl culture filtrate, respectively. This was repeated with all combinations of strains and fungi. The plates were incubated in a growth chamber (16/8 h photoperiod at 21/16°C) and scored after two weeks. Relative efficiency was estimated according to the amount of culture filtrate needed to completely inhibit fungal growth. Culture filtrate pH was measured to assure that no differences in pH were responsible for the inhibition.
In Vitro Protection of Plants
Bacillus UCMB-5715T, UCMB-5033, UCMB-5036, UCMB-5113, and L. maculans spore solutions were prepared, and B. napus seeds were surface sterilized as described above. Seeds were treated with the bacterial spore solution as mentioned earlier. Multiwell plates with MS media (Duchefa, Harlem, Netherlands) were filled with two seeds in each well. To each well, except for the negative control, 20 μl of L. maculans spore solution (106 spores ml-1) was added. A positive control was also included comprising of L. maculans spores added to seeds that had been treated with water instead of bacteria. The plates were incubated in a growth chamber (16/8 h photoperiod at 21/16°C), and surviving seedlings were counted after 10 days.
Statistical Analysis
Two-sample t-tests and Andersson Darling normal variance tests were made using Minitab 14. P values were calculated and values <0.05 were used as a level for significant differences between control and Bacillus treatment and as a between-experimental repeats.
Results
The pathogen screenings revealed that treatment of oilseed rape seeds with Bacillus strains UCMB-5113 and UCMB-5036 resulted in significant protection against all fungal pathogens tested (Fig. 1). Bacillus strain UCMB-5033 showed protection against V. longisporum and efficient protection to L. maculans, but had no effect toward B. cinerea and A. brassicae. The use of B. endophyticus strain UCMB-5715T did not result in any significant protection against the pathogens except V. longisporum, but this effect was very close to being not significant. The protection seemed to be a combination of lower infection frequency and less severe disease symptoms on treated plants.
Effects of Bacillus on oilseed rape protection to different pathogens. Plants were grown on soil and challenged with different pathogens and scored as described in the Materials and Methods section using different scales. (a) Alternaria brassicae (white bars, N = 30), Botrytis cinerea (black bars, N = 25). Asterisks (*) depicts significant differences (P < 0.05) from control. Variation bars on the histograms correspond to standard deviation. (b) Leptosphaeria maculans (white bars, N = 35), Verticillium longisporum (black bars, N = 18–21). Asterisks (*) depicts significant differences (P < 0.05) from control.
To study direct effects of bacteria toward fungi, the potential production of antifungal substances in the growth medium of the Bacillus strains were tested on fungal growth in vitro. The effect of addition of growth medium was more of an all or nothing reaction (Table 1). Either the fungi and the control grew, or otherwise, the wells showed no growth at all even after three weeks. This would suggest that the antifungal substances present in the growth medium either kills or prevents germination of the fungal spores in a concentration-dependent matter. UCMB-5036 was the only strain that produced antifungal substances that showed a strong growth retardation effect against all fungi tested. UCMB-5033 and UCMB-5113 media showed effects that varied between no visible effects to a strong effect on pathogen growth. UCMB-5715T medium was only effective against A. brassicae. None of the bacterial strains affected the pH of their growth media.
Phenological analysis of plants was undertaken to observe any fitness effects due to Bacillus inoculation. The fitness study showed a significantly higher survival rate for the seeds treated with UCMB-5033, UCMB-5036, and UCMB-5113 providing a growth promoting effect (Table 2). Otherwise, there were no significant differences between treatments with the exception of the UCMB-5715T strain that showed deleterious or growth-retarded symptoms on oilseed rape plants (Table 2). No visible signs of infection such as chlorosis or yellowing were observed on plants that did germinate after bacterial treatment. The germination efficiency was the same as the seeds harvested from the different plants including control plants.
Effects of Bacillus strains to L. maculans inoculation on oilseed rape seeds were also tested on in vitro cultivated plants as this could provide a more convenient high-throughput screening. Treatment with all strains except for UCMB-5715T gave significantly higher number of surviving oil seed rape plants (Fig. 2) similar to the soil experiments. However, UCMB-5113 had stronger disease suppression with plants on soil compared with agar grown seedlings (Fig. 2).
Effects of Bacillus on oilseed rape seeds infected by Leptosphaeria maculans. Oilseed rape seeds were inoculated with Bacillus spores, transferred to PDA agar and challenged with Leptosphaeria maculans spores. Survival was scored after 10 days. Asterisks (*) depicts significant differences (P < 0.05) from control (N = 35).
Discussion
Biocontrol is an interesting durable and potential environmental friendly alternative for plant protection compared with chemical control and its many negative effects on the environment. A number of Bacillus strains representing different genotypes were tested for the first time on oilseed rape for their potential usefulness for disease protection and also to evaluate fitness effects on plants. Several fungal pathogens constituting a serious problem to Brassica cultivation were tested in this report. A. brassicae, B. cinerea, L. maculans, and V. longisporum all constitute severe problems to Brassicas but have widely different infection mechanisms, which provide a good opportunity to also study the mechanism for Bacillus control, if present.
Bacillus strains UCMB-5036 and UCMB-5113 were found to confer protection to oilseed rape against all pathogens tested in this study. Protection was confirmed in spite of the very different infection strategies used by the different pathogens. V. longisporum is a soil-borne pathogen that infects roots whereas the other pathogens tested are foliar pathogens [4, 9, 11, 26]. It is tempting to speculate that the difference in protection observed between the Bacillus strains is dependent on the fungal lifestyles. UCMB-5033 only provides significant protection against V. longisporum and the hemibiotroph, L. maculans, but does not provide protection toward the necrotrophic fungi, B. cinerea and A. brassicae. The UCMB-5715T strain did not confer protection to any of the pathogens tested on oilseed rape.
The production of antifungal substances gave a complex answer, indicating production of structurally different or different amounts of antifungal compounds among the Bacillus strains (Table 1). Fungicides produced by the bacteria does not seem to strictly correlate with the resistance as all strains showed antifungal production but no protection, or protection but no antifungal production, except for UCMB-5036 that had antibiotic effect against all fungi tested. It has earlier been shown that the correlation of antiobiotic production on nutrient rich medium as LB media and other medias differ significantly, and this might also be a reason why no correlation is seen in this study [31]. Production of antifungals is probably one of many ways these strains protect the plant, but not the only explanation. Another possibility is inducible defense of plants where IR dependent on jasmonate and phytoalexins has been shown to be effective against pathogens in Arabidopsis [27]. The discrepancies between protection in planta and in vitro suggest that the protection observed here could at least, in part, be dependent on IR, which will be the subject of future research.
The agar-screening assay using plant seeds confirmed the protection mediated by UCMB-5033, 5036, and 5113 to L. maculans using pure isolates in vitro, but if induction of production of antifungal compounds were responsible for the effect in planta, it could not be evaluated from our investigation. Moreover, biocontrol capacity of the strains relays on their ability to survive on roots and colonize plants. UCMB-5033, 5036, and 5113 treated seeds all showed significantly higher survival rate than control in the greenhouse experiments. This could also be an indicator that seed treatment with these bacteria makes the plants more tolerant. The exception was strain UCMB-5715T that clearly delayed plant development.
Despite the close phylogenetic relationship of the studied B. amyloliquefaciens strains, the antifungal effect pattern differs between all the strains and the protective capability, which indicates that the interactions between plants and bacteria seem to be strain and species specific. The finding that B. endophyticus strain UCMB-5715T delayed development to B. napus is interesting especially as this bacterium was isolated from the vascular tissue of cotton plants. This also indicates that the colonization of endophytes is very species specific, as shown previously for other systems [7, 25] and that results for potential biocontrol strains not can be generalized but need to be tested in each context. Differences between colonization patterns by endophytes can be seen even on cultivar level as demonstrated for oilseed rape [9]. B. amyloliquefaciens strains, UCMB-5036 and UCMB-5113, showed a broad protective capability on B. napus against fungi that has a necrotrophic or biotrophic lifestyle, and are foliar or soil spread pathogens. This gives these two bacteria a good potential as future biocontrol agents, although further studies are needed of various properties before field experiments can be performed.
References
Asaka, O, Shoda, M (1996) Biocontrol of Rhizoctonia solani damping-off of tomato with Bacillus subtilis rb14. Appl Environ Microbiol 62: 4081–4085
Bais, HP, Fall, R, Vivanco, JM (2004) Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 134: 307–319
Benhamou, N, Kloepper, JW, Quadt-Hallman, A, Tuzun, S (1996) Induction of defense-related ultrastructural modifications in pea root tissues inoculated with endophytic bacteria. Plant Physiol 112: 919–929
Bohman, S, Staal, J, Thomma, BPHJ, Wang, M, Dixelius, C (2004) Characterisation of an Arabidopsis–Leptosphaeria maculans pathosystem: resistance partially requires camalexin biosynthesis and is independent of salicylic acid, ethylene and jasmonic acid signalling. Plant J 37: 9–20
Bostock, RM (2005) Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu Rev Phytopathol 43: 545–580
Denison, RF, Kiers, ET (2004) Lifestyle alternatives for rhizobia: mutualism, parasitism, and forgoing symbiosis. FEMS Microbiol Lett 237: 187–193
Dunn, AK, Klimowicz, AK, Handelsman, J (2003) Use of a promoter trap to identify Bacillus cereus genes regulated by tomato seed exudate and a Rhizosphere resident, Pseudomonas aureofaciens. Appl Environ Microbiol 69: 1197–1205
Garbeva, P, van Veen, JA, van Elsas, JD (2004) Microbial diversity in soil: selection microbial populations by plant and soil type and implications for disease suppressiveness. Annu Rev Phytopathol 42: 243–270
Granér, G, Persson, P, Meijer, J, Alstrom, S (2003) A study on microbial diversity in different cultivars of Brassica napus in relation to its wilt pathogen, Verticillium longisporum. FEMS Microbiol Lett 224: 269–276
Handelsman, J, Stabb, EV (1996) Biocontrol of soilborne plant pathogens. Plant Cell 8: 1855–1869
Howlett, BJ, Idnurm, A, Pedras, MSC (2001) Leptosphaeria maculans, the causal agent of blackleg disease of Brassicas. Fungal Genet Biol 33: 1–14
Iavicoli, A, Boutet, E, Buchala, A, Metraux, J-P (2003) Induced systemic resistance in Arabidopsis thaliana in response to root inoculation with Pseudomonas fluorescens CHA0. Mol Plant Microbe Interact 16: 851–858
Idriss, EE, Makarewicz, O, Farouk, A, Rosner, K, Greiner, R, Bochow, H, Richter, T, Borriss, R (2002) Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effect. Microbiol 148: 2097–2109
Leifert, C, Li, H, Chidburee, S, Hampson, S, Workman, S, Sigee, D, Epton, HAS, Harbour, A (1995) Antibiotic production and biocontrol activity by Bacillus subtilis CL27 and Bacillus pumilus CL45. J Appl Bacteriol 78: 97–108
Lindow, SE, Brandl, MT (2003) Microbiology of the Phyllosphere. Appl Environ Microbiol 69: 1875–1883
Lucy, M, Reed, E, Glick, BR (2004) Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 86: 1–25
Marcroft, SJ, Sprague, SJ, Salisbury, PA, Howlett, BJ (2004) Potential for using host resistance to reduce production of pseudothecia and ascospores of Leptosphaeria maculans, the blackleg pathogen of Brassica napus. Plant Pathol 53: 468–474
Nejad, P, Johnson, PA (2000) Endophytic bacteria induce growth promotion and wilt disease suppression in oilseed rape and tomato. Biol Control 18: 208–215
Ongena, M, Duby, F, Jourdan, E, Beaudry, T, Jadin, V, Dommes, J, Thonart, P (2005) Bacillus subtilis M4 decreases plant susceptibility towards fungal pathogens by increasing host resistance associated with differential gene expression. Appl Microbiol Biotechnol 67: 692–698
Reva, ON, Dixelius, C, Meijer, J, Priest, FG (2004) Taxonomic characterization and plant colonizing abilities of some bacteria related to Bacillus amyloliquefaciens and Bacillus subtilis. FEMS Microbiol Ecol 48: 249–259
Reva, ON, Smirnov, VV, Pettersson, B, Priest, FG (2002) Bacillus endophyticus sp. nov., isolated from the inner tissues of cotton plants (Gossypium sp.). Int J Syst Evol Microbiol 52: 101–107
Risøen, PA, Rønning, P, Hegna, IK, Kolstø, A-B (2004) Characterization of a broad range antimicrobial substance from Bacillus cereus. J Appl Microbiol 96: 648–655
Ryu, C-M, Farag, MA, Hu, C-H, Reddy, MS, Wei, H-X, Pare, PW, Kloepper, JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100: 4927–4932
Ryu, C-M, Murphy, JF, Mysore, KS, Kloepper, JW (2004) Plant growth-promoting rhizobacteria systemically protect Arabidopsis thaliana against Cucumber mosaic virus by a salicylic acid and NPR1-independent and jasmonic acid-dependent signaling pathway. Plant J 39: 381–392
Smith, KP, Handelsman, J, Goodman, RM (1999) Genetic basis in plants for interactions with disease-suppressive bacteria. Proc Natl Acad Sci USA 96: 4786–4790
Thomma, BPHJ (2003) Alternaria spp.: from general saprophyte to specific parasite. Mol Plant Pathol 4: 225–236
Ton, J, De Vos, M, Robben, C, Buchala, A, Metraux, J-P, Van Loon, LC, Pieterse, CMJ (2002) Characterization of Arabidopsis enhanced disease susceptibility mutants that are affected in systemically induced resistance. Plant J 29: 11–21
van Loon, LC, Bakker, PAHM, Pieterse, CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36: 453–483
Walker, R, Powell, AA, Seddon, B (1998) Bacillus isolates from the spermosphere of peas and dwarf French beans with antifungal activity against Botrytis cinerea and Pythium species. J Appl Microbiol 84: 791–801
Walker, TS, Bais, HP, Deziel, E, Schweizer, HP, Rahme, LG, Fall, R, Vivanco, JM (2004) Pseudomonas aeruginosa—plant root interactions. pathogenicity, biofilm formation, and root exudation. Plant Physiol 134: 320–331
Whipps, JM (1987) Effect of media on growth and interactions between a range of soilborne glasshouse pathogens and antagonistic fungi. New Phytol 107: 127–142
Wulff, EG, Mguni, CM, Mansfeld-Giese, K, Fels, J, Lubeck, M, Hockenhull, J (2002) Biochemical and molecular characterization of Bacillus amyloliquefaciens, B. subtilisand B. pumilus isolates with distinct antagonistic potential against Xanthomonas campestris pv. campestris. Plant Pathol 51: 574–584
Wulff, EG, Mguni, CM, Mortensen, CN, Keswani, CL, Hockenhull, J (2002) Biological control of black rot (Xanthomonas campestris pv. campestris) of Brassicas with an antagonistic strain of Bacillus subtilis in Zimbabwe. Eur J Plant Pathol 108: 317–325
Yu, GY, Sinclair, JB, Hartman, GL, Bertagnolli, BL (2002) Production of iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem 34: 955–963
Zehnder, G, Kloepper, J, Tuzun, S, Yao, C, Wei, G, Chambliss, O, Shelby, R (1997) Insect feeding on cucumber mediated by rhizobacteria-induced plant resistance. Entomol Exp Appl 83: 81–85
Zehnder, GW, Yao, C, Murphy, JF, Sikora, ER, Kloepper, JW (2000) Induction of resistance in tomato against cucumber mosaic cucumovirus by plant growth-promoting rhizobacteria. BioControl 45: 127–137
Acknowledgements
This study was supported by grants from SLU-IMOP, Helge Ax:son Johnsons Stiftelse and Stiftelsen Oscar och Lili Lamms minne. We are grateful to Prof. Christina Dixelius and Gunilla Swärdh for provision of fungal strains and advice on cultivation and to Prof. Christina Dixelius for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Danielsson, J., Reva, O. & Meijer, J. Protection of Oilseed Rape (Brassica napus) Toward Fungal Pathogens by Strains of Plant-associated Bacillus amyloliquefaciens . Microb Ecol 54, 134–140 (2007). https://doi.org/10.1007/s00248-006-9181-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-006-9181-2