Abstract
Background
Juvenile idiopathic inflammatory myopathy is a rare yet potentially debilitating condition. MRI is used both for diagnosis and to assess response to treatment. No study has evaluated the performance of US elastography in the diagnosis of this condition in children.
Objective
To assess the performance of compression–strain US elastography in detecting active myositis in children with clinically confirmed juvenile idiopathic inflammatory myopathy and to compare its efficacy to MRI.
Materials and methods
Children with juvenile idiopathic inflammatory myopathy underwent non-contrast MR imaging as well as compression–strain US elastography of the quadriceps muscles. Imaging findings from both modalities were compared to each other as well as to the clinical determination of active disease based on physical examination and laboratory data. Active myositis on MR was defined as increased muscle signal on T2-weighted images. Elastography images were defined as normal or abnormal based on a previously published numerical scale of muscle elastography in normal children. Muscle echogenicity was graded as normal or abnormal based on gray-scale sonographic images.
Results
Twenty-one studies were conducted in 18 pediatric patients (15 female, 3 male; age range 3–19 years). Active myositis was present on MRI in ten cases. There was a significant association between abnormal MRI and clinically active disease (P = 0.012). US elastography was abnormal in 4 of 10 cases with abnormal MRI and in 4 of 11 cases with normal MRI. There was no association between abnormal elastography and either MRI (P > 0.999) or clinically active disease (P > 0.999). Muscle echogenicity was normal in 11 patients; all 11 had normal elastography. Of the ten patients with increased muscle echogenicity, eight had abnormal elastography. There was a significant association between muscle echogenicity and US elastography (P < 0.001). The positive and negative predictive values for elastography in the determination of active myositis were 75% and 31%, respectively, with a sensitivity of 40% and specificity of 67%.
Conclusion
Compression–strain US elastography does not accurately detect active myositis in children with juvenile idiopathic inflammatory myopathy and cannot replace MRI as the imaging standard for detecting myositis in these children. The association between abnormal US elastography and increased muscle echogenicity suggests that elastography is capable of detecting muscle derangement in patients with myositis; however further studies are required to determine the clinical significance of these findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Juvenile idiopathic inflammatory myopathies (JIIM) represent a heterogeneous group of systemic connective tissue diseases characterized by chronic muscle inflammation. Juvenile dermatomyositis (JDM), the most common cause of JIIM, represents approximately 85% of cases and has an incidence of 3 per million children per year [1]. JDM is more common in girls, has a median age of onset of 7.5 years and is characterized by a heliotrope rash and Gottron papules. Its prognosis is variable, with some patients making a complete recovery with appropriate therapy; however most patients have waxing and waning chronic disease. Mortality is low for JDM (2–3%). Less common causes of JIIM in children include myositis overlapping with another autoimmune disease (“overlap myositis”), such as systemic lupus erythematosus or scleroderma (6–12%), and polymyositis (4–8%). Unlike JDM, polymyositis is characterized by the absence of a rash. Both polymyositis and overlap myositis have higher mortality rates than JDM [2]. Prompt diagnosis and treatment of JIIM is crucial.
The diagnosis of JDM is based on the modified criteria of Bohan and Peter [3–5]. Although these criteria do not include imaging studies, MRI has been validated as an accurate indicator of muscle edema and active disease [6, 7] and is typically incorporated into the diagnostic workup of children with suspected myositis. MRI has also proved useful in assessing disease activity and therapeutic response in children with an established diagnosis of JIIM. However, limitations of MRI, including high cost, long study time, and potential need for patient sedation, have raised interest in the use of other imaging modalities for the diagnosis and follow-up of children with JIIM.
US strain elastography evaluates tissue deformation or compressibility from an external force [8, 9]. It has been used extensively in assessing tendon pathology, based on the detection of alteration in the normal US elastography appearance of the tendon [10–14]. Recent publications describe the use of US elastography in assessing muscles of healthy children [15] and in children with spastic cerebral palsy [16, 17]. However the potential benefits of US elastography in children with active myositis have not been evaluated. Myositis affects the microvascular endothelium of muscle, resulting in swelling of vascular endothelium, obliteration of vessel lumena, perivascular inflammation, and degeneration of muscle fibers, all of which distort normal muscle architecture [18]. We postulated that the alteration in normal muscle architecture would lead to an alteration of muscle elasticity, which would be detectable by US elastography.
We therefore undertook a prospective study to determine the utility of US elastography in detecting active myositis in children with clinically confirmed JIIM and to compare its efficacy with MRI.
Materials and methods
Study population
Between June 2012 and January 2014, children, adolescents and young adults (ages 2–21 years) with a diagnosis of probable or definite juvenile dermatomyositis (JDM) (as determined by Bohan and Peter [3–5] criteria), overlap myositis or polymyositis, were recruited from the pediatric rheumatology clinic and were included regardless of their level of disease activity. After receiving approval from the institutional review board, one of three board-certified pediatric rheumatologists performed a complete evaluation of each patient. Physical examination included an assessment of muscle strength using the Childhood Myositis Assessment Scale (CMAS) [19], and measurement of serum levels of creatine phosphokinase, lactate dehydrogenase, and alanine aspartate.
Clinical assessment of disease activity
The Pediatric Rheumatology International Trials Organization (PRINTO), an internationally recognized research network, has established criteria for determining achievement of inactive disease state in children with juvenile idiopathic inflammatory myopathies (JIIM) [20]. For the purpose of this study, we based clinical disease activity on the modified PRINTO criteria. Patients were considered clinically inactive if they had achieved all of the following: (1) normalization of all muscle enzymes, (2) normal muscle strength (determined by CMAS ≥48) and (3) physician global assessment of disease activity ≤0.2 (on a 0.0–10.0-cm visual analog scale) [21, 22]. Patients who did not meet these criteria were determined to have ongoing clinically active disease. Imaging findings did not contribute to determination of clinical disease activity. Patients with systemic lupus erythematosus underwent additional laboratory studies, including C3, C4 and anti-double-stranded deoxyribonucleic acid (DNA) antibodies. We also collected information regarding disease duration.
Imaging assessment
All subjects underwent MR imaging of the pelvis and upper thighs within 2 weeks of their clinical evaluation. Imaging was performed using 3-T units (Philips Healthcare, Andover, MA) or 1.5-T units (GE Healthcare, Waukesha, WI) with a long torso coil. Axial T1-weighted, axial fat-saturated T2-weighted, and coronal short tau inversion recovery (STIR) sequences were obtained.
Immediately following MRI, patients underwent compression–strain US elastography of the quadriceps muscles. All studies were performed by the same sonographer, with 3 years of US elastography experience. Imaging was performed using an iU22 machine equipped with compression–strain elastography (Philips Healthcare, Bothell, WA). The proximal third of the quadriceps muscle was scanned in the long axis using an L12-5 linear-array transducer with the transducer positioned anteriorly over the thigh along the plane of a line between the patella and hip. Patients were scanned in the supine position with the legs in extension. Manual compression of the tissues using the US transducer was performed to obtain the elastography image. A real-time compression feedback bar on the US display ensured that adequate manual compression was applied. A color map representing tissue elasticity was superimposed on the gray-scale sonographic image, with red indicating the most elastic tissues, green indicating tissues with intermediate elasticity, and blue indicating the least elastic tissues.
Imaging assessment of disease activity
MRI and US elastography images were evaluated by two radiologists in consensus. Radiologists were blinded to the results of the other imaging modality as well as to clinical disease activity. MRI was defined as positive for active myositis if edema was present in the quadriceps muscles on fluid-sensitive sequences, while the absence of quadriceps muscle edema on MRI was defined as inactive myositis. The presence of muscle atrophy was evaluated and defined as fatty infiltration of muscle on the T1-weighted sequence.
The echogenicity of the interrogated quadriceps muscle was assessed on gray-scale sonographic images. Muscle echogenicity was considered normal if it was less than or equal to the echogenicity of the subcutaneous fat; it was considered abnormal if the muscle echogenicity was greater than that of the subcutaneous fat.
To evaluate quadriceps muscle elasticity, the color pattern of the quadriceps muscles was assessed. Elasticity was assessed based on a comparison with previously published findings of muscle elasticity in a cohort of normal children of similar age and gender [15]. Muscle elasticity was defined as normal if the dominant color in the muscle was green, with small amounts of either red or blue interspersed within the muscle. The presence of greater than 50% red or blue in the muscle was defined as abnormal.
Statistics
Statistical analysis was conducted using STATA software, version 11.2 (College Station, TX). Descriptive statistics were computed to summarize each variable, using mean and standard deviations for normally distributed continuous variables, median and interquartile ranges (IQR) for non-normally distributed continuous variables, and frequencies for categorical variables. All tests were two-sided and P-values less than 0.05 were considered statistically significant. Association with imaging modalities was assessed by bivariate analyses using Pearson chi-square for categorical variables and Student’s t-test for continuous variables. In cases of non-normally distributed data, Fisher exact and Wilcoxon rank sum tests were performed. We calculated the sensitivity, specificity and positive and negative predictive values for assessment of active disease by MRI and US elastography using physician global assessment of disease activity as the gold standard.
Results
Patient characteristics (Table 1)
Eighteen subjects with JIIM (15 female and 3 male, age range 3–19 years) were recruited, including 15 with JDM, 2 with systemic lupus erythematosus overlap myositis and 1 with polymyositis.
Comparison of imaging modalities with clinical disease activity (Table 2)
Twenty-one studies were performed in 18 patients (three patients had two sets of imaging studies over the course of the study period because of disease flare). Clinically active disease was present in 15 studies (71%) at the time of imaging.
Active myositis was present on MRI in 10 cases, while normal muscle signal on MRI was present in 11 cases. No cases demonstrated muscle atrophy. There was a significant association between abnormal MRI and physician global assessment of disease activity (P = 0.012), with a trend toward a significant association with CMAS score (P = 0.066). Although patients with abnormal MRI findings appeared to have a shorter disease duration (21 months; IQR 6, 50) compared to those with normal MRI findings (38 months; IQR 11, 48), there was no statistically significant association between MRI findings and disease duration (P = 0.724).
Muscle echogenicity on gray-scale US was normal in 11 patients and abnormally echogenic in ten patients. There was no significant association between muscle echogenicity and physician global assessment of disease activity (P = 0.635) or disease duration (P = 0.438).
US elastography did not correlate with either clinical disease activity as determined by physician global assessment of disease activity (P > 0.999) or CMAS score (P = 0.686). Although abnormal appearance on US elastography was more common in children with longer disease duration (31 months, [IQR 6, 49], compared to 20 months [IQR 12, 48] for patients with normal US elastography appearance) this was also not statistically significant (P = 0.942).
Association between magnetic resonance imaging and ultrasound elastography (Table 3)
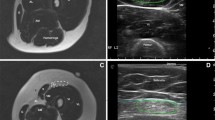
Using US elastography, decreased quadriceps muscle elasticity was present in 4 of the 10 (40%) cases in which muscle edema was present on MRI (Fig. 1), and in 4 of the 11 (36.4%) cases in which muscle signal intensity was normal on MRI (Fig. 2). In the remainder of cases, US elastography was normal (Fig. 3). No patient demonstrated increased muscle elasticity. There was no association between abnormal appearance on US elastography and muscle edema detected on MRI (P > 0.999).
Abnormal US elastography, abnormal MRI. Imaging in a 7-year-old girl with clinically active juvenile dermatomyositis. a Coronal short tau inversion recovery (STIR) MR image (echo time [TE] 33 ms, repetition time [TR] 2,750 ms) demonstrates patchy areas of muscle edema consistent with myositis. b Longitudinal gray-scale US image of the left quadriceps muscle (left) with superimposed color elastogram (right) demonstrates predominantly blue color in the muscle, consistent with abnormal elastography appearance. Note the feedback compression bar on the bottom right, with the presence of green in this bar indicating that adequate compression has been applied
Abnormal US elastography, normal MRI. Imaging in an 11-year-old girl with clinically inactive juvenile dermatomyositis. a Coronal STIR image (TR/TE 2,017/46 ms) demonstrates no muscle edema. b Longitudinal gray-scale US image of the left quadriceps muscle (left) with superimposed color elastogram (right) demonstrates predominantly blue color in the muscle, consistent with abnormal elastography appearance. STIR short tau inversion recovery, TE echo time, TR repetition time
Normal elastography, abnormal MRI. Imaging in an 11-year-old girl with clinically active juvenile dermatomyositis. a Coronal STIR image (TR/TE 3,367/45 ms) demonstrates patchy areas of muscle edema consistent with myositis. b Longitudinal gray-scale US image of the left quadriceps muscle (left) with superimposed color elastogram (right) demonstrates predominantly green color in the muscle, with scattered areas of red and blue, consistent with the appearance of normal muscle. STIR short tau inversion recovery, TE echo time, TR repetition time
Association between muscle echogenicity and ultrasound elastography
All 11 patients with normal muscle echogenicity had normal US elastography of the muscle, while eight of the ten patients with increased muscle echogenicity had abnormal US elastography appearance of the muscle. There was a highly significant association between muscle echogenicity and US elastography findings (P < 0.001).
Ultrasound elastography sensitivity and specificity
Using physician global assessment of disease activity as the gold standard for the assessment of active myositis, US elastography had a sensitivity and specificity of 40% and 67%, respectively. In contrast, MRI had improved sensitivity of 67% and specificity of 100%. The positive and negative predictive values for US elastography in the detection of myositis were 75% and 31%, respectively. When using MRI, positive and negative predictive values were 100% and 55%, respectively.
Discussion
Juvenile idiopathic inflammatory myopathy (JIIM) is a potentially debilitating and progressive condition that typically presents during the first decade of life. Since the 1970s the introduction of corticosteroids as the mainstay of treatment has led to significant improvement in functional outcomes and mortality. Despite the vastly improved prognosis, complications can be severe, and adequate assessment of disease activity is crucial. MRI has become an integral component in the diagnosis and monitoring of disease status in children with inflammatory myositis and provides a noninvasive method to assess muscle edema (active disease) [6, 7, 23, 24]. However, young children often have difficulty tolerating the exam without sedation, which has generated interest in the validation of other imaging techniques for the diagnosis and long-term assessment of myositis.
US elastography has proved useful in the evaluation of a variety of musculoskeletal conditions, ranging from tendon pathology [10–14] to muscle spasticity in children [16, 17]. It combines the portability, low cost and short study time of sonography with the ability to assess tissue elasticity. Validation of this technique for the assessment of myositis in children could allow this imaging modality to replace or at least complement MRI in the diagnosis and follow-up of this condition.
One study has evaluated the efficacy of US elastography in detecting active myositis. In a study of 24 adults with polymyositis and dermatomyositis and active myositis (age range 24–67 years, mean 54.8 years), Botar-Jid et al. [25] reported decreased US elasticity of the muscles of the arms and legs in a majority of their subjects. In contrast, our study in children demonstrates that US elastography performs poorly when used to assess for active myositis in this population. Although adult and juvenile dermatomyositis share certain features, such as skin rash and muscle inflammation, there are significant differences in the clinical manifestation of these diseases between these populations, suggesting that they may represent distinct entities [26]. This may explain our differing results from those obtained by Botar-Jid et al. [25]. Additionally, the longer disease duration in most adults with dermatomyositis may contribute to the divergent findings.
Subjects with active myositis detected on MR imaging exhibited both normal muscle elasticity as well as decreased muscle elasticity on US elastography, while subjects with normal MR imaging and no evidence of active myositis also demonstrated both normal and decreased muscle elasticity. US elastography demonstrated poor sensitivity and specificity in diagnosing active myositis when compared with physician assessment of disease activity, as well as poor positive and negative predictive values for the detection of active disease. Abnormal MRI findings were significantly associated with overall clinically active disease in this study, as shown in prior reports demonstrating the utility of MRI in the evaluation of JIIM [6, 7, 23, 24]; however this was not true of US elastography.
Although one might expect a change in normal elasticity of the muscle in the presence of myositis, our data suggest otherwise. The pathological changes that occur in myositis on the microscopic level — including swelling of vascular endothelium, obliteration of vessel lumena, perivascular inflammation, and degeneration of muscle fibers [18] — did not result in an observable difference in muscle elasticity as determined by US elastography. Technical or mechanical factors in current US elastography systems may limit the sensitivity of this modality in the detection of small changes in muscle elasticity. Additionally, unlike MRI, US elastography can only interrogate a relatively small area. This may also limit the sensitivity of US elastography because affected areas of muscle inflammation may not be uniformly distributed in the muscle. The areas of signal abnormality seen on MRI in patients with active disease correspond to areas of muscle edema. Thus, abnormalities are best detected on fluid-sensitive sequences. US elastography may not be able to detect small changes in fluid related to muscle inflammation. It is still unclear whether US elastography might help in the detection of changes in muscle architecture and content that occur with long-standing disease.
Increased muscle echogenicity is often seen in diseased muscle and may reflect muscle edema in the acute phase of disease [27] and fibrosis [28] or fatty replacement in chronic disease [29]. We found no association between abnormal muscle echogenicity and disease duration in our study. However, our study does demonstrate a significant association between abnormal muscle echogenicity and abnormal muscle US elastography. This suggests that US elastography may be useful in the detection of derangement of muscle structure in patients with myositis. Further work correlating US elastography and gray-scale sonography with muscle biopsy results might elucidate the significance of these findings.
Limitations of this study include a small sample size; however, given the rarity of JIIM the inclusion of 18 patients in a short study period is notable. Additionally, although uncommon in current practices, the results from this study would have been enhanced by comparison with muscle biopsy specimens. There are also a number of limitations associated with US elastography. These include lack of standardization of the degree of manual compression applied to produce the elastography image, which leads to an inherent limitation in the reproducibility of compression–strain US elastography. Additionally, US elastography was performed in one anatomical location, and therefore could only assess disease activity in a small subset of the muscle. However, patients with JDM often have heterogeneous areas of affected muscle, and therefore it is possible that the area of muscle interrogated on the US elastography studies was not an area of active disease. Similarly, although the proximal third of the quadriceps muscle was interrogated, standardization of the exact imaging location among patients was not possible. Finally, the qualitative assessment of the US elastography images includes some subjectivity in interpretation.
Although we found that compression–strain elastography does not accurately detect active myositis in children, it is possible that other elastography techniques, including shear-wave elastography and MR elastography, may be useful in the assessment of this condition. Of note, a single study of nine patients with idiopathic inflammatory myopathy demonstrated a role for MR elastography in the detection of active myositis [30]. Future studies utilizing shear-wave elastography and MR elastography could demonstrate a role for elastography in the assessment of active myositis in children with JIIM.
Conclusion
Compression–strain US elastography performs poorly in detecting active myositis. Although it is a promising technique, at present US elastography cannot replace MRI as the imaging standard for detecting myositis in children with juvenile idiopathic inflammatory myopathy. The association between abnormal US elastography and increased muscle echogenicity suggests that elastography can be used in the detection of derangement of muscles in patients with JIIM; however further studies are required to determine the clinical significance of these findings.
References
Robinson AB, Reed AM (2011) Juvenile dermatomyositis. In: Kliegman RM, Stanton B, St. Geme J et al (eds) Nelson textbook of pediatrics, 19th edn. Saunders, Philadelphia, pp 846–850
Rider LG, Katz JD, Jones OY (2013) Developments in the classification and treatment of the juvenile idiopathic inflammatory myopathies. Rheum Dis Clin North Am 39:877–904
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292:344–347
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (second of two parts). N Engl J Med 292:403–407
Brown VE, Pilkington CA, Feldman BM et al (2006) An international consensus survey of the diagnostic criteria for juvenile dermatomyositis (JDM). Rheumatology 45:990–993
Hernandez RJ, Keim DR, Sullivan DB et al (1990) Magnetic resonance imaging appearance of the muscles in childhood dermatomyositis. J Pediatr 117:546–550
Hernandez RJ, Sullivan DB, Chenevert TL et al (1993) MR imaging in children with dermatomyositis: musculoskeletal findings and correlation with clinical and laboratory findings. AJR Am J Roentgenol 161:359–366
Garra BS (2007) Imaging and estimation of tissue elasticity by ultrasound. Ultrasound Q 23:255–268
Klauser AS, Peetrons P (2010) Developments in musculoskeletal ultrasound and clinical applications. Skeletal Radiol 39:1061–1071
De Zordo T, Chhem R, Smekal V et al (2010) Real-time sonoelastography: findings in patients with symptomatic Achilles tendons and comparison to healthy volunteers. Ultraschall Med 31:394–400
Tan S, Kudaş S, Özcan AS et al (2012) Real-time sonoelastography of the Achilles tendon: pattern description in healthy subjects and patients with surgically repaired complete ruptures. Skeletal Radiol 41:1067–1072
Sconfienza LM, Silvestri E, Cimmino MA (2010) Sonoelastography in the evaluation of painful Achilles tendon in amateur athletes. Clin Exp Rheumatol 28:373–378
De Zordo T, Fink C, Feuchtner GM et al (2009) Real-time sonoelastography findings in healthy Achilles tendons. AJR Am J Roentgenol 193:W134–138
De Zordo T, Lill SR, Fink C et al (2009) Real-time sonoelastography of lateral epicondylitis: comparison of findings between patients and healthy volunteers. AJR Am J Roentgenol 193:180–185
Berko NS, Fitzgerald EF, Amaral TD et al (2014) Ultrasound elastography in children: establishing the normal range of muscle elasticity. Pediatr Radiol 44:158–163
Kwon DR, Park GY, Lee SU et al (2012) Spastic cerebral palsy in children: dynamic sonoelastographic findings of medial gastrocnemius. Radiology 263:794–801
Vasilescu D, Vasilescu D, Dudea S et al (2010) Sonoelastography contribution in cerebral palsy spasticity treatment assessment, preliminary report: a systematic review of the literature apropos of seven patients. Med Ultrason 12:306–310
Wedderburn LR, Varsani H, Li CK et al (2007) International consensus on a proposed score system for muscle biopsy evaluation in patients with juvenile dermatomyositis: a tool for potential use in clinical trials. Arthritis Rheum 57:1192–1201
Lovell DJ, Lindsley CB, Rennebohm RM et al (1999) Development of validated disease activity and damage indices for the juvenile idiopathic inflammatory myopathies. II. The childhood myositis assessment scale (CMAS): a quantitative tool for the evaluation of muscle function. The juvenile dermatomyositis disease activity collaborative study group. Arthritis Rheum 42:2213–2219
Lazarevic D, Pistorio A, Palmisani E et al (2013) The PRINTO criteria for clinically inactive disease in juvenile dermatomyositis. Ann Rheum Dis 72:686–693
Felson DT, Anderson JJ, Boers M et al (1995) American college of rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 38:727–735
Rider LG, Feldman BM, Perez MD et al (1997) Development of validated disease activity and damage indices for the juvenile idiopathic inflammatory myopathies: I. Physician, parent, and patient global assessments. Juvenile dermatomyositis disease activity collaborative study group. Arthritis Rheum 40:1976–1983
Tomasová Studynková J, Charvát F, Jarosová K et al (2007) The role of MRI in the assessment of polymyositis and dermatomyositis. Rheumatology 46:1174–1179
Fraser DD, Frank JA, Dalakas M et al (1991) Magnetic resonance imaging in the idiopathic inflammatory myopathies. J Rheumatol 18:1693–1700
Botar-Jid C, Damian L, Dudea SM et al (2010) The contribution of ultrasonography and sonoelastography in assessment of myositis. Med Ultrason 12:120–126
Tansley SL, McHugh NJ, Wedderburn LR (2013) Adult and juvenile dermatomyositis: are the distinct clinical features explained by our current understanding of serological subgroups and pathogenic mechanisms? Arthritis Res Ther 8:211
Zamorani MP, Valle M (2007) Muscle and tendon. In: Bianchi S, Martinoli C (eds) Ultrasound of the musculoskeletal system. Springer, Berlin Heidelberg, pp 45–96
Hu CF, Chen CP, Tsai WC et al (2012) Quantification of skeletal muscle fibrosis at different healing stages using sonography: a morphologic and histologic study in an animal model. J Ultrasound Med 31:43–48
Reimers K, Reimers CD, Wagner S et al (1993) Skeletal muscle sonography: a correlative study of echogenicity and morphology. J Ultrasound Med 12:73–77
McCullough MB, Domire ZJ, Reed AM et al (2011) Evaluation of muscles affected by myositis using magnetic resonance elastography. Muscle Nerve 43:585–590
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berko, N.S., Hay, A., Sterba, Y. et al. Efficacy of ultrasound elastography in detecting active myositis in children: can it replace MRI?. Pediatr Radiol 45, 1522–1528 (2015). https://doi.org/10.1007/s00247-015-3350-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-015-3350-8