Abstract
Objective
Ultrasound (US) technologies are rapidly advancing, offering several refined transducer technologies as well as soft and hardware facilities. The aim of this article is to outline US developments, from B-mode technologies over Doppler advances to more sophisticated technologies, and their potential clinical impact in the field of musculoskeletal (MSK) imaging.
New advances
When using B-mode ultrasound, compound imaging and beam-steering are of help to decrease anisotropy in tendons and ligaments, that are less well depicted due to their oblique course. Doppler imaging has become sensitive in the detection of flow in small vessels, which is of particular value in rheumatologic conditions, tumour and overuse assessment. The use of US microbubble contrast agents improves detection of low-volume blood flow in smaller vessels by increasing the signal-to-noise ratio and thereby facilitating detection of angiogenetic vessels in inflammatory conditions or tumours. The use of US blood pool contrast agents enables molecular imaging in real-time, and thus the diagnostic potential of US is expanded, opening up a new field of US applications. Objective quantification of altered tissue (e.g., synovial proliferation, tumours) is still demanding and might be improved by the use of three-dimensional imaging and software tools as parametric evaluation. Real-time sonoelastography (EUS) is a new development for visualization of tissue elasticity by measurement of tissue displacement in terms of tissue stiffness changes, promising new insights into tendon disorders. Image fusion is an exciting development that enables superimposition of CT/MRI data sets on real-time US scanning. This technique might be helpful in guiding injections under real-time conditions even in regions less easily accessible by US as, for instance, the axial skeleton, and can additionally provide an interesting tool for teaching MSK imaging and ways to guide interventions.
Conclusion
In summary, exciting developments are expanding the applications of US in the MSK field, offering the advantages of real-time performance, high tissue resolution and relative speed at a reasonable cost.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapid advances in US technologies in the past 10 years have contributed to tremendous improvement in image quality from this diagnostic modality [1, 2]. Refined transducer technology, broadband transducers, compound imaging, improved focusing and transducers with large and small fields of view are available for different applications and are refining the B-mode image quality, as well as information gained from Doppler imaging. Additionally, new applications such as real-time sonoelastography (EUS) and image fusion have made US a high-ranking imaging modality in the assessment of MSK disorders.
B-mode technologies
Near-field imaging by the use of higher frequencies and harmonic imaging have greatly contributed to improved joint and tendon surface visibility as compared with the visibility achieved with conventional B-mode US. Harmonic imaging has been found to be superior to conventional US for diagnosis of subscapularis tendon abnormalities [3]. When using spatial compound imaging, electronic US beam steering of a transducer array during real-time acquisition results in improved delineation of lesions, because images are generated from different view angles. This is of special value in assessing structures with specular surface echoes such as tendons, nerves and muscles, because one of the several view angles will be perpendicular to the tissue, generating a higher echo amplitude even at an insonation angle causing anisotropy on conventional B-mode US [2]. Anisotropic artefacts have long been recognised as a problem for MSK imaging since anisotropic structures such as tendons and ligaments, due to their oblique course, are less well depicted (Fig. 1a,b). To overcome anisotropy, instead of manual transducer angulation and tilting, a newer steering-based option, which can be activated by pressing a button on the keyboard, tilts and angulates the US beam in order to steer the whole B-mode image itself. This technique can also help better differentiate insertional tendinosis, where often a part of the fibres run an oblique course causing hypoechoic patterns of uncertain pathological value.
Generally, the use of higher frequencies in imaging MSK structures leads to improved resolution, which is important for the assessment of superficially located joint capsules, nerves and tendons. However, higher frequencies (up to 18 MHz) cause reduction of penetration depth, and this is a major limitation; therefore, accurate adjusted settings, precise placement of focal zones and appropriate adjustment of penetration depth are mandatory for tapping the full potential of new US technologies.
Coupling mediums greatly reduce compression on superficial structures because of their high elasticity and high water content, and for this reason are especially helpful in the assessment of superficially located regions of interest. They greatly reduce tissue, fluid and vessel compression as well when using B-mode and colour Doppler US (CDUS) and improve image resolution. If gel pads are used, settings should be accordingly checked and adjusted. Coupling mediums with 10-mm thickness are suitable when frequencies with up to 10–14 MHz are used; in the higher frequency ranges of up to 15–18 MHz, thinner coupling mediums are more effective (3–4 mm) (Fig. 2a-d).
a Gel pad coupling media (stars) with 10 mm thickness are suitable for frequencies of 10-14 MHz; when using frequencies up to 18 MHz, thinner coupling mediums are more effective. b Palmar axial scan of MCP joint in a patient with Dupuytren’s disease. Using the thicker coupling medium (stars), low vascularity in the hypoechoic subcutaneous thickening can be appreciated. c Using a thinner coupling medium (star) high intralesional vascularity can be depicted
Elastography
Elastography is based on the principle that the compression of tissue produces strain (displacement). This strain is lower in tissue that is hard, and higher in tissue that is soft. As pathological tissue often presents with the same echogenicity as surrounding tissue, recently sonoelastography (EUS) has been evaluated in tendon disorders. Differentiation of isoechoic lesions due to edema, haemorrhage, progressive tendinosis or mucoid degeneration and partial tears might be challenging when conventional B-mode US is the imaging modality employed. EUS is an imaging method giving information concerning tissue elasticity in addition to information obtained from conventional B-mode US.
Physical examination and manual palpation are a part of clinical examination for assessing pathology with changes in tissue elasticity; EUS is a new development to demonstrate tissue stiffness under real-time conditions by measurement of tissue displacement. Ophir et al. first described the principle of strain imaging (“elastography”) in 1991 and in 1999, Pesavento et al. developed a fast cross-sectional technique, based on real-time elastographical imaging with clinical practicability [4–6]. The principle of EUS is as follows: tissue compression produces displacement within the tissue, which is less in harder tissue than in softer tissue. EUS is capable of visualizing different displacements by comparing an image pair before and after compression is applied on tissue [4–6]. Displacement is calculated in real-time by a modified US scanner in order that different grades of elasticity can be displayed over the conventional ultrasound image by using different colouring, e.g., yellow to red representing softer tissue in contrast to green and blue, which is found in harder tissue. New technical developments (e.g., for calculation of axial and lateral displacement of tissue structures under compression) allow for better spatial resolution, reduction of artefacts, and increased accuracy so that EUS can be usefully integrated in routine examinations [7].
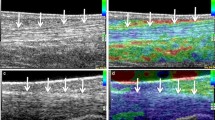
Using conventional US, it is sometimes difficult or even impossible to distinguish pathological from surrounding healthy tissue, as they often present with the same echogenicity [8]. Inflammation or tumours leading to changes in tissue elasticity have already been evaluated in breast [9, 10], thyroid [11] and prostate cancers [7] and lymph node characterization [12]. In MSK imaging, differentiation of tendon alterations might be difficult since edema, haemorrhage, mucoid degeneration or partial tears present as isoechoic alterations. Estimation of elasticity for characterization of Achilles tendons in healthy volunteers and extensor tendon insertion in patients complaining of lateral epicondylitis, has shown promising results in initial studies [13, 14].
Real-time sonoelastography was valuable in the detection of intratendinous and peritendinous alterations of lateral epicondylitis and facilitated differentiation between healthy and symptomatic extensor tendon [13]. Preliminary results revealed distinct softening of the symptomatic Achilles tendons when compared to healthy volunteers presenting with hard tendons structures [15] (Fig. 3a,b). However, this new technique has disadvantages and presents technical challenges. One major limitation is operator dependency because pressure is applied by using a free-hand technique, which might affect reproducibility. Moderating the pressure exerted with the ultrasound probe to avoid overly high and low pressures seems important. Near-proportional relationship between pressure exerted and tissue strain should be maintained; this can be monitored using the visual indicator scale on the machine, because when the pressure decreases or increases below a certain level, the pattern of the elasticity image starts to change drastically. Tissue shifting due to unilateral compression is another potential limitation, the use of a gel standoff pad can help to alleviate this problem.
In summary, the clinical value of EUS might be seen in cases where identical gray-scale values need to be better differentiated regarding tissue softening, and for delineation of the extent and degree of tendinosis, which might improve earlier detection of tendon degeneration and consequently impact on therapeutic decisions. However, these preliminary results need to be confirmed by establishing pathohistologic correlation.
Doppler technologies
Colour/power Doppler US (CDUS-PDUS)
Power Doppler imaging has improved technically leading to better sensitivity in the detection of low flow in smaller and superficially located vessels, which is of interest in rheumatologic conditions, tumour and overuse assessment. For PDUS, settings are still crucial, and the use of a low pulse repetition frequency, low wall filter, medium persistence, appropriate colour velocity scale and restricted window (color box) to the vascular area studied will help avoid artefacts such as aliasing and enable pulsed wave spectral Doppler analysis to confirm true arterial or venous flow.
In rheumatological inflammatory disease, PDUS/CDUS has been shown to be capable of detecting vascularity in synovial proliferations, to be more sensitive than clinical assessment of disease activity by improved differentiation of active from inactive joint processes and improved grading of disease activity, all of which can impact on therapy and help assess response to treatment [16–18]. PDUS as an adjunct to gray-scale sonography has also been useful in the assessment of tendon neovascularization in patients with chronic achillodynia, because the presence of blood flow was associated with stronger pain, discomfort and physical restriction [19, 20].
Contrast enhanced ultrasound (CEUS)
As angiogenesis occurs in most of these diseases, depiction of angiogenetic microvessels is of interest. CDUS-PDUS techniques have limited ability to detect slow flow and flow in very small vessels. The addition of microbubble-based US contrast agents (CEUS) improves the detection of low-volume blood flow by increasing the signal-to-noise ratio. This opens a new field of applications in MSK imaging, because US has the diagnostic potential to allow for molecular imaging in real-time by using US contrast media, which are blood pool enhancers. Microbubbles can demonstrate vascularity in terms of activity or inactivity in synovial tissue, which is the target of new treatment regimens in rheumatologic diseases. This is of importance because vascularization of the hypertrophied synovium correlates with disease activity [21–23] (Fig. 4a-d).
a-d Dorsal longitudinal scan of MCP joint a Before contrast injection; synovial proliferation between arrows. b After contrast injection, showing enhancement in most parts of the synovial proliferation (white dotted areas, outlined by arrow heads); synovial proliferation between arrows. c Parametric evaluation with region of interest (between arrow heads) in the enhancing part of the synovial proliferation, showing a high peak enhancement curve; synovial proliferation between arrows. d Parametric evaluation with region of interest in the unenhanced part of the synovial proliferation (between arrow heads), showing no enhancement in the time intensity curve; synovial proliferation between arrows
Absence of vascularization correlates with absence of destructive progression, whereas clinical improvement does not necessarily mean a cessation of erosive progression. This underlines the importance of imaging in rheumatological diseases since it assesses changes in vascularity, which is of crucial importance [24–26]. Moreover, erosive destruction occurs as early as the first 6 months of disease in untreated patients suffering from rheumatoid arthritis (RA) [27]. Therefore, early diagnosis and thorough follow-up of RA using a sensitive imaging method capable of quantifying vascularization is of crucial importance [28]. New treatment options (biologicals, TNF inhibitors) target the microvascular level, and treatment follow-up needs easily available sensitive vascular imaging in daily routine. The impact of CEUS in routine diagnosis and follow-up of inflammatory rheumatic diseases is not yet established, but several studies have shown significantly improved detection of vascularity by using US contrast agents. At present, CEUS is of particular interest for clinical studies in monitoring new anti-inflammatory drugs used to treat rheumatological diseases.
In tendons, improved sensitivity achieved by using an US contrast agent enabled the demonstration of intratendinous and peritendinous vascularity, which normally is not apparent with conventional Doppler imaging techniques [19, 20]. Interestingly, by using CEUS, insights have been gained in the rotator cuff, where an age-related decrease in the vascular supply of the tendon was observed, which may predispose to the development of rotator cuff tendinopathy and, ultimately, attritional tears [29–31]. Furthermore, several important aspects of the vascular response 3 months after supraspinatus repair could be quantified, which highlights the potential of CEUS in gaining new insight into in vivo dynamic assessment of tendon vascularity.
First-generation contrast media were used with CDUS/PDUS and a high mechanical Index (MI) scanning protocol, resulting in an earlier and a higher level of microbubble destruction. The use of second-generation contrast media is based on nonlinear acoustic effects of US interaction with microbubbles, displaying microbubble enhancement in gray-scale. Contrast harmonic imaging is a form of nonlinear imaging that takes advantage of stimulated echoes produced by microbubbles that undergo resonant oscillations when interacting with the insonating acoustic wave and in turn, produce higher-order oscillations (harmonics) that can be detected by a broadband transducer. The echoes result from resonance of the transmitted acoustic frequency. Contrast-specific US modes based on the higher harmonic emission capabilities of second-generation contrast agents allow imaging with gray-scale US and avoid Doppler-specific artefacts such as blooming and aliasing. At the same time, the possibility of using US power with a very low mechanical index (MI = 0.06-0.1) enables continuous imaging without the need for time intervals between scans for contrast replenishment, since at higher MI values (>1), bubbles will be destroyed. Mechanical index (MI) is a measure of acoustic power. Loss of bubbles is by breakdown of the phospholipid membrane and exhalation of the gas in the lungs. This technique maximizes contrast and spatial resolution and enables evaluation of the microcirculation, thus prompting the evolution of contrast US from vascular imaging to the imaging of perfused tissues. Very low mechanical index (MI) and low acoustic output optimize the detection of perfusion in microvessels.
The method of administration of second-generation contrast agents so far has been that of a bolus injection. This enables quantitative assessment of several parameters such as time intensity, maximum peak enhancement, area under the curve, p and wash out. Continuous infusion of second-generation contrast agents might be of interest in cases where examination time is prolonged. However, for a successful application of this method, adequate infusion devices are needed to avoid too rapid dissolving of contrast medium. Their value has to be established in future studies.
Possible future developments might be therapeutic targeting of microbubbles for targeted delivery in rheumatological diseases. Furthermore, it would be desirable to have sensitive disease activity assessment which would help make appropriate therapeutic decisions. Earlier diagnosis of rheumatoid arthritis might be facilitated by employing US or MRI instead of X-ray, currently the gold standard in imaging modality. Whereas the latter is useful in the detection of marked erosive changes, the former imaging modes enable detection of earlier changes, and thus contribute to early diagnosis and early therapeutic intervention.
Further developments
Objective quantification of vascularity
Operator experience and expertise may strongly influence the final results of US examination [32, 33]. With the increased use of US for detection of inflammatory processes in the MSK system, there has been a growing awareness that standardization and validation of this method are not yet adequate. Koski et al. as well as Szkudlarek et al. reported a good to excellent intra-reader agreement, but only a moderate to good inter-reader agreement when evaluating dynamic images of healthy and rheumatic joints assessed with PDUS. They suggest that sonographic training and more definitions of normal and abnormal US images are needed to raise the level of the results [34, 35].
Subjective grading before and after the application of contrast media can be a practicable tool in clinical routine because it is relatively easy, quick to perform, and reliable [36, 37]. However, objective quantitative analysis software might increase discriminant validity that could be of importance in clinical trials. Since there are few published reports of objective quantification of CEUS in the musculoskeletal system, intra- and inter-observer reliability of this method has not yet been proven sufficiently [38–40]. Several studies have been published using parametric imaging softwares to quantify perfusion of different tissues. Primarily developed for the quantification of myocardial perfusion, currently it is used, e.g., in studies of perfusion quantification in liver and breast tissue ([41, 42] First studies with qualitative and quantitative quantification of supraspinatus tendon enhancement were performed by determining regions of interest with ultrasound imaging quantification and analysis software [29–31]. Objective quantification software tools should be evaluated in further musculoskeletal US applications, to clarify whether computer-based quantification is superior to subjective vascularity grading, especially in therapeutic follow-up of rheumatological diseases.
Three-dimensional imaging
Measurement of altered tissue vascularity and lesion extent (e.g., synovial proliferation, tumours) is a challenging task where volume estimation is desirable. Here, the use of three-dimensional imaging (3D imaging) could be helpful, which can be carried out in B-mode, Doppler mode and in CEUS for a better understanding of pathology and improved measurements after acquiring volumetric data. The dedicated 3D transducer is larger than a standard probe since it provides assessment of each scanning plane. It is held firmly freehand over a selected region, and by tilting the scan head with a mechanized drive along the z-axis, it sweeps over this region automatically to record serial slices for volume acquisition. Data are provided immediately, stored as a cine loop. The monitor displays reconstructed slices according to longitudinal, transverse and coronal planes, and can be parallel or rotational shifted to enhance depth perception and provide true 3D perspective. Various rendering algorithms including maximum intensity projection, transparent, surface and Doppler renderings and highly advanced contrast rendering are currently available [43].
By using conventional two dimensional (2D) US, only a part of the inflamed joint compartment might be imaged, which consequently impacts on follow-up examinations of synovitis in, e.g., rheumatic diseases. During a quick sweep over the entire joint or including even several small joints (e.g., finger joints), 3D imaging offers a complete three-dimensional registration over the entire region, which can be postprocessed immediately in the US system for computerised objective quantification (Fig. 5).
Three-dimensional imaging a Longitudinal parasagittal dorsal scan showing synovitis (outlined) in a second MCP joint at the bare area by using a dedicated 3D probe. b Axial plane, synovial proliferation is outlined. Erosions are better depicted in the axial plane, because localized very laterally (stars). c Coronal plane; d complete three-dimensional postprocessing of the synovial proliferation, calculating a volume of 0.84 cm3
Three-dimensional imaging of PDUS for quantification in the assessment of arthritis during anti-inflammatory treatment performed by two independent investigators demonstrated good to excellent interobserver reliability [44, 45]. Three-dimensional imaging was further useful in the assessment of muscle injuries and of synovitis in knees with osteoarthritis [46, 47].
A further potential use of real-time 3D (4D) imaging is in guiding tumor biopsies; it enables better visualization of the biopsy procedures by using multiplanar views, a combination of cross-sectional and rendered images, which may be of particular help for biopsy of lesions with close relation to relevant anatomical structures [48]. However, until now, 3D evaluation is imitated because it is more time consuming, and image quality results in lower resolution than standard B-and PDUS scans, what might be the reason, why this imaging option is not implemented in MSK clinical routine yet.
Fusion imaging
Fusion imaging that superimposes CT/MRI data sets on to real-time US scanning is an exciting development. Fusion of different imaging data sets for multi-modality matching is already used in nuclear medicine, radiotherapy and neurosurgery. By incorporating information from previously performed CT or MRI scans into real-time US, image fusion has already shown to be of value in an experimental study of US-guided targeted liver ablation [49–51].
In MSK interventions, precise needle placement in peripheral joints and, for instance, sacroiliac joints [52, 53], can be achieved under US and MRI guidance; in cases where avoidance of radiation exposure is imperative, these modalities, can replace fluoroscopy or CT guidance (Fig. 6). However, open MRI scanner for such interventions are not ubiquitously available; furthermore, US beam penetration can be limited due to several reasons, especially in regions where bones or bony spurs are present, so that this technique has limited use, for instance, in spinal interventions.
Image fusion (Virtual Navigator 1.0, Esaote Biomedica, Genoa, Italy). a Overlapping of CT data set with real-time US of the sacroiliac joint (star: iliac bone, arrow: median sacral crest, cross: spinous process). b Overlapping of CT data set with real-time US of the left facet joint L2, bony contours do not match perfectly as yet, fine tuning will be performed for final overlapping. Important landmarks can be differentiated already. Star: spinous process, cross: entrance to facet joint, arrow: superior articular process. (N.B. registration points at the left facet joint)
Incorporation of information from cross-sectional imaging such as MRI or CT into US-guided real-time interventions could open up new horizons for US-guided MSK interventions in selected cases [54]. Image fusion software is integrated in high-end US machines, allowing them to combine cross-sectional imaging methods and real-time US. After overlapping both imaging modalities, the US scan incorporates all information from the previously performed CT or MRI scan, which has been shown to be of value for targeting liver lesions for ablation in an experimental study [55].
Virtual real-time sonography seems promising for MSK imaging to guide injections into the sacroiliac joint and for injection of facet joints. By using a navigator technology relying on bony extra- and intraarticular landmarks which are provided by the CT scan, the needle insertion can be guided sonographically for intraarticular injection.
The US system characteristics such as spatial dimension, orientation, probe and field of view, provide basic settings for image fusion, permitting correct representation in size and orientation of CT scans. For correct overlapping of US and CT, internal landmarks are defined on both imaging techniques and are placed on bony contours of anatomic structures such as the spinous process of the fifth lumbar vertebra, posterior superior iliac spine, posterior inferior iliac spine, posterior sacral foramen and SI joint.
An electromagnetic tracking system, comprising a transmitter and a small receiver mounted on the US probe, provides information on the position and orientation of the US probe in relation to the transmitter. During navigation, the system extracts in real-time a CT slice at the location and orientation parallel to the US beam, displaying both images on the screen. CT data are automatically updated each time the position of the transducer is altered; therefore, direct real-time overlapping of both imaging modalities is possible (Fig. 7). If injections have to be performed bilaterally or repeated over time e.g. in the sacroiliac joint, real-time needle positioning under US guidance with image fusion using a CT data set acquired once for multiple injections could be valuable, especially in juvenile spondyloarthritis patients in whom limited radiation exposure is desired.
Use of needle tracker in cases with limited needle visualization due to axial scanning plane might be of value as a teaching tool. a The target (left facet joint) is marked by a region of interest (arrow). b Green line (=needle tracker) correctly targets towards facet joint just after entering the skin to estimate correct needle positioning in cases where direct longitudinal visualization of the needle might be difficult
Teaching
Dedicated teaching and training of young investigators and radiological junior staff is important for the future of US generally [56] and this is true of MSK US also. Recently, a study by Galiano et al. reported facilitated periradicular and facet joint injections in the lumbar and cervical spine by using a homemade US-guided and CT navigation-assisted technique and discussed their value for teaching purposes [57]. Therefore, a commercially available software that is integrated into the US systems to allow for image fusion and real-time navigation might become a helpful teaching instrument in radiological departments.
Very recently, preliminary evaluations were done to guide interventions by using a “needle tracker” (not commercially available software, Esaote Biomedica, Genoa, Italy), which might be a valuable teaching tool in targeting oriented needle guidance. This might enhance teaching perspectives on free hand interventions involving needle positioning and needle angulations under real-time conditions. A magnetic device on top of a syringe provides information regarding needle position and angulations and reflects its alignment with internal markers, which are placed into the targeted area.
Interdisciplinary communication is crucial when rapid advances in technologies take place, as in the field of US. New developments in US imaging such as image fusion have several potential applications in MSK disorders. An interdisciplinary approach between radiologists, medical specialists and generalists will enable dissemination of information on such technological developments, which should, in the final analysis, result in optimal patient management employing state-of-the art knowledge and equipment.
Conclusions
In summary, exciting developments are expanding the range of applications of US in the field of MSK imaging, offering the advantages of real-time performance, high tissue resolution, and relative speed at reasonable costs.
References
Claudon M, Tranquart F, Evans DH, et al. Advances in ultrasound. Eur Radiol. 2002;9:331–3.
Lin CD, Nazarian LN, O’Kane Pl, et al. Advantages of real-time compound sonography of the musculoskeletal system versus conventional sonography. AJR. 2002;171:1629–31.
Strobel K, Zanetti M, Nagy L, Hodler J. Suspected rotator cuff lesions: tissue harmonic imaging versus conventional US of the shoulder. Radiology. 2004;230(1):243–49.
Ophir J, Cespedes I, Ponnekanti H, et al. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–34.
Varghese T, Ophir J, Konofagou E, et al. Tradeoffs in elastographic imaging. Ultrason Imaging. 2001;23:216–48.
Pesavento A, Perrey C, Krueger M, et al. A time efficient and accurate strain estimation concept for ultrasonic elastography using iterative phase zero estimation. IEEE Trans Ultrason Ferroelectr Freq Contr. 1999;46:1057–67.
Pallwein L, Mitterberger M, Struve P, et al. Comparison of sonoelastography guided biopsy with systematic biopsy: impact on prostate cancer detection. Eur Radiol. 2007;17:2278–85.
Frey H. Realtime elastography: a new ultrasound procedure for the reconstruction of tissue elasticity. Radiologe. 2003;43:850–5.
Garra BS, Cespedes EI, Ophir J, et al. Elastography of breast lesions: initial clinical results. Radiology. 1997;202:79–86.
Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–50.
Lyshchik A, Higashi T, Asato R, et al. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005;237:202–11.
Lyshchik A, Higashi T, Asato R, et al. Cervical lymph node metastases: diagnosis at sonoelastography-initial experience. Radiology. 2007;243:258–67.
De Zordo T, Lill SR, Fink C, Feuchtner GM, Jaschke W, Bellmann-Weiler R, et al. Value of real-time sonoelastography in lateral epicondylitis: comparison of findings between patients and healthy volunteers. AJR Am J Roentgenol. 2009;193(1):180–5.
De Zordo T, Fink C, Feuchtner GM, Smekal V, Reindl M, Klauser AS. Real-time sonoelastography findings in healthy Achilles tendons. AJR Am J Roentgenol. 2009;193(2):W134–8.
Klauser A, De Zordo T,Pallwein L,et al. Value of real-time sonoelastography in Achilles tendon comparison of findings between healthy volunteers and patients with symptomatic Achilles tendons. Abstract, RSNA Chicago, IL;Dec 2007: SSG16–04.
Hau M, Kneitz C, Tony HP, Keberle M, Jahns R, Jenett M. High resolution ultrasound detects a decrease in pannus vascularisation of small finger joints in patients with rheumatoid arthritis receiving treatment with soluble tumour necrosis factor alpha receptor (etanercept). Ann Rheum Dis. 2002;61(1):55–8.
Walther M, Harms H, Krenn V, Radke S, Kirschner S, Gohlke F. Synovial tissue of the hip at power Doppler US: correlation between vascularity and power Doppler US signal. Radiology. 2002;225(1):225–31.
Naredo E, Möller I, Cruz A, Carmona L, Garrido J. Power Doppler ultrasonographic monitoring of response to anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58:2248–56.
Zanetti M, Metzdorf A, Kundert HP, et al. Achilles tendons: clinical relevance of neovascularization diagnosed with power Doppler US. Radiology. 2003;227(2):556–60.
Reiter M, Ulreich N, Dirisamer A, Tscholakoff D, Bucek RA. Colour and power Doppler sonography in symptomatic Achilles tendon disease. Int J Sports Med. 2004;25(4):301–5.
Koch A. Review: angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum. 1998;41(6):951–62.
Bodolay E, Koch A, Kim J, Szegedi G, Szekanecz Z. Angiogenesis and chemokines in rheumatoid arthritis and other systemic inflammatory rheumatic diseases. J Cell Mol Med. 2002;6(3):357–76
Taylor P. VEGF and imaging of vessels in rheumatoid arthritis. Arthritis Res. 2002;4(Suppl 3):S99–107.
Ostergaard M, Hansen M, Stoltenberg M, et al. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42(5):918–29.
Conaghan P, O’Connor P, McGonagle D, et al. Elucidation of the relationship between synovitis and bone damage: a randomized magnetic resonance imaging study of individual joints in patients with early rheumatoid arthritis. Arthritis Rheum. 2003;48(1):64–71.
McQueen F, Stewart N, Crabbe J, et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann Rheum Dis. 1999;58(3):156–63.
Nell V, Machold K, Eberl G, Stamm T, Uffmann M, Smolen J. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford). 2004;43(7):906–14.
Bajaj S, Lopez-Ben R, Oster R, Alarcón GS. Ultrasound detects rapid progression of erosive disease in early rheumatoid arthritis: a prospective longitudinal study. Skeletal Radiol. 2007;36(2):123–8.
Gamradt SC, Gallo RA, Adler RS, Maderazo A, Altchek DW, Warren RF, Fealy S.Vascularity of the supraspinatus tendon three months after repair: characterization using contrast-enhanced ultrasound. J Shoulder Elbow Surg. 2009 Jun 12.
Adler RS, Fealy S, Rudzki JR, Kadrmas W, Verma NN, Pearle A, et al. Rotator cuff in asymptomatic volunteers: contrast-enhanced US depiction of intratendinous and peritendinous vascularity. Radiology. 2008;248(3):954–61.
Rudzki JR, Adler RS, Warren RF, Kadrmas WR, Verma N, Pearle AD, et al. Contrast-enhanced ultrasound characterization of the vascularity of the rotator cuff tendon: age- and activity-related changes in the intact asymptomatic rotator cuff. J Shoulder Elbow Surg. 2008;17(Suppl 1):96S–100.
Delle Sedie A, Riente L, Bombardieri S. Limits and perspectives of ultrasound in the diagnosis and management of rheumatic diseases. Mod Rheumatol. 2008;18(2):125–31.
Le Corroller T, Cohen M, Aswad R, Pauly V, Champsaur P. Sonography of the painful shoulder: role of the operator’s experience. Skeletal Radiol. 2008;37(11):979–86.
Koski J, Saarakkala S, Helle M, et al. Assessing the intra- and inter-reader reliability of dynamic ultrasound images in power Doppler ultrasonography. Ann Rheum Dis. 2006;65(12):1658–60.
Szkudlarek M, Court-Payen M, Jacobsen S, Klarlund M, Thomsen H, Østergaard M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003;48(4):955–62.
Klauser A, Frauscher F, Schirmer M, Halpern E, Pallwein L, Herold M, et al. The value of contrast-enhanced color Doppler ultrasound in the detection of vascularization of finger joints in patients with rheumatoid arthritis. Arthritis Rheum. 2002;46(3):647–53.
Klauser A, Demharter J, De Marchi A, et al. Contrast enhanced gray-scale sonography in assessment of joint vascularity in rheumatoid arthritis: results from the IACUS study group. Eur Radiol. 2005;15(12):2404–10.
Schueller-Weidekamm C, Krestan C, Schueller G, Kapral T, Aletaha D, Kainberger F. Power Doppler sonography and pulse-inversion harmonic imaging in evaluation of rheumatoid arthritis synovitis. AJR Am J Roentgenol. 2007;188(2):504–8.
Song I, Althoff C, Hermann K, et al. Contrast-enhanced ultrasound in monitoring the efficacy of a bradykinin receptor-2 antagonist in painful knee osteoarthritis compared to magnetic resonance imaging. Ann Rheum Dis. 2009;68(1):75–83.
Song I, Althoff C, Hermann K, et al. Knee Osteoarthritis Efficacy of a new method of contrast-enhanced musculoskeletal ultrasonography in detection of synovitis in patients with knee osteoarthritis in comparison with magnetic resonance imaging. Ann Rheum Dis 2007 May 14. (Epub ahead of print)
Ricci P, Cantisani V, D’Onofrio M, et al. Behavior of hepatocellular adenoma on real-time low-mechanical index contrast-enhanced ultrasonography with a second-generation contrast agent. J Ultrasound Med. 2008;27(12):1719–26.
Ricci P, Cantisani V, Ballesio L, et al. Benign and malignant breast lesions: efficacy of real time contrast-enhanced ultrasound vs. magnetic resonance imaging. Ultraschall Med 2007;28(1):57–62.
Brandl H, Gritzky A, Haitzinger M. 3 D Ultrasound: a dedicated system. Eur Radiol. 1999;9:331–3.
Albrecht K, Grob K, Lange U, Müller-Ladner U, Strunk J. Reliability of different Doppler ultrasound quantification methods and devices in the assessment of therapeutic response in arthritis. Rheumatology (Oxford). 2008;47(10):1521–6.
Strunk J, Strube K, Rumbaur C, Lange U, Müller-Ladner U. Interobserver agreement in two- and three-dimensional power Doppler sonographic assessment of synovial vascularity during anti-inflammatory treatment in patients with rheumatoid arthritis. Ultraschall Med. 2007;28(4):409–15.
Serafin-Król M, Król R, Ziólkowski M, Jedrzejczyk M, Marianowska A, Mlosek R, et al. Potential value of three-dimensional ultrasonography in diagnosing muscle injuries in comparison to two-dimensional examination: preliminary results. Orthop Traumatol Rehabil. 2008;10(2):137–45.
Ju JH, Kang KY, Kim IJ, Yoon JU, Kim HY, Park SH. Three-dimensional ultrasonographic application for analyzing synovial hypertrophy of the knee in patients with osteoarthritis. J Ultrasound Med. 2008;27(5):729–36.
Albrecht H, Stroszczynski C, Felix R, Hünerbein M. Real time 3D (4D) ultrasound-guided percutaneous biopsy of solid tumours. Ultraschall Med. 2006;27(4):324–8.
Ma CM, Paskalev K. In-room CT techniques for image-guided radiation therapy. Med Dosim. 2006;31:30–9.
Grunert P, Darabi K, Espinosa J, Filippi R. Computer-aided navigation in neurosurgery. Neurosurg Rev. 2003;26:73–99.
Pereira PL, Günaydin I, Trübenbach J, et al. Interventional MR imaging for injection of sacroiliac joints in patients with sacroiliitis. AJR Am J Roentgenol. 2000;175:265–6.
Pekkafahli MZ, Kiralp MZ, Başekim CC, et al. Sacroiliac joint injections performed with sonographic guidance. J Ultrasound Med. 2003;22:553–9.
Klauser A, De Zordo T, Feuchtner G, Sögner P, Schirmer M, Gruber J, Sepp N, Moriggl B. Feasibility of ultrasound-guided sacroiliac joint injection considering sonoanatomic landmarks at two different levels in cadavers and patients. Arthritis Rheum 2008;15;59(11):1618–1624
Sofka CM. Current applications of advanced cross-sectional imaging techniques in evaluating the painful arthroplasty. Skeletal Radiol. 2007;36(3):183–93.
Crocetti L, Lencioni R, De Beni S, et al. Targeting liver lesions for radiofrequency ablation: an experimental feasibility study using a CT-US fusion imaging system. Invest Radiol. 2008;43:33–9.
Lockhart ME. The role of radiology in the future of sonography. AJR. 2008;190:8141–2.
Galiano K, Obwegeser AA, Bale R, et al. Ultrasound-guided and CT-navigation-assisted periradicular and facet joint injections in the lumbar and cervical spine: a new teaching tool to recognize the sonoanatomic pattern. Reg Anesth Pain Med. 2007;32:254–7.
Support
No financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klauser, A.S., Peetrons, P. Developments in musculoskeletal ultrasound and clinical applications. Skeletal Radiol 39, 1061–1071 (2010). https://doi.org/10.1007/s00256-009-0782-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-009-0782-y