Abstract
Background
There is currently a lack of suitable objective endpoints to measure disease progression in Duchenne muscular dystrophy (DMD). Emerging research suggests that diffusion tensor imaging (DTI) has potential as an outcome measure for the evaluation of skeletal muscle injury.
Objective
The objective of this study was to evaluate the potential of DTI as quantitative magnetic resonance imaging (MRI) markers of disease severity in DMD.
Materials and methods
Thirteen consecutive boys (8.9 years ± 3.0 years) with DMD were evaluated using DTI. Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) were compared with clinical outcome measures of manual muscle testing and MRI determinations of muscle fat fraction (MFF) in the right lower extremity.
Results
Both MRI measures of FA and ADC strongly correlated with age and muscle strength. Values for FA positively correlated with age and negatively correlated with muscle strength (r = 0.78 and −0.96; both P ≤ 0.002) while measures of ADC negatively correlated age, but positively correlated with muscle strength (r = −0.87 and 0.83; both P ≤ 0.0004). Additionally, ADC and FA strongly correlated with MFF (r = −0.891 and 0.894, respectively; both P ≤ 0.0001). Mean MMF was negatively correlated with muscle strength (r = −0.89, P = 0.0001).
Conclusion
DTI measures of muscle structure strongly correlated with muscle strength and adiposity in boys with DMD in this pilot study, although these markers may be more reflective of fat replacement rather than muscle damage in later stages of the disease. Further studies in presymptomatic younger children are needed to assess the ability of DTI to detect early changes in DMD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Duchenne muscular dystrophy (DMD) is the most common form of inherited muscular dystrophy in children, affecting 1 in 3,500 boys [1]. The disease is caused by mutations in the X-linked dystrophin gene, which is important in linking the cytoskeleton and cell membrane of muscle myofibers [2]. DMD is characterized by a rapid, progressive destruction of skeletal muscle, leading to systemic muscle weakness. Most patients are diagnosed in early childhood at approximately 5 years of age, with loss of developmental milestones [3]. Weakness proceeds in a proximal to distal pattern, leading to loss of ambulation in the early teenage years [3]. Death from cardiopulmonary complications typically occurs in early adulthood. To date, there is neither a cure nor definitive therapy for slowing disease progression [4–6].
A major challenge in developing therapeutic agents for the treatment of DMD is the lack of reliable objective endpoints to measure disease progression. This is especially difficult in DMD because testing is performed at a young age where cooperation becomes an issue and cognitive deficits are seen in approximately one-third of patients [7]. While the greatest experience is with manual muscle tests, there are concerns regarding intra- and interexaminer variability because of subjectivity and dependence on patient effort [8]. Measures of muscle strength using dynamometry are more quantitative but have similar limitations in terms of patient effort and have been shown to be less sensitive in detecting small changes [9]. Measurements of creatine phosphokinase levels can vary tremendously and do not always correlate with disease activity [10]. Lastly, muscle biopsy, currently the gold standard to monitor disease and interventions, is invasive and its accuracy is limited to a small area of muscle [11–15].
Magnetic resonance imaging (MRI) has tremendous potential as an objective outcome measure for the evaluation of muscle since the major histological features associated to muscle injury (i.e. volume loss, irregular contour, fatty infiltration, edema and fibrosis) in DMD can be depicted using MRI techniques. Conventional MRI has focused on qualitative evaluation of fat infiltration and edema of muscle using T1 and STIR techniques, respectively [16]. Quantitative MRI techniques, such as chemical-shift encoded water-fat MRI (hereafter referred to as water-fat MRI), T2 relaxometry and magnetic resonance spectroscopy, have also been shown to reliably assess muscle fat infiltration in DMD patients [17–25].
Emerging research indicates that diffusion tensor imaging (DTI) MR techniques can be utilized to evaluate altered skeletal muscle structure. DTI is based on the principle that the diffusion of water in tissue is restricted by membranes and other cellular constituents, resulting in anisotropic diffusion, which is lower than free diffusion and orientation-dependent for elongated structure [26]. Since anisotropy is a feature of skeletal muscle fibers, DTI measures could serve as surrogate measures of muscle structure and architecture. Specifically, a number of quantitative diffusion patterns can be used to characterize the diffusion in skeletal muscle, most commonly fractional anisotropy (FA) and apparent diffusion coefficient (ADC). Over the past decade, investigators have studied the application of DTI in characterizing muscle architecture [27], evaluating muscle injury [28] and regeneration [29], and identifying the effects of denervation on muscle structure [30].
To our knowledge, studies examining the value of DTI as a quantitative MRI marker of disease severity in DMD are rare. The purpose of this study was to compare DTI metrics of FA and ADC with clinical muscle strength measurements and established imaging determinations of muscle fat fraction (MFF) using water-fat MRI [20, 23, 24]. An additional objective was to provide a more comprehensive characterization of the extent of disease, by examining the entire lower extremity at the same time point.
Materials and methods
Study subjects
The study cohort was comprised of 13 consecutive boys (mean age: 8.9 years ± 3.0 years, range: 6–17 years) with DMD who were recruited from the patient population of a pediatric Muscular Dystrophy Association Clinic. Inclusion criteria required a diagnosis of DMD confirmed with DNA testing, no history of recent exercise and the ability to remain still for the MRI exam. Subjects were excluded if they had a history of chronic illness other than DMD, including any other neuromuscular, metabolic or endocrine disorder that could alter bone or muscle metabolism. Additionally, children younger than 5 years of age were excluded because of the likely need for sedation. The study was compliant with the Health Insurance Portability and Accountability Act and approved by the hospital’s Institutional Review Board for clinical investigations. Written informed consent was obtained from all parents. For subjects older than 7 years, subject assent was also obtained.
Muscle strength evaluations
Subjects were evaluated by a pediatric neurologist (L. R.-P.) with 7 years of experience and specialized training in neuromuscular physiology and musculoskeletal testing, who was blinded to the MRI results. Muscle strength was measured through standard manual muscle testing (MMT) following the traditional 5-point scale [31]. The muscles assessed included the hip flexors, extensors, abductors, adductors, knee flexors and extensors, ankle dorsiflexors, plantarflexors, inverters and everters. Following Medical Research Council (MRC) guidelines [11], a total muscle strength score was calculated as % MRC = (Sum of grade scores x 10) / (# muscles tested x 5), in addition to examining individual muscles.
MRI
All MRI scans were performed on a 3.0-Tesla Achieva MRI scanner (Achieva R3.2; Philips Healthcare, Cleveland, OH). With the patient in the supine position, axial images of the right thigh and lower leg were obtained using a 16-channel torso array coil. The DTI sequence utilized a multislice spin-echo single-shot echo planar imaging sequence (TR/TE: 2,479/43 ms; 15 diffusion directions + 1 baseline; b = 0, 250, 500 s/mm2). Slice thickness was 6 mm, with a 1.5- mm in-plane resolution. Adiabatic fat suppression was performed using Spectral Attenuated Inversion Recovery. Parallel imaging sensitivity encoding (SENSE) with an acceleration of 2 was utilized to shorten the scan time, which was approximately 6 min. Axial water-fat MRI of the right thigh and lower leg was performed using the 3-D spoiled-gradient-echo multi-echo T2*-corrected mDIXON pulse sequence provided by the manufacturer (TR/TE: 10/1.48 ms; flip angle = 3°). A six-echo generalization of the traditional in/opposed phase two-echo Dixon water-fat imaging technique was performed [27]. Isotropic spatial resolution was obtained, with a slice thickness of 1 mm and 1-mm in-plane resolution. SENSE acceleration of 2 was utilized to shorten the scan time, which was approximately 2 min. The DTI and mDixon scans were colocalized and covered the same anatomical extent.
Image analysis
Slice selection was chosen and regions of interest (ROIs) were drawn by two pediatric radiologists in consensus, with 3 (S.P.) and 30 (V.G.) years of experience in pediatric musculoskeletal imaging. The evaluators were blinded to the muscle strength results. We have previously examined the interobserver and test-retest repeatability of DTI and water-fat MRI for evaluation of lower extremity skeletal muscle in healthy children and found the interobserver repeatability as measured by intraclass coefficient to be excellent for DTI measures of ADC (0.94) and FA (0.93), as well as for water-fat MRI measures of MFF (0.96) [32]. Test-retest repeatability was also excellent for DTI measures of ADC (0.88) and FA (0.75) and water-fat MRI measures of MFF (0.73), although less so compared with interobserver repeatability.
For each dataset, ADC, FA and MFF were individually calculated for the following muscles: pelvis (sartorius, gluteus maximus, tensor fascia lata, iliopsoas), thigh (rectus femoris, vastus medialis, vastus lateralis, vastus intermedius, semimembranosus, semitendinosus, biceps femoris, adductor magnus and adductor longus) and lower leg (anterior tibialis, posterior tibialis, peroneus longus, gastrocnemius and soleus). The image slice that provided maximal transverse cross-sectional area of each muscle to be measured was selected and ROIs were drawn circumferentially around the muscle using a freehand technique. The slice location and ROI for each muscle was copied and pasted to each dataset to ensure perfect correlation among the different sequences. From these quantitative measures of each individual muscle, mean ADC, FA and MFF values of all the muscles in the lower extremity were calculated.
DTI datasets were analyzed using commercially available software provided by the manufacturer (FiberTrak; Philips Healthcare, Cleveland, Ohio). The DTI datasets were co-registered with the higher resolution water-fat MRI images for anatomical reference. Fiber tracking was performed with a single ROI line propagation technique using the following parameters: FA threshold, 0.12; direction threshold, 6.75°. Quantitative ADC and FA values were automatically generated by the software.
The post-processed water-fat MRI dataset included coregistered fat, water, in-phase and opposed-phase image series, and quantitative fat fraction and T2* maps [33]. Fat fraction maps were generated by calculating the proton density ratios between fat and the sum of water and fat and multiplying by 100 to get a range of 0-100%. Prior studies have demonstrated the accuracy of the generated fat fraction maps when using a multi-peak spectral model of fat and a small excitation flip angle [34]. Average MFF values for each ROI were calculated using processing software as provided by the manufacturer.
Statistical analysis
Statistical analysis was carried out with statistical software (Stata 12, 2011; Stata, College Station, Texas) by using the t-test for paired samples and logistic linear regression analyses. All values are expressed as the mean ± standard deviation, and were considered significant if the P-value was <0.05.
Results
Table 1 describes the age, anthropometric parameters, MRC score, and the averaged values for total ADC, FA and MFF. Table 2 provides the simple correlations among these variables. There were no significant correlations between height, weight and BMI percentile and the averaged values for total MFF, ADC and FA. Additionally, there were no significant associations between anthropometric parameters and measures of muscle strength.
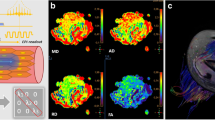
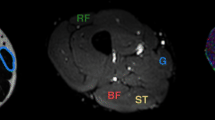
Qualitative T1-weighted images and quantitative MRI water-fat MRI images of the right thigh clearly depict increased muscle fat infiltration with age (Fig. 1). As expected, age was positively correlated with mean MFF (r = 0.92, P < 0.001) and negatively correlated with muscle strength (r = −0.82, P < 0.001), compatible with known progression of muscle weakness and fatty replacement of the muscle as children with DMD age. Figure 2 depicts tractography images of sampled muscles in the right lower thigh of subjects of different ages. Qualitatively, the fiber tracts appear decreased in number, length and organization with age. When quantitatively comparing DTI measures with age, there was a negative correlation between age and mean ADC (r = −0.87, P < 0.001), but a positive correlation between age and mean FA (r = 0.78, P = 0.002).
DTI tractography of rectus femoris (green), biceps femoris (red) and gracilis (blue) muscles in a 6-year-old boy, an 8-year-old boy and a 12-year-old boy, superimposed on anatomical T1-weighted images, demonstrating decreasing number, length and organization of fiber tracks with age. a Early ambulatory 6-year-old with DMD. b Late ambulatory 8-year-old with DMD. c Early non-ambulatory 12-year-old with DMD
T1-weighted and color-coded water-fat MRI of the right mid-thigh of the same patients in Figure 1. a Axial T1-weighted image of the right mid-thigh in a 6-year-old boy in the early ambulatory stage DMD. b Axial T1-weighted image of the right mid-thigh in a 8-year-old boy in the late ambulatory stage and (c) axial T1-weighted image of the right mid-thigh in a 12-year-old boy in the early non-ambulatory stage. d Axial color-coded water-fat MRI of the right mid-thigh in the same patient as (a) depicts no quantitative difference in muscle fat infiltration. e Axial color-coded water-fat MRI of the right mid-thigh in the same patient as (b) shows areas of up to 50% fatty infiltration in the quadriceps and adductor magnus muscles. f Axial color-coded water-fat MRI of the right mid-thigh in the same patient as (c) with >50% fatty infiltration in all muscles groups excluding the gracilis muscle (thin white arrow) and semitendinosus muscle (thick white arrow)
Correlations between muscle strength, as measured by the MRC score, and MRI measures of MFF, FA and ADC were made for the ambulatory patients. The single non-ambulatory patient was excluded from this part of the analysis. Strong correlations were found between muscle strength, as measured by the MRC score, and DTI measures of muscle structure; negative with mean FA (r = −0.84, P < 0.01), but positive with mean ADC (r = 0.57, P = 0.04). Mean MMF also correlated with muscle strength (r = −0.74, P < 0.01).
Measures of MFF were significantly negatively correlated with mean ADC (r = −0.891, P <0.001) and positively correlated with mean FA (r = 0.894, P < 0.001). Mean ADC and mean FA were also significantly negatively correlated (r = −0.886, P < 0.001).
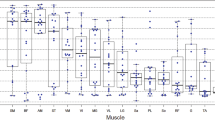
Table 3 describes the values for MFF, ADC and FA for each muscle of the lower extremity measured. When separated into a proximal muscle group (quadriceps, hamstrings, gracilis, sartorius, tensor fascia lata, iliopsoas and gluteus maximus) and a distal muscle group (anterior and posterior tibialis, peroneus longus and gastrocnemius), there were significant differences in mean ADC, FA and MFF values between the proximal thigh muscles and distal leg muscles. The proximal muscle group had higher MFF (P = 0.02) and FA (P < 0.01) and a lower ADC (P = 0.04) compared with the distal muscle group.
Within the thigh, values for 1) MFF values were highest in the gluteus maximus (52.2%) and lowest in the sartorius (18.5%), 2) FA were highest in the tensor fascia lata (0.518) and lowest in the semitendinosus (0.381), and 3) ADC were highest in the vastus medialis (1.429) and lowest in the gluteus maximus (1.047). Within the lower leg, values for 1) MFF were highest in the peroneus longus muscle (28.2%) and lowest in the posterior tibialis (13.0%), 2) FA were highest in the anterior tibialis (0.411) and lowest in the soleus (0.309), and 3) ADC were highest in the posterior tibialis (1.602) and lowest in the gastrocnemius (1.287).
Discussion
Previous investigators have explored the potential of quantitative MR imaging markers of disease progression in DMD, particularly in the evaluation of muscle fat infiltration. For example Gaeta et al. [20] demonstrated significant correlations between MFF measures from water-fat MRI and clinical measures of muscle strength and functional ability. Similarly, Kim et al. [35] studied T2 mapping as a quantitative measure of fat infiltration in skeletal muscle qualitatively visualized on T1-weighted images and found similar significant correlations. However, the major disadvantage of these fat quantification methods is that they evaluate disease progression in the chronic phase of the disease after normal muscle structure has been lost and replaced by fat. To better assess subtle response to new therapies in clinical trials that may slow muscle breakdown before irreversible fatty replacement has occurred, additional MRI biomarkers targeting early muscle injury are needed.
In this study, we investigate the potential of DTI as quantitative MRI markers of disease severity in DMD. We used DTI tractography to determine mean FA and ADC values of individual muscles and quantify disease severity in the lower extremity of boys with DMD. We found strong correlations between DTI measures of muscle and both clinical measures of manual muscle testing and MRI measures of muscle adiposity. Consistent with knowledge indicating greater disease severity in proximal muscle groups [20, 22], we observed increased muscle damage, as measured by FA and ADC, most prominently in the gluteus maximus muscles, followed by the quadriceps, calves and then hamstrings.
Despite the significant heterogeneity of normal ADC and FA values for thigh and leg muscles reported in the literature, the values obtained in the current study are at the upper limits or exceed the normal ranges reported using similar protocols [28, 32, 36–39]. For example, as described previously, the range of FA values in our study was 0.309 - 0.518 and the range of ADC values was 1.047 × 10−3 mm2/s – 1.429 × 10−3 mm2/s, with the normal ranges in the lower extremity in other studies ranging from 0.18-0.33 for FA and 1.31 × 10−3 mm2/s – 1.80 × 10−3 mm2/s for ADC. Although comparison with small datasets of normal controls from other studies reported in the literature is limited, such differences support the notion that DTI can assess structural changes in diseased muscles, beyond the normal variation associated with differences in technique.
Unexpectedly, we found that increased age in patients with DMD correlated positively with FA and negatively with ADC, while muscle strength correlated negatively with FA and positively with ADC. FA measures the degree of anisotropy within skeletal muscle tissue while ADC measures the magnitude of diffusion of water within skeletal muscle. With muscle injury, we would expect decreased FA and increased ADC given the loss of the normal muscle architecture and the release of water from damaged muscle cells. This relationship was shown in a study by Zaraiskaya et al. [28], who found that traumatic muscle injury was associated with decreased FA and increased ADC values. A possible explanation for the incongruous results in our study is that DTI measures are influenced by muscle adiposity. Qi et al. [40] first described decreased ADC values in the later stages of muscle necrosis and infiltration by fat and fibrous tissue in patients with chronic polymyositis. They postulated that the decreased ADC values could be explained by reduced water content. More recently, Williams et al. [41] reported that ADC is artificially decreased and FA is artificially increased in the setting of greater than 45% fat signal. However, they also found that FA is paradoxically increased, likely related to error from random noise because of the signal loss from using a fat-suppressed echo-planar imaging technique of predominantly fat-replaced tissue. Therefore, ADC and FA values in this study may partially reflect fat infiltration in the region of interest rather than loss of muscle structure. Interestingly, the qualitative tractography images shown in Fig. 2 show an expected decrease in fiber length, number and architecture that did not correspond with the quantitative ADC and FA values, suggesting DTI may still provide useful information. Studies evaluating both DTI and water-fat MRI before and after the development of muscle fat infiltration are needed to establish the degree to which muscle structure and fat infiltration influence DTI determinations.
Several general limitations of our study must be acknowledged. Quantitative DTI measures were not compared with muscle biopsy, which is the gold standard for visualization of muscle fiber architecture. However, serial muscle biopsy without direct benefit is an unacceptable procedure for children. Other limitations of this study are the small sample size and the older age of the study cohort. Although the subjects encompassed a broad age range and disease severity, the relatively small cohort studied was not sufficiently powered to detect small differences. By design, we purposely excluded children younger than 5 years of age, who likely have little muscle damage, to avoid the need for sedation, thereby precluding our ability to evaluate the presymptomatic grade of disease severity. Further studies in younger children are needed to assess the ability of MRI to detect early changes in DMD. This study is also limited secondary to a lack of a normative age-based dataset for which to distinguish normal values from abnormal values. Normal values from the only prior study evaluating DTI measures of skeletal muscle in healthy children are limited by the small sample size of seven subjects [30]. Finally, although the majority of studies of MRI and DMD evaluate the bilateral proximal lower extremities [1], we decided to examine the entire unilateral lower extremity at the same time point because DMD tends to be a symmetrical process. This was to provide a more comprehensive characterization of the extent of disease and to shorten examination time.
Conclusion
This pilot study suggests that DTI can provide noninvasive markers of muscle damage that reflect disease severity in patients with DMD, although these markers may be more reflective of fat replacement rather than muscle damage in later stages of the disease. Further studies in presymptomatic younger children are needed to assess the ability of DTI to detect early changes in DMD.
References
Bushby K, Finkel R, Birnkrant DJ et al (2009) Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 9:77–93
Hoffman EP, Brown RH Jr, Kunkel LM (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51:919–928
Dubowitz V (1995) The muscular dystrophies. Muscle disorders in childhood. Saunders, London, pp 39–42
Kapsa R, Kornberg AJ, Byrne E (2003) Novel therapies for Duchenne muscular dystrophy. Lancet Neurol 2:299–310
Bogdanovich S, Perkins KJ, Krag TO et al (2004) Therapeutics for Duchenne muscular dystrophy: current approaches and future directions. J Mol Med (Berl) 82:102–115
Tidball JG, Spencer MJ (2003) Skipping to new gene therapies for muscular dystrophy. Nat Med 9:997–998
Cyrulnik SE, Fee RJ, Batchelder A et al (2008) Cognitive and adaptive deficits in young children with Duchenne muscular dystrophy (DMD). J Int Neuropsychol Soc 14:853–861
Escolar DM, Henricson EK, Mayhew J et al (2001) Clinical evaluator reliability for quantitative and manual muscle testing measures of strength in children. Muscle Nerve 24:787–793
Schrama PP, Stenneberg MS, Lucas C et al (2014) Intra-examiner reliability of hand-held dynamometry in the upper extremity: a systematic review. Arch Phys Med Rehabil. doi:10.1016/j.apmr.2014.05.019
Stern LM, Caudrey DJ, Perrett LV et al (1984) Progression of muscular dystrophy assessed by computed tomography. Dev Med Child Neurol 26:569–573
Scott OM, Hyde SA, Goddard C et al (1982) Quantitation of muscle function in children: a prospective study in Duchenne muscular dystrophy. Muscle Nerve 5:291–301
Brooke MH, Fenichel GM, Griggs RC et al (1983) Clinical investigation in Duchenne dystrophy: 2. Determination of the “power” of therapeutic trials based on the natural history. Muscle Nerve 6:91–103
Kilmer DD, Abresch RT, Fowler WM Jr (1993) Serial manual muscle testing in Duchenne muscular dystrophy. Arch Phys Med Rehabil 74:1168–1171
Brooke MH, Griggs RC, Mendell JR et al (1981) Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve 4:186–197
Pourmand R (ed) (2004) Diagnostic tests in neuromuscular disease. Saunders, Philadelphia, PA
Marden FA, Connolly AM, Siegel MJ et al (2005) Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skeletal Radiol 34:140–148
Forbes SC, Walter GA, Rooney WD et al (2013) Skeletal muscles of ambulant children with Duchenne Muscular Dystrophy: validation of multicenter study of evaluation with MR imaging and MR spectroscopy. Radiology 269:198–207
Fischmann A, Hafner P, Gloor M et al (2013) Quantitative MRI and loss of free ambulation in Duchenne muscular dystrophy. J Neurol 260:969–974
Finanger EL, Russman B, Forbes SC et al (2012) Use of skeletal muscle MRI in diagnosis and monitoring disease progression in Duchenne muscular dystrophy. Phys Med Rehabil Clin N Am 23:1–10, ix
Gaeta M, Messina S, Mileto A et al (2012) Muscle fat-fraction and mapping in Duchenne muscular dystrophy: evaluation of disease distribution and correlation with clinical assessments. Preliminary experience. Skeletal Radiol 41:955–961
Gaeta M, Scribano E, Mileto A et al (2011) Muscle fat fraction in neuromuscular disorders: dual-echo dual-flip-angle spoiled gradient-recalled MR imaging technique for quantification–a feasibility study. Radiology 259:487–494
Kim HK, Laor T, Horn PS et al (2010) T2 mapping in Duchenne muscular dystrophy: distribution of disease activity and correlation with clinical assessments. Radiology 255:899–908
Wren TA, Bluml S, Tseng-Ong L et al (2008) Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: preliminary study. AJR Am J Roentgenol 190:W8–12
Hollingsworth KG, Garrood P, Eagle M et al (2013) MR imaging in Duchenne muscular dystrophy: longitudinal assessment of natural history over 18 months. Muscle Nerve 48:586–588
Kan HE, Scheenen TW, Wohlgemuth M et al (2009) Quantitative MR imaging of individual muscle involvement in facioscapulohumeral muscular dystrophy. Neuromuscul Disord 19:357–362
Basser PJ, Jones DK (2002) Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed 15:456–467
Heemskerk AM, Strijkers GJ, Vilanova A et al (2005) Determination of mouse skeletal muscle architecture using three-dimensional diffusion tensor imaging. Magn Reson Med 53:1333–1340
Zaraiskaya T, Kumbhare D, Noseworthy MD (2006) Diffusion tensor imaging in evaluation of human skeletal muscle injury. J Magn Reson Imaging 24:402–408
Heemskerk AM, Damon DM (2007) Diffusion tensor MRI assessment of human skeletal muscle architecture. Curr Med Imaging Rev 3:152–160
Zhang J, Zhang G, Morrison B et al (2008) Magnetic resonance imaging of mouse skeletal muscle to measure denervation atrophy. Exp Neurol 212:448–457
Kendall HO, Kendall FP, Wadsworth GE (1971) Muscles, testing and function. Williams and Wilkins, Baltimore, MD
Ponrartana S, Andrade K, Wren TA et al (2014) Repeatability of diffusion tensor imaging for the evaluation of lower extremity skeletal muscle. AJR Am J Roentgenol 202:W567–574
Reeder SB, McKenzie CA, Pineda AR et al (2007) Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging 25:644–652
Bley TA, Wieben O, Francois CJ et al (2010) Fat and water magnetic resonance imaging. J Magn Reson Imaging 31:4–18
Kim HK, Laor T, Horn PS et al (2010) Quantitative assessment of the T2 relaxation time of the gluteus muscles in children with Duchenne muscular dystrophy: a comparative study before and after steroid treatment. Korean J Radiol 11:304–311
Kermarrec E, Budzik JF, Khalil C et al (2010) In vivo diffusion tensor imaging and tractography of human thigh muscles in healthy subjects. AJR Am J Roentgenol 195:W352–356
Sinha S, Sinha U, Edgerton VR (2006) In vivo diffusion tensor imaging of the human calf muscle. J Magn Reson Imaging 24:182–190
Galban CJ, Maderwald S, Uffmann K et al (2005) A diffusion tensor imaging analysis of gender differences in water diffusivity within human skeletal muscle. NMR Biomed 18:489–498
Budzik JF, Le Thuc V, Demondion X et al (2007) In vivo MR tractography of thigh muscles using diffusion imaging: initial results. Eur Radiol 17:3079–3085
Qi J, Olsen NJ, Price RR et al (2008) Diffusion-weighted imaging of inflammatory myopathies: polymyositis and dermatomyositis. J Magn Reson Imaging 27:212–217
Williams SE, Heemskerk AM, Welch EB et al (2013) Quantitative effects of inclusion of fat on muscle diffusion tensor MRI measurements. J Magn Reson Imaging 38:1292–1297
Conflicts of interest
Dr. T. G. Perkins and Mr. J. M. Chia are employees of Philips Healthcare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ponrartana, S., Ramos-Platt, L., Wren, T.A.L. et al. Effectiveness of diffusion tensor imaging in assessing disease severity in Duchenne muscular dystrophy: preliminary study. Pediatr Radiol 45, 582–589 (2015). https://doi.org/10.1007/s00247-014-3187-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-014-3187-6