Abstract
The purpose of this ethics approved trial was to correlate quantitative MRI with functional abilities in both ambulant and non-ambulant Duchenne muscular dystrophy (DMD). Twenty patients with genetically confirmed DMD were recruited. Physical assessment was performed using the motor function measurement (MFM) scale. Axial 3T MRI scans of the thighs were acquired using T1-weighted in- and opposed-phase images (TR = 20 ms, TE1 = 2.45 ms, TE2 = 3.68 ms, flip angle = 15°) to calculate the relative fat fraction according to the two-point Dixon method in the knee extensors, flexors, and adductor muscles. The average MFM was 65.3 % and correlated negatively to age (r 2 = 0.60). Overall mean fat fraction correlated positively to age (r 2 = 0.51–0.64). An average of 5 % increase in mean fat fraction per year was calculated. Mean fat fraction of the quadriceps showed a high negative correlation (r 2 = 0.93) to the D1 (standing position and transfers) component of the MFM. A cutoff for mean fat fraction of 50 % predicted loss of ambulation with a sensitivity of 100 % and a specificity of 91 %. Therefore, quantitative muscle MRI seems to be a promising endpoint for short clinical trials evaluating the effect of newer treatments on the time of loss of ambulation in DMD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Duchenne muscular dystrophy (DMD) is a recessive X-linked disease of childhood and the most common pediatric muscular dystrophy, affecting about 1 in every 3,500 male births. The disease is caused by loss of function mutations in the dystrophin gene located on the X-chromosome (Xp21). Mutations lead to the absence of or truncation in the protein dystrophin, an important structural component of the muscle cell membrane: this abnormality results in structural fragility, membrane permeability, metabolic crisis, and progressive myocyte degeneration. Mutations in the DMD gene result in either Duchenne muscular dystrophy, the milder Becker muscular dystrophy, or X-linked dilated cardiomyopathy [1]. Boys with DMD experience a progressive loss of strength and function that leads to wheelchair dependence, cardiorespiratory compromise, and early death. Recently, several therapeutic trials with promising results have been published [2, 3]. However, biomarkers and outcome measures, especially for short-term effects, are still largely lacking.
Loss of independent ambulation has a large impact on the daily life of the patient as well as the supporting family and is one of the major factors influencing morbidity. It would therefore be helpful to predict the time until the loss of ambulation to give the patient and his caregivers sufficient time to adapt their lifestyle and surroundings. Trials to establish clinical measurements as prognostic parameters were recently conducted [4], however most clinical parameters show a high observer dependency.
As a result, quantitative MRI (qMRI) of skeletal muscles has been introduced in patients with neuromuscular diseases, especially in patients with DMD [5, 6] and has been shown to provide excellent correlation to clinical parameters in several diseases [7–9].
Most trials in patients with DMD, however, rely on semiquantitative visual scores to evaluate disease progression [10, 11]. In a study on 20 DMD patients, we compared qMRI of the fat fraction in diseased muscles with established clinical parameters.
Materials and methods
Twenty patients with genetically confirmed DMD were recruited. Written informed consent was obtained from all patients or their caretakers. The local ethics committee approved all measurements.
Physical assessment was performed by two physiotherapists (T.W. and R.G.) in consensus using the motor function measurement (MFM) scale, covering the whole range of abilities in both ambulant and non-ambulant patients. This scale comprises items from the following three dimensions: standing position and transfers (D1), axial and proximal motor function (D2), and distal motor function (D3). MFM has been shown to have a high inter-rater reliability, does not require any special equipment and is well tolerated by patients [12]. Both evaluators had received special training in MFM evaluation in Lyon, France, 3 years before and have performed MFM evaluations regularly since.
All MRIs were performed on a 3 Tesla scanner (Magnetom Verio, Siemens Healthcare, Erlangen, Germany), with two 16-element body array coils and a spine coil. One operator with more than 10 years experience in research MRI performed all exams according to a pre-specified protocol. Localization comprised a series of scout images in three orthogonal directions and additional scout images parallel and orthogonal to the right/left femur in 14/6 cases, respectively (depending on patient), as well as scout images through the hip and knee joint space. In the case of atypical patient positioning due to contractions, additional localizers were acquired. A single axial slice group was chosen at 50 % distance from knee to hip joint space. Two three dimensional (3D) gradient echo sequences with different TE for in-phase and opposed-phase imaging were acquired (30 slices, TR = 20 ms, TE1 = 2.45 ms, TE2 = 3.68 ms, flip angle = 15°, acquisition time 2:49 min). A field of view of 400 × 400 mm and a 384 × 384 matrix were used yielding 1 mm in plane resolution with 3 mm slice thickness. Two saturation bands were placed above the slab to avoid inflow artefacts from arterial blood. The protocol was completed within 15–20 min dependent on the amount of localizer angulation necessary.
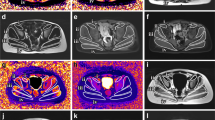
For every patient, regions of interest (ROI) were drawn in thigh muscles (Fig. 1) and care was taken to areas of chemical shift artefact. Three ROIs were chosen to evaluate the knee extensors (quadriceps), knee flexors (hamstrings) and adductor muscle groups. All ROIs were drawn by one co-author (M.S.) and independently checked for quality on raw images and calculated maps by a radiologist with 5 years experience in neuromuscular imaging (A.F.) and by one physicist performing the evaluations (M.G.). All MRI evaluations were performed blinded to clinical status and MFM measurements.
In the two-point Dixon method [13], two images at identical positions are acquired such that water and fat protons are in-phase and opposed-phase, respectively. A combination of these images yields water (w) and fat (f) images. Relative fat content maps were generated from the pixelwise fat fraction given by f/(f + w). ROIs were placed on the maps and the mean fat content was calculated for each muscle group.
Independent ambulation was assessed according to the method proposed by Vuillerot et al. [4], who calculated a hypothetical score of 25 % for the D1 subscale of the MFM as cutoff point to establish loss of independent ambulation.
Statistical analysis was performed by the corresponding author using JMP 8.0.2 (SAS Institute, Cary, NC, USA). Correlations were calculated using Spearman’s rank coefficient. Categorical data were compared using Fischer’s exact test.
Results
We included 20 DMD patients aged 5–23 (median 10.2 years, mean 11.2 years). The average MFM was 65.3 % (Table 1) and showed a strong negative correlation to age (r 2 = 0.60, p < 0.001), with most prominent changes (r 2 = 0.61, p < 0.001) in the D1 component, measuring standing position and transfer, whereas the D3 component, assessing distal motor function showed no correlation (r 2 = 0.01, p = 0.75). Assessment of independent ambulation according to Vuillerot corresponded perfectly to patient reports: no patient with a D1 component below 25 % reported independent ambulation, while all patients above 25 % showed some degree of independent ambulation.
Average cross sectional area of the ROI was 6,248 mm2 (599–11,618 mm2) for the quadriceps, 3,374 mm2 (521–9,955 mm2) for the hamstrings and 2,450 mm2 (467–5,758 mm2) for the adductors.
Fat fractions of the major muscle groups were increased (Table 1). These changes were symmetrical in each patient as indicated by a correlation of 0.99 between the legs. There was a positive correlation between fat fractions and age of 0.61 and 0.64 for the right and left quadriceps, respectively. The corresponding values for the hamstrings were 0.55 and 0.57, and for the adductors 0.51 and 0.53, indicating a moderate correlation (p < 0.001 for all correlations). While the average increase over all patients could be calculated with high confidence (small dotted lines in 2b), variability between patients resulted in larger confidence intervals for each individual patient (large dotted lines in 2).
In the MFM scale, ambulation is mainly assessed with the D1 component, which showed a high negative correlation to the fat fractions in the left and right quadriceps of 0.93 and 0.91 (Fig. 2a) and of 0.86 and 0.85 in the left and right hamstrings, respectively (p < 0.001). When a 50 % cutoff for the fat fraction was used, loss of ambulation could be predicted with a sensitivity of 100 %, and a specificity of 91 and 100 % for the left and right leg, respectively (p < 0.0001). One patient lost ambulation (D1 = 10 %) although he presented with a left quadriceps fat fraction of 54 %.
Average increase of fatty replacement for the individual muscle groups could be calculated as:
translating into an annual 5 % increase in the fat content of the quadriceps and hamstrings starting from an age between 3 and 4 years.
Discussion
Due to its high prevalence and rapid progression, DMD was used as a model for imaging in muscular dystrophies early on. Huang et al. [7] measured T1 and T2 values in the anterior tibial muscle and found increased T2 times and reduced T1 times in patients with DMD as compared to healthy controls. Liu et al. [11] developed a visual grading system, measuring the number of involved muscles per region and found an excellent correlation to functional parameters.
Kinali et al. compared visual analyses of fatty replacement in the calves of 34 DMD patients to histologic analyses of the extensor digitorum brevis. They found a correlation between the semiquantitative MRI score and age as well as a correlation to the time since loss of independent ambulation [10]. No quantitative analysis was performed.
Quantitative MRI
While visual scores show a high inter-reader variability, qMRI is expected to be more reproducible and therefore more objective and more reliable for the assessment of fatty atrophy in DMD. Excellent reproducibility of qMRI was demonstrated in healthy volunteers [14] as well as in patients with oculopharyngeal muscle dystrophy [8, 15]. In the latter case, qMRI was also more sensitive in detecting progressive changes than clinical evaluation and visual analysis of MRI scans. While qMRI is influenced by exercise, the changes introduced even with exercise until muscular fatigue are far below the changes we observed in our study collective [16]. Kim et al. [17] examined T2 maps and visual fatty atrophy in the gluteus muscles in 11 boys with DMD before and after 12 months of steroid treatment and found increased fatty atrophy in two patients and increasing T2 values in five patients, stable results in two and decreasing T2 values in four patients. T2 values are increased by fatty replacement and by inflammatory changes. This might explain the heterogeneous results by Kim et al., as steroid treatment can influence both components in variable degrees. We therefore consider the fat fraction to be a more robust and reliable parameter, especially for long-term disease monitoring. The correlation of the MFM score to fatty atrophy found in our study was higher than correlations of T2 values to clinical function scores or the Gowers test [18]. Alternatively, separate evaluation of T2 values for the fat and water components might provide additional information but require longer scanning time [19], which might be difficult in children with DMD. Gaeta et al. examined 20 patients with DMD using two-point Dixon fat measurements in several muscles of the thigh and hip. They found the highest FF in the gluteus maximus and adductor magnus muscle and the lowest in the semitendinosus muscle [20]. Fat fractions correlated to patient age, as well as to the MRC score for muscular strength and the timed Gowers test for standing up. As only patients who were independently ambulant and cooperative were included, they were unable to correlate their data to the loss of ambulation [20].
Predicting the loss of ambulation
Vuillerot et al. [4] used the MFM score to predict loss of ambulation in patients with DMD and estimated that a total score of 70 % and a D1 subscore of 40 % will predict loss of ambulation within 1 year. Variability in their data, however, was too large to make predictions over longer periods. Clinical scores also have the disadvantage of being dependent on patient cooperation. In contrast, qMRI offers an observer independent measurement which is highly repeatable and can be performed on most clinical scanners. The coefficient of repeatability of 0.9 % calculated by Sinclair et al. [9] for FF measurement with MRI indicates that even small trials can detect a differences as small as 2 % in FF with a high statistical power.
For our study we proposed a cutoff value of 50 % for loss of ambulation, as semiquantitative evaluations in orthopaedic patients indicate irreparable damage with a fat fraction above 50 % indicated by a Goutallier grade of 3 or above [21]. We calculated an average increase in muscular fat of about 5 % per year. This would mean that the time until loss of ambulation could be approximated as:
According to this calculation, a difference in fat content of 2 % should be detectable after 6 months. In addition it might be possible to calculate the increased time to loss of ambulation with novel therapies. If for example, FF with a novel therapy would increase by 1 % over a 6-month trial period (as compared to 2.5 % during the natural course), this would indicate that a 5-year old boy receiving this treatment could remain ambulatory until an age of 15 years.
However, greater variability between patients and the known variability in the clinical course indicate that age at disease onset, or muscular content at disease onset or the progression of fatty atrophy have some degree of inter-individual variability. Future trials should therefore be performed with repeated measurements on individual patients to assess the rate of increase in muscle fat content individually.
Using an individual progression rate, qMRI might also provide the necessary biomarker for small group or individual patient trials [22, 23], as it is noninvasive and has a high reproducibility.
Limitations
Our study has limitations, the major being the lack of a control group to evaluate change in fatty atrophy over time. Data from previous trials, however, demonstrate that the overall fat fraction in healthy volunteers is stable [15]. Another limitation arises from the relatively small number of patients included in our study. A further limitation lies in potential confounding effects of the two-point Dixon method. As only two measurements are used, field inhomogeneities and phase shifts might introduce a potential error in the calculated relative fat content. One possible solution is the use of a third measurement, the so-called three-point Dixon method, which was not available on our scanner at the time of this study. We used an implementation for the two-point Dixon technique that accounts for phase errors from magnetic field inhomogeneities. A potential issue of gradient echo-based two-point Dixon imaging is that T1 and T2* relaxation may cause inaccurate fat and water images [24], This leads to a potential overestimation of low FF and an underestimation of high FF by the two-point Dixon method.
In conclusion, we were able to correlate fat fractions in the major muscle groups of the thigh to age and clinical parameters of physical abilities in DMD patients using qMRI. This allowed an estimation of the natural course of the disease and estimation of the remaining time until loss of ambulation based on a single qMRI scan. This might be a helpful prognostic tool in the prediction of loss of ambulation and might also serve as an outcome measure in future therapeutic trials. A longitudinal study, however, should be performed to validate these findings.
Abbreviations
- DMD:
-
Duchenne muscular dystrophy
- F:
-
Fat
- FF:
-
Fat fraction
- MRI:
-
Magnetic resonance imaging
- MFM:
-
Motor function measurement
- qMRI:
-
Quantitative magnetic resonance imaging
- ROI:
-
Regions of interest
- TE:
-
Echo time
- TR:
-
Repetition time
- W:
-
Water
References
Manzur AY, Muntoni F (2009) Diagnosis and new treatments in muscular dystrophies. J Neurol Neurosurg Psychiatr 80:706–714. doi:10.1136/jnnp.2008.158329
Buyse GM, Goemans N, van den Hauwe M, Thijs D, de Groot IJ, Schara U, Ceulemans B, Meier T, Mertens L (2011) Idebenone as a novel, therapeutic approach for Duchenne muscular dystrophy: results from a 12 month, double-blind, randomized placebo-controlled trial. Neuromuscul Disord 21:396–405. doi:10.1016/j.nmd.2011.02.016
Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C, Guglieri M, Ashton E, Abbs S, Nihoyannopoulos P, Garralda ME, Rutherford M, McCulley C, Popplewell L, Graham IR, Dickson G, Wood MJ, Wells DJ, Wilton SD, Kole R, Straub V, Bushby K, Sewry C, Morgan JE, Muntoni F (2009) Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol 8:918–928. doi:10.1016/S1474-4422(09)70211-X
Vuillerot C, Girardot F, Payan C, Fermanian J, Iwaz J, De Lattre C, Berard C (2010) Monitoring changes and predicting loss of ambulation in Duchenne muscular dystrophy with the motor function measure. Dev Med Child Neurol 52:60–65. doi:10.1111/j.1469-8749.2009.03316.x
Pichiecchio A, Uggetti C, Egitto MG, Berardinelli A, Orcesi S, Gorni KO, Zanardi C, Tagliabue A (2002) Quantitative MR evaluation of body composition in patients with Duchenne muscular dystrophy. Eur Radiol 12:2704–2709. doi:10.1007/s00330-002-1392-4
Garrood P, Hollingsworth K, Eagle M, Aribisala BS, Birchall D, Bushby K, Straub V (2009) MR imaging in Duchenne muscular dystrophy: quantification of T1-weighted signal, contrast uptake, and the effects of exercise. J Magn Reson Imaging 30:1130–1138. doi:10.1002/jmri.21941
Huang Y, Majumdar S, Genant HK, Chan WP, Sharma KR, Yu P, Mynhier M, Miller RG (1994) Quantitative MR relaxometry study of muscle composition and function in Duchenne muscular dystrophy. J Magn Reson Imaging 4:59–64
Fischmann A, Gloor M, Fasler S, Haas T, Rodoni Wetzel R, Bieri O, Wetzel S, Heinimann K, Scheffler K, Fischer D (2011) Muscular involvement assessed by MRI correlates to motor function measurement values in oculopharyngeal muscular dystrophy. J Neurol 258:1333–1340. doi:10.1007/s00415-011-5937-9
Sinclair C, Morrow J, Fischmann A, Hanna M, Reilly M, Yousry T, Golay X, Thornton J (2011) Skeletal muscle MRI-determined fat fraction and myometric strength in inclusion body myositis and Charcot-Marie-Tooth disease Type 1A. Neuromuscul Disord 21:S5. doi:10.1016/S0960-8966(11)70014-1
Kinali M, Arechavala-Gomeza V, Cirak S, Glover A, Guglieri M, Feng L, Hollingsworth KG, Hunt D, Jungbluth H, Roper HP, Quinlivan RM, Gosalakkal JA, Jayawant S, Nadeau A, Hughes-Carre L, Manzur AY, Mercuri E, Morgan JE, Straub V, Bushby K, Sewry C, Rutherford M, Muntoni F (2011) Muscle histology vs MRI in Duchenne muscular dystrophy. Neurology 76:346–353. doi:10.1212/WNL.0b013e318208811f
Liu GC, Jong YJ, Chiang CH, Jaw TS (1993) Duchenne muscular dystrophy: MR grading system with functional correlation. Radiology 186:475–480
Bérard C, Payan C, Hodgkinson I, Fermanian J, Group MFMCS (2005) A motor function measure for neuromuscular diseases. Construction and validation study. Neuromuscul Disord 15:463–470
Dixon WT (1984) Simple proton spectroscopic imaging. Radiology 153:189–194
Sinclair C, Morrow J, Yousry T, Reilly M, Hanna M, Golay X, Thornton J (2010) Inter-scan reproducibility of quantitative neuromuscular MRI. Neuromuscul Disord 20:S28
Fischmann A, Hafner P, Fasler S, Gloor M, Bieri O, Studler U, Fischer D (2012) Quantitative MRI can detect subclinical disease progression in muscular dystrophy. J Neurol. doi:10.1007/s00415-011-6393-2
Fischmann A, Kaspar S, Reinhardt J, Gloor M, Stippich C, Fischer D (2012) Exercise might bias Skeletal-Muscle Fat Fraction Calculation from Dixon Images. Neuromuscul Disord 22. doi: 10.1016/j.nmd.2012.05.014
Kim HK, Laor T, Horn PS, Wong B (2010) Quantitative assessment of the T2 relaxation time of the gluteus muscles in children with Duchenne muscular dystrophy: a comparative study before and after steroid treatment. Korean J Radiol 11:304–311. doi:10.3348/kjr.2010.11.3.304
Kim HK, Laor T, Horn PS, Racadio JM, Wong B, Dardzinski BJ (2010) T2 mapping in Duchenne muscular dystrophy: distribution of disease activity and correlation with clinical assessments. Radiology 255:899–908. doi:10.1148/radiol.10091547
Janiczek RL, Gambarota G, Sinclair CD, Yousry TA, Thornton JS, Golay X, Newbould RD (2011) Simultaneous T(2) and lipid quantitation using IDEAL-CPMG. Magn Reson Med 66:1293–1302. doi:10.1002/mrm.22916
Gaeta M, Messina S, Mileto A, Vita GL, Ascenti G, Vinci S, Bottari A, Vita G, Settineri N, Bruschetta D, Racchiusa S, Minutoli F (2012) Muscle fat-fraction and mapping in Duchenne muscular dystrophy: evaluation of disease distribution and correlation with clinical assessments: preliminary experience. Skeletal Radiol 41:955–961. doi:10.1007/s00256-011-1301-5
Goutallier D, Postel JM, Bernageau J, Lavau L (1994) Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 304:78–83
Catlin N, Bettelheim K, Henderson I (2011) Individual patient (n = 1) “trials” in Duchenne dystrophy. Neuromuscul Disord 21:525–526. doi:10.1016/j.nmd.2011.05.003
Aartsma-Rus A (2011) The risks of therapeutic misconception and individual patient (n = 1) “trials” in rare diseases such as Duchenne dystrophy. Neuromuscul Disord 21:13–15. doi:10.1016/j.nmd.2010.09.012
Skinner TE, Glover GH (1997) An extended two-point Dixon algorithm for calculating separate water, fat, and B0 images. Magn Reson Med 37:628–630
Acknowledgments
D.F. was supported by a grant from the Lorenzo-Piaggio Foundation, Switzerland, and the University Children's Hospital, Basel. The sponsors had no influence on study design, data evaluation or publication. The Department of Radiology is supported by a grant from Bracco (Switzerland). The sponsor played no role in matters of design, collection, analysis, interpretation of data and writing of the report. We would like to thank Anthony Tyndall for his help proofreading the manuscript. We also would like to thank the reviewers, whose comments have improved the quality of the manuscript considerably.
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standard
This study has been approved by the appropriate ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Additional information
P. Hafner and A. Fischmann contributed equally to this work.
Rights and permissions
About this article
Cite this article
Fischmann, A., Hafner, P., Gloor, M. et al. Quantitative MRI and loss of free ambulation in Duchenne muscular dystrophy. J Neurol 260, 969–974 (2013). https://doi.org/10.1007/s00415-012-6733-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6733-x