Abstract
Background
Secretin—a hormone that stimulates pancreatic exocrine secretion—is described to improve visualization of the pancreatic duct by magnetic resonance cholangiopancreatography (MRCP). In our pediatric practice, however, we have not observed substantial benefit with the use of secretin.
Objective
To determine whether secretin dilates and improves visualization of the pancreatic duct in pediatric MRCP.
Materials and methods
Retrospective evaluation of secretin-enhanced MRCPs performed over a 15-month period. One reviewer measured the pancreatic duct pre- and post-secretin and two reviewers, blinded to the administration of secretin, assessed image quality and subjective duct visibility. Similar assessments of the biliary tree served as internal controls.
Results
We reviewed 20 MRCPs in 17 children. Following secretin administration, there was a small (0.3 mm) but statistically significant increase in pancreatic duct diameter (P = 0.002) and small (<0.2 mm) but significant increase in intrahepatic bile duct diameter (P = 0.0104). On subjective review, there was no significant difference in image quality or duct visibility based on the administration of secretin.
Conclusion
Secretin induces dilatation of the pancreatic duct but the value of that effect in pediatric MRCP is suspect given the small change in duct diameter and the lack of improvement in image quality and duct visibility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance cholangiopancreatography (MRCP) has gained favor as a non-invasive means of evaluating the pancreatic and biliary systems in both adults and children [1]. These heavily T2-weighted images allow visualization of small-caliber fluid-filled ductal structures of the liver and pancreas. The addition of secretin-enhanced MRCP sequences has been shown to improve visualization of the pancreatic duct and associated anatomic variation and pathology and also aids in the assessment of pancreatic exocrine function [2–9]. Although most of these data are based on studies in adults, studies in children have shown similar results [2, 6, 10].
Despite these reports, we have anecdotally noted that, in routine clinical use, secretin has little observable effect on pancreatic ductal diameter in our pediatric population and thus appears to add little incremental diagnostic value. Given that the administration of secretin adds approximately US $500 to the charge billed to the patient for a routine MRCP examination at our institution, adds logistical complexity (requiring nursing and pharmacy support) and has a potential risk of allergic or other adverse reaction, we set out to assess the effect of secretin administration on the pancreatic duct during MRCP.
We hypothesized that there would be no observable effect of secretin on the pancreatic duct in children. Specifically, there would be no objectively measurable dilatation of the pancreatic duct or subjectively improved visibility of the pancreatic duct following administration of secretin.
Materials and methods
Following approval by the Institutional Review Board at our tertiary care children’s hospital, we performed a HIPAA-compliant retrospective review of all secretin-enhanced MRCP examinations performed at our institution over a 15-month period (March 2010–June 2011). Eligible examinations were identified through a query of the hospital electronic medical record system. Patient examinations were included in this study if images were acquired according to our standard protocol (detailed below) with both pre- and post-secretin images. MRCP examinations in which secretin was not administered or in which only post-secretin images were acquired were excluded.
Imaging technique
All MR images were acquired on a 1.5-T HDx scanner equipped with eight receiver channels with a gradient performance of 50 mT/m (GE Healthcare, Waukesha, WI) using an eight-channel cardiac array coil. Children were sedated as needed, depending on the expected ability of the child to remain still and tolerate approximately 30 min of imaging. The field of view was adjusted to best fit the child’s size and stay within the minimum coil parameters. The imaging sequences were prescribed as shown (Table 1). Intravenous contrast was administered only when clinically indicated such as in the assessment of cholangitis or vascular anatomy. No oral contrast material was administered.
Secretin administration and post-secretin imaging
Secretin is administered in cases where it is requested by the referring physician and in cases where, after discussion between the referring physician and interpreting radiologist, either believes that the addition of secretin might improve detectability of pancreatic ductal anatomy or pathology.
After completing the pre-secretin sequences, a 0.2-μgm/kg IV dose of secretin (ChiRhoStim®; ChiRhoClin, Burtonsville, MD) up to a maximum of 16 μgm was administered slowly over approximately 1 min. Post-secretin images were then acquired immediately following secretin administration.
Dynamic post-secretin images were not obtained, as the referring clinicians at our institution use MRCP for assessment of duct structural anatomy and pathology and not as a means to assess pancreatic exocrine function.
Objective image review
All MRCP examinations were reviewed in their clinical form by a single reviewer (A.T.T.) with direct comparison of the pre- and post-secretin MRCP images. Duct diameters were measured in the axial plane perpendicular to the long axis of the duct at the same location on the pre- and post-secretin 3-D fast spin-echo images (Fig. 1). Duct measurements were obtained at the following locations: pancreatic head, pancreatic body, pancreatic tail, common bile duct just above the level of the pancreatic head, and right and left intrahepatic ducts within 1 cm of their confluence. Spatial resolution was calculated based on imaging parameters employed in the 3-D fast spin-echo images. Duodenal fluid content was also quantified prior to and following secretin administration according to the system described by Matos et al. [7]. This system grades duodenal fluid content relative to anatomical landmarks, assigning a grade of 0 to no fluid in the duodenum, grade 1 to fluid confined to the duodenal bulb, grade 2 to fluid within the bulb and partially filling the duodenum to the level of the genu inferius, and grade 3 to filling of the duodenum beyond the level of the genu inferius [7].
Magnetic resonance cholangiopancreatography (MRCP) in a 10-year-old boy with familial pancreatitis. Coned down image from axial 3-D FSE MRCP sequence shows measurement of the pancreatic duct at the level of the pancreatic tail. Prominent side branch ducts are present in the pancreatic tail, consistent with the history of a chronic form of pancreatitis
Subjective image review
Following objective review, all images were de-identified and the T1, T2 and balanced steady-state free-precession (bSSFP) images were discarded. The remaining pre- and post-secretin MRCP images for each case were separated into two distinct studies and the separated image files were ordered randomly. Two additional reviewers (A.J.T. and D.J.P., both pediatric abdominal imagers) independently reviewed the de-identified, randomized images blinded to patient information, imaging data, and secretin administration status. They subjectively assessed the overall image quality and the visibility of the same duct segments as measured in the objective portion of the study. Overall image quality was graded as poor, sufficient, good, or excellent. All ducts were classified as not visible, partially visible or visible. The impact of secretin administration on overall image interpretation was not assessed.
Clinical review
Patient medical records were reviewed for demographic information and clinical history. Records were also reviewed to identify children with histories or imaging indications suggestive of chronic pancreatitis, because chronic pancreatitis is known to dampen the dilatory effect of secretin on the pancreatic ductal system and to be associated with decreased exocrine function of the pancreas [11].
Statistical analysis
Changes in pancreatic duct segments (both objective and subjective) were evaluated individually for each segment and as a composite of the segments. Analysis of the intrahepatic biliary ducts was based on a composite of the right and left ducts (diameter of right intrahepatic duct + diameter of left intrahepatic duct). Subanalyses of children with histories suggestive or diagnostic of chronic pancreatitis were performed to assess for a confounding effect by disease. Comparison of pre- and post-values for each duct segment and composite segments were examined using a paired t-test for normally distributed outcomes and signed rank test for skewed outcomes. Agreement between raters on image quality was assessed using kappa statistics. Results were considered statistically significant if P < 0.05.
Results
A total of 20 secretin-enhanced MRCP examinations in 17 children were reviewed with indications for the examinations detailed in Table 2. The mean (± SD) patient age at the time of the examination was 13.3 ± 5.3 years and ten of the children were boys. Twelve (12/20; 60%) examinations were performed in children who carried a clinical diagnosis of recurrent pancreatitis, chronic pancreatitis or hereditary pancreatitis. Pathological proof of chronic pancreatitis was not available in any of these cases.
Objective image review
After the administration of secretin, there was a small (0.3-mm maximum, <20% duct diameter) but statistically significant increase in pancreatic duct diameter (P = 0.002 for composite pancreatic duct) (Table 3). Example pre- and post-secretin images are shown in Fig. 2. There was no significant increase in diameter of the common bile duct (P = 0.3661) after secretin administration but there was a significant increase in the composite diameter of the intrahepatic ducts (mean summed increase = 0.355 ± 0.129 mm, P = 0.0104).
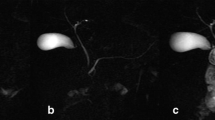
MRCP images in a 17-year-old girl with Crohn disease. a Coned down image from axial 3-D FSE MRCP sequence prior to secretin administration. Pancreatic duct (arrow) measured 2.1 mm. b Coned down image from axial 3-D FSE MRCP sequence after secretin administration. Pancreatic duct (arrow) measured 2.4 mm. c, d Coned down images from coronal 2-D SSFSE sequence prior to (c) and following (d) secretin administration with arrows indicating approximately the same duct segment seen in (a) and (b). Note that, although the duct is larger in caliber on the post-secretin image in both the coronal and axial planes, the same duct segment is visible both pre- and post-secretin
Calculated spatial resolution for the 3-D fast spin-echo images range 0.94–1.4 mm with a mean of 1.2 mm.
Duodenal fluid content increased significantly following secretin administration (P = 0.0001) with median filling scores of 1 (interquartile range = 0.5–2) pre-secretin and 2 (interquartile range = 2–3) post-secretin [7].
Among the subgroup of children with chronic pancreatitis, a significant increase in duodenal fluid content was observed (P = 0.0078) similar to the overall population. Ductal changes were also similar in this subgroup of children, with a significant increase in composite pancreatic duct diameter (P = 0.0214) and in composite intrahepatic duct diameter (P = 0.0039) following secretin administration. The mean change in pancreatic and biliary duct diameter following secretin administration was not significantly different between the subgroups of children with and without chronic pancreatitis (P = 0.9229 pancreatic, P = 0.5805 biliary).
Subjective image review
Inter-rater agreement on image quality was limited with poor agreement (κ = 0.0345) on the quality of the pre-secretin MRCP images and only fair agreement (κ = 0.2131) on the quality of the post-secretin images. However, within each observer, there was no significant difference in image quality based on the administration of secretin (Table 4).
Inter-rater agreement on the subjective visibility of duct segments was slightly better. Specifically, there was moderate agreement on the visibility of pancreatic duct segments both pre- and post-secretin (κ = 0.4576 and κ = 0.5302 respectively), substantial agreement on visibility of the common bile duct pre-secretin (κ = 0.6694), moderate agreement on common bile duct visibility post-secretin (κ = 0.5082), and perfect agreement (κ = 1 for both) on visibility of the intrahepatic ducts pre- and post-secretin. Although more duct segments were visible following secretin administration (Table 5), overall subjective segmental duct visibility was not significantly different based on the administration of secretin (Table 6).
Discussion
Magnetic resonance cholangiopancreatography is a technique that has many potential advantages in the pediatric population. This non-invasive modality allows assessment of pancreatic and biliary ductal anatomy without direct instrumentation and the associated technical challenges, cost and risks. The addition of secretin to pediatric MRCP imaging protocols can theoretically improve the diagnostic value of this technique by increasing visibility of the pancreatic ducts. The mechanism by which secretin is stated to have this effect is through ductal dilatation resulting from a combination of increased exocrine secretion by the pancreas and changes in tone at the sphincter of Oddi [12–15].
Studies in adults have demonstrated both objective and subjective improvements in evaluation of the pancreatic duct following secretin administration. Objective increases in the diameter of the pancreatic duct are reported to be in the range of 0.5–1.2 mm following secretin administration [7, 12, 16]. Reported subjective improvements following secretin administration have included improvements in overall image quality as well as improved visualization of the pancreatic duct either in its entirety or at specific segments [4, 7–9, 16]. This improved visualization of the pancreatic duct has been described to enhance detection of duct pathology and anatomic variation including duct disruption, duct stricture, pancreas divisum and anomalous pancreaticobiliary junction [5, 8].
Based upon the reported benefit in adults, secretin has also been used in MRCP in children. To date, three studies have looked specifically at the diagnostic value of secretin in pediatric MRCP. The first study prospectively evaluated three secretin-enhanced MRCP examinations as part of a larger series and described a subjective improvement in the conspicuity of the pancreatic duct in 2/3 of the patients following secretin administration [2]. The second study of 15 children reported a similar subjective improvement in visualization of the pancreatic duct as well as an objective increase in the mean ductal diameter of the pancreatic duct at three locations: pancreatic head (1.2-mm increase), pancreatic body (1-mm increase) and pancreatic tail (0.9-mm increase) [6]. In the most recent pediatric secretin MRCP study, Delaney et al. [10] reviewed 41 secretin-enhanced MRCPs and reported a significant improvement in the visibility of the pancreatic duct as well as an increase in the mean duct diameter of 0.6 mm in the pancreatic head, 0.4 mm in the pancreatic body, and 0.5 mm in the pancreatic tail following secretin administration.
Our data differ from the findings of these studies. In the 20 MRCP examinations we reviewed, neither image quality nor subjective visibility of the pancreatic duct (or duct segments) was improved significantly with the administration of secretin. Objectively, there was a statistically significant increase in pancreatic duct diameter, but the actual increase in duct diameter was only 0.3 mm throughout the length of the pancreatic duct, less than the changes described in other papers.
There are several possible reasons for our discrepant results. First, at our institution, post-secretin MRCP imaging consists primarily of static 3-D slab FSE images oriented in the axial plane with an image acquisition time of approximately 400 seconds. These are acquired immediately following secretin administration. We do not perform dynamic imaging of the pancreatic duct as described in previous studies in the pediatric literature [2, 6, 10]. It is possible that by imaging in this manner we are temporally missing the maximal effect of secretin on the pancreatic duct or that the time required to acquire this sequence results in signal averaging that partly masks the duct dilatation. Unfortunately because of the retrospective nature of this study and the limited data available in the medical record we cannot assess this temporal question. That being said, the plasma half-life of secretin is in the range of 3–5 min, which means that the effect of the drug on the pancreatic duct should persist through the time required to acquire this sequence. Moreover, the significant increase in duodenal fluid content observed on the post-secretin images in our population suggests that the drug is having its desired effect and that we are imaging during that effect and should be seeing changes in the pancreatic duct diameter.
Heterogeneity in patient population might also influence the results of our study. In adults, data in the literature suggest that ductal dilatation might not be seen following secretin administration in patients with chronic pancreatitis because of decreased exocrine function of the gland and fibrosis of the duct [11]. If this effect were at play in the substantial proportion of our population with clinically diagnosed chronic pancreatitis, then that might explain the lack of observed effect on ductal diameter. However, this does not appear to be the case in our study because subanalysis of children with clinically diagnosed chronic pancreatitis showed no significant difference in ductal change following secretin administration from the other study patients. Moreover, the observed increase in duodenal fluid after secretin administration in these children suggests a lack of substantial exocrine insufficiency related to pancreatitis that might mask the medication effect and skew the study findings. The importance of these results is in demonstrating that an abnormal response to secretin in patients with chronic pancreatitis is not masking the effect of secretin in the remainder of the population but these results also raise the question of the severity of chronic pancreatitis in these children.
Differences in study design might be the most important possible explanation for our findings. Our study represents the first reported blinded assessment of the effect of secretin on pancreatic duct visibility in pediatric MRCP. In our study, the subjective reviews were performed in a blinded, randomized fashion without direct side-by-side comparison of pre- and post-secretin images. Previous studies have looked at the pre- and post-secretin images together, perhaps introducing bias, a possibility recognized in those publications [10]. This effect is apparent when one reviews the paired pre- and post-secretin images in Fig. 2. In addition to the objective dilatation of the pancreatic duct, the duct is clearly larger in caliber and more easily seen on the post-secretin images. However, the duct over this segment was fully visible on the pre-secretin images and both images would have been scored equally (duct = visible) when reviewed in a blinded fashion.
Whatever the reason(s) for our findings, they raise questions about the added diagnostic value of secretin in pediatric MRCP, particularly given its added cost. It is entirely possible that children are different from adults in some way that limits the effect/value of secretin. Previous studies have acknowledged that pediatric MRCP as a whole is more difficult than adult MRCP because of the small caliber of pediatric pancreatic and biliary ductal structures [1].
In addition to enlargement of the pancreatic duct, there was a statistically significant increase in diameter of the main right and left intrahepatic ducts. Most reports of secretin-enhanced MRCP have not reported a change in bile duct diameter following secretin administration. There are two ways to look at this finding: first, it is possible that the less-than-0.2-mm dilatation of the right and left biliary ducts reflects measurement error. If this is the case, it casts doubt on the significance of the 0.3-mm dilatation of the pancreatic duct. The other possible explanation is that this effect reflects pathology or a normal physiological process. Some authors view biliary duct dilation or increased fluid signal in the biliary tree following secretin administration as a pathological finding related to reflux of pancreatic secretions into the biliary tree [17]. However, secretin is known to stimulate secretion of bicarbonate-rich fluid from the biliary epithelium, perhaps accounting for the observed biliary ductal dilatation [18, 19]. Whatever the explanation for this finding, it is clear that further research into secretin-enhanced MRCP in children is needed.
This study has several limitations, the most important of which is its retrospective design. A well-designed, blinded prospective study is needed to adequately evaluate the diagnostic value of secretin in children. One effect of the retrospective design of this study is that we are limited to evaluating images obtained according to our clinical protocol, which might not adequately capture the peak effect of secretin on the pancreatic duct. If assessing the maximal effect of secretin on the pancreatic duct is of diagnostic value, dynamic post-secretin imaging might better serve this purpose. To this point, however, clinicians at our institution are more interested in ductal anatomy, which is better defined by high-resolution 3-D images than in dynamic exocrine functional information. Small sample size is also a limitation of this study. It is possible that the observed lack of statistical significance for some of the analyses is a function of the small number of children in the population (limited statistical power). Measurement constraints are a further limitation that applies not only to this study but also to those previously reported in the literature. Measurements are limited by pixel size, which is determined by the image matrix. Differences in the range of 0.3 mm, as seen in our study, are below the resolution of the acquired images (mean resolution = 1.2 mm) and therefore within the range of measurement error. Additionally, although duct measurements were obtained in a consistent fashion throughout the study, the measurements were performed in the axial plane rather than in the true short axis and might not represent the true cross-sectional diameter of the duct. Finally, this study focused on the effect of secretin on duct diameter and visibility. We did not assess whether there was value related to secretin administration in terms of assessing pathology or providing a clinical diagnosis. While changes in visibility of the pancreatic duct would be expected to be correlated with or predictive of interpretive benefit, this was not assessed directly.
Conclusion
Based upon the results of this study, we conclude that, although secretin does result in minimal dilatation of the pancreatic duct when used in pediatric MRCP, the effect that dilatation has on visualization and assessment of the pancreatic duct is suspect. The observed dilatation is small (mean of 0.3 mm, <20% duct diameter) and there is no significant corresponding improvement in overall image quality or subjective visibility of the pancreatic duct. We acknowledge that these findings differ from those of prior studies and that further research is needed to assess the possible clinical value of secretin-enhanced MRCP. As a result of this study we are carefully assessing the clinical indications for use of secretin at our institution. In future cases where secretin is administered we might alter our imaging protocol to include dynamic post-secretin imaging of the pancreatic duct and plan to continue to assess the effect of secretin on duct diameter and visibility.
References
Chavhan GB, Babyn PS, Manson D et al (2008) Pediatric MR cholangiopancreatography: principles, technique, and clinical applications. Radiographics 28:1951–1962
Arcement CM, Meza MP, Arumanla S et al (2001) MRCP in the evaluation of pancreaticobiliary disease in children. Pediatr Radiol 31:92–97
Hosoki T, Hasuike Y, Takeda Y et al (2004) Visualization of pancreaticobiliary reflux in anomalous pancreaticobiliary junction by secretin-stimulated dynamic magnetic resonance cholangiopancreatography. Acta Radiol 45:375–382
Manfredi R, Costamagna G, Brizi MG et al (2000) Severe chronic pancreatitis versus suspected pancreatic disease: dynamic MR cholangiopancreatography after secretin stimulation. Radiology 214:849–855
Manfredi R, Costamagna G, Brizi MG et al (2000) Pancreas divisum and “santorinicele”: diagnosis with dynamic MR cholangiopancreatography with secretin stimulation. Radiology 217:403–408
Manfredi R, Lucidi V, Gui B et al (2002) Idiopathic chronic pancreatitis in children: MR cholangiopancreatography after secretin administration. Radiology 224:675–682
Matos C, Metens T, Deviere J et al (1997) Pancreatic duct: morphologic and functional evaluation with dynamic MR pancreatography after secretin stimulation. Radiology 203:435–441
Matos C, Metens T, Deviere J et al (2001) Pancreas divisum: evaluation with secretin-enhanced magnetic resonance cholangiopancreatography. Gastrointest Endosc 53:728–733
Nicaise N, Pellet O, Metens T et al (1998) Magnetic resonance cholangiopancreatography: interest of IV secretin administration in the evaluation of pancreatic ducts. Eur Radiol 8:16–22
Delaney L, Applegate KE, Karmazyn B et al (2008) MR cholangiopancreatography in children: feasibility, safety, and initial experience. Pediatr Radiol 38:64–75
Sanyal R, Stevens T, Novak E et al (2012) Secretin-enhanced MRCP: review of technique and application with proposal for quantification of exocrine function. AJR 198:124–132
Cappeliez O, Delhaye M, Deviere J et al (2000) Chronic pancreatitis: evaluation of pancreatic exocrine function with MR pancreatography after secretin stimulation. Radiology 215:358–364
Akisik MF, Sandrasegaran K, Aisen AA et al (2006) Dynamic secretin-enhanced MR cholangiopancreatography. Radiographics 26:665–677
Geenen JE, Hogan WJ, Dodds WJ et al (1980) Intraluminal pressure recording from the human sphincter of Oddi. Gastroenterology 78:317–324
Laugier R (1994) Dynamic endoscopic manometry of the response to secretin in patients with chronic pancreatitis. Endoscopy 26:222–227
Hellerhoff KJ, Helmberger H 3rd, Rosch T et al (2002) Dynamic MR pancreatography after secretin administration: image quality and diagnostic accuracy. AJR 179:121–129
Sai JK, Ariyama J, Suyama M et al (2002) Occult regurgitation of pancreatic juice into the biliary tract: diagnosis with secretin injection magnetic resonance cholangiopancreatography. Gastrointest Endosc 56:929–932
Song HK, Kim MH, Lee SK et al (2003) Progressive bile duct and gallbladder dilation on MRCP after secretin stimulation: physiologic or pathologic finding? Gastrointest Endosc 58:165, author reply 165–166
Alvaro D, Gigliozzi A, Fraioli F et al (1997) Hormonal regulation of bicarbonate secretion in the biliary epithelium. Yale J Biol Med 70:417–426
Acknowledgments
This publication was supported by an Institutional Clinical and Translational Science Award, NIH/NCATS grant number 8UL1TR000077-04. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trout, A.T., Podberesky, D.J., Serai, S.D. et al. Does secretin add value in pediatric magnetic resonance cholangiopancreatography?. Pediatr Radiol 43, 479–486 (2013). https://doi.org/10.1007/s00247-012-2561-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-012-2561-5