Abstract
Chronic liver diseases are rare in children, but encompass a wide spectrum of disorders that may all be complicated by liver fibrosis and therefore by portal hypertension. They may be classified according to the level of portal flow obstruction: prehepatic, intrahepatic or suprahepatic. Most of them, except presinusoidal diseases, may progress to cirrhosis that carries additional risks of impaired liver function and development of hepatocellular carcinoma. Imaging plays an important role in guiding the diagnosis and biopsy and for follow-up during treatment. US, with high-frequency transducers and Doppler, is the first modality of choice, directs the rest of the investigations and guides interventional radiology. MDCT has made great progress and has replaced angiography for diagnostic purposes. MRI is indicated for parenchyma and nodule characterization and for biliary tract evaluation. To avoid liver biopsy, several elasticity imaging techniques have been developed and have to be evaluated for accuracy and convenience in children. The role of each modality with main imaging findings is described in extrahepatic portal vein obstruction, hepatoportal sclerosis, congenital hepatic fibrosis, cirrhosis and Budd-Chiari syndrome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic liver diseases are a rare occurrence in children but encompass a wide spectrum of disorders, including malformations, viral, metabolic, genetic, drug-induced, vascular and autoimmune diseases. All these diseases may be complicated by liver fibrosis and therefore by portal hypertension and can be classified according to the level of portal flow obstruction: prehepatic, intrahepatic or suprahepatic (Table 1) [1, 2]. Most of them, except classically prehepatic and presinusoidal intrahepatic diseases, may progress to cirrhosis that is defined as the histological development of regenerative nodules surrounded by fibrous bands, in response to chronic liver injury. As a consequence, cirrhosis carries additional risks of impaired liver function and development of hepatocellular carcinoma (HCC) [3].
Chronic liver diseases may present with hepatomegaly or splenomegaly on clinical examination, abnormal biological findings such as elevated liver enzymes or abnormal coagulation tests, or may have an acute presentation with acute liver failure, ascites, gastrointestinal bleeding or jaundice. They may also present with cardiopulmonary complications such as pulmonary arteriovenous shunting and pulmonary hypertension and spontaneous bacterial peritonitis or with hepatic encephalopathy (rare in childhood). The clinical presentation and initial laboratory data in many of these diseases are similar and a definitive diagnosis often relies on specialized laboratory investigation and histological examination of liver tissue by means of liver biopsy. Imaging however plays an important role to direct the diagnosis, guide the biopsy and for follow-up after treatment, especially for decision making of palliative surgical procedure or liver transplantation.
In this review, we would like first to provide general principles about the indications and imaging techniques for diagnosis and follow-up of chronic liver diseases in children (including image-guided biopsy), Secondly to describe the role of each modality with the main imaging findings in extrahepatic portal vein obstruction, hepatoportal sclerosis, congenital hepatic fibrosis (CHF), cirrhosis and Budd-Chiari syndrome. Finally we will address the question of the assessment of liver fibrosis with the advent of recent elasticity imaging techniques.
Imaging techniques in pediatric chronic liver diseases
US
US with high resolution transducers and colour Doppler remains the first modality of choice and can direct subsequent investigations. US examination of the liver should follow a standardized approach in order to be reproducible and objective for communication with experts. Every US examination should include sonogram of the hepatic pedicle, portal bifurcation and confluence of the hepatic veins, the gallbladder, inferior vena cava (IVC), longitudinal sonogram through the aorta and measurement of the spleen. An examination of the whole abdomen should also be performed including evaluation of the kidneys, and the pancreas.
Choice of US transducer should be adapted not only to the age of the child, but also to the size and beam attenuation of the liver. However, after global evaluation of the whole liver with a curved-array transducer, use of a high-frequency linear-array transducer is recommended for further analysis of the echogenicity of the parenchyma (Fig. 1).
Value of high-frequency transducer. Twelve-year-old boy presenting with acute liver failure and haemolytic anemia. US examination showed findings of portal hypertension with thickening of the lesser omentum and splenomegaly, normal portal vein and hepatic veins. The liver parenchyma appears fairly homogeneous with a curved array transducer (a) but with a linear high-frequency transducer there are multiple nodules (b). The diagnosis of Wilson disease was confirmed by laboratory investigation
Colour Doppler and spectral waveform analysis allow haemodynamic assessment of the hepatic vessels and provide rapid information that may not be evident on more complex imaging techniques such as multi-detector (MD) CT or MRI especially in young children [4].
A positive diagnosis of portal hypertension relies on US demonstration of hepatofugal veins that represent portosystemic communication and can be seen outside the liver in the thickened lesser omentum, at the splenic hilum, between the spleen and the left kidney or inside the liver as the paraumbilical vein or ductus venosus [5]. Presence of intrahepatic hepatofugal collaterals indicates that the level of portal obstruction is intra- or suprahepatic but not prehepatic. On the contrary hepatopetal collaterals with or without portal vein thrombosis are usually present in prehepatic and presinusoidal blocks but not in cirrhosis or suprahepatic blocks. Therefore, on a carefully performed US with evaluation of all hepatic vessels, biliary tract and parenchyma, the aetiology of portal hypertension can be suggested.
MDCT
There has been recent progress in CT with the advent of MDCT that provides great improvement in scan speed, spatial resolution and reconstruction algorithms, allowing (even in children) rapid, high resolution and multiplanar imaging [6]. However a risk-benefit analysis is mandatory to avoid increase in radiation dose, especially in children with chronic disease. Our dose reduction strategy includes reviewing any outside examinations by importing the CD with DICOM data onto our workstation, discussing the indications of every MDCT and tailoring a protocol for every examination with the ALARA principles: the use of multiphase examination has to be limited; the phase without contrast can be avoided in most cases; an arterial phase has to be performed in pretransplantation work-up and for diagnosis of hypervascular nodules but in most of the preoperative indications only a portal phase is needed; the field of view has to be collimated to the area of interest; and the dose has to be adapted to the child’s weight. Our present parameters for abdominal MDCT are listed in Table 2.
Sedation and fasting are no longer necessary in most of the cases and we perform intravenous injection of 2 ml/kg of low osmolar contrast medium with about 300 mg/ml iodine concentration. The injection rate is 1–2 ml/s and is automatic whenever possible. The acquisition should be performed with very thin sections between 0.5 and 1.5 mm to allow optimal multiplanar reconstructions and maximum intensity projection images.
The main indications of MDCT include assessment of hepatic vasculature before surgical shunt, creation of a transjugular intrahepatic portosystemic shunt (TIPS) and liver transplantation (Fig. 2). Reporting at a workstation with the surgeon is recommended and the most useful reconstructions are generally the coronal plane for the portal and superior mesenteric vein and hepatopetal collaterals and the axial plan for the splenic vein. Although MR angiography (MRA) is a valid alternative because it involves no ionizing radiation, it requires sedation and monitoring in young children and provides less spatial resolution than MDCT for vascular work-up. Therefore in our experience, MDCT has replaced conventional angiography for diagnostic purposes in most cases of chronic liver diseases.
MDCT evaluation before liver transplantation. Examination performed without sedation in a 4-month-old infant presenting with decompensated cirrhosis due to biliary atresia, allowing complete work-up of the hepatic vasculature. The arterial phase (a) shows an enlarged modal hepatic artery (arrow) arising from the celiac trunk. On the portal phase (b), the portal vein is very hypoplastic (arrow) but the spleno-mesenteric confluence is patent. There is also evidence (c) of a large spontaneous splenorenal shunt (*) that was closed surgically during transplantation
MRI
MRI has emerged as an important imaging modality for assessing liver parenchyma and the biliary tract because of its ability to provide a nuanced depiction of different tissues properties and to evaluate the bile ducts without contrast medium injection and independently of liver function. It requires sedation in children younger than 5 or 6 years of age, but the introduction of faster sequences with respiratory compensation technique has allowed quality imaging of the entire liver with high intrinsic soft-tissue contrast. The main indications in chronic liver disease are evaluation of fatty infiltration, iron content of the liver, characterization of hepatic nodules and assessment of the biliary tract.
Our protocol of investigation of chronic liver disease includes axial T2- and T1-weighted sequences with and without fat suppression and dual gradient-recalled-echo sequences with out-of-phase and in-phase image acquisitions that are very helpful for assessing diffuse or intralesional steatosis (Fig. 3). After intravenous gadolinium injection dynamic multiphasic three-dimensional fat-suppressed sequences provide a good analysis of nodule vascularity. However, it has to be stressed that gadolinium injection is contraindicated in cases of renal failure that may be associated with chronic liver disease in the so-called hepatorenal syndrome because of the risk of systemic nephrogenic fibrosis [7]. To visualise findings of sclerosing cholangitis or supportive features of CHF we routinely use both 2-D single-shot fast spin-echo and 3-D fast spin-echo cholangiopancreatography sequences that yield variable results and complementary findings [8]. In addition, coronal and axial balanced steady-state sequences are fast to acquire and display an overview of the hepatic vessels, portal vein system and bile ducts [9].
Focal steatosis demonstrated by MRI. Fifteen-year-old boy treated for autoimmune hepatitis since 3 years of age with US detection of a nodule in segment 5 of the liver and subsequent characterization with MRI. T1-W images in- (a) and out- (b) of-phase and T2-W image (c) with fat suppression (d). There is striking loss of signal intensity in the images with fat suppression (b and d) allowing the diagnosis to be made
Image-guided liver biopsy
The main indication for liver biopsy in chronic liver disease in children is for diagnosis prior to treatment, when the cause of the disorder is not established by characteristic laboratory or imaging findings. Moreover it may have to be performed in an emergency to rule out contraindications for liver transplantation in cases that present with acute hepatic failure. It may also be indicated to evaluate progression of fibrosis under treatment and if there is suspicion of HCC.
Image guidance of the biopsy may be required for a focal lesion or to avoid large vessel puncture in cases of dysmorphic or reduced transplanted liver. In our experience, in nearly all cases, liver biopsy can be accurately performed in children under US guidance and this technique is preferred to CT guidance whenever possible because of its ease and absence of radiation for the child and the radiologist.
Indications for transjugular liver biopsy include severe or uncorrectable coagulopathy, ascites or both. Thanks to the use of US guidance for internal jugular vein access and also for the real-time control of the trajectory of the automated core biopsy needle, transjugular liver biopsy has become a safe procedure and can be performed even in infants (Fig. 4) [10]. The main difficulty of the technique is the placement of a large 9 French introducer into the internal jugular vein of an infant with coagulation disorder. Micropuncture and progressive dilatation are recommended.
US guidance of a transjugular liver biopsy performed in a 4-month-old infant presenting with hepatic failure and ascites. The trajectory of the automatic core biopsy needle is easily visible and can be followed (arrows) making the procedure safer. The diagnosis of respiratory chain disorder was made
An alternative to the transjugular liver biopsy technique is the coaxial percutaneous biopsy with track embolization, which may be used in cases of mild coagulation disorder and when a focal lesion is targeted [11].
Main paediatric chronic liver diseases
Extrahepatic portal vein obstruction (EHPVO)
Also known as portal cavernoma or cavernomatous transformation of the portal vein, EHPVO is the second most frequent cause of portal hypertension in children, cirrhosis being the leading cause. Predisposing factors include umbilical venous catheterization with or without concurrent infection, omphalitis, abdominal sepsis such as liver abscesses or appendicitis, abdominal surgery, malignancies, splenectomy, sickle cell anemia and hypercoagulable states [12]. In very rare instances, thrombosis is discovered in the acute phase with imaging displaying an enlarged portal vein partly or completely filled with echogenic clots. However, in most cases the aetiology remains unknown and the main presenting signs are splenomegaly, gastrointestinal bleeding and more rarely ascites. The liver is functionally normal in most cases and does not exhibit cirrhosis or major fibrosis histologically [1].
On US examination the liver appears normal-size or small. The normal portal vein and branches are not visible and are replaced by multiple tortuous collateral veins with hepatopetal flow, the so-called cavernomatous transformation of the portal vein (Fig. 5). In some cases, a large collateral vein may simulate a normal portal vein but the clues to the diagnosis are the tortuosity of the vein and presence of surrounding small channels. Hepatofugal collaterals may be displayed in the lesser omentum which appears thickened on a longitudinal scan through the aorta and also in the vicinity of the enlarged spleen. In rare instances and along with biological cholestasis, there may be associated intrahepatic bile duct dilatation, only identified with colour Doppler and due to compression of the common bile duct by the cavernoma [13].
Imaging of EHPVO. Five-year-old girl presenting with massive upper gastrointestinal bleeding and splenomegaly on physical examination. US shows multiple tortuous veins replacing the portal vein (a) and also the intrahepatic portal vein confluence (b) (cavernoma), allowing the diagnosis of portal vein obstruction. MDCT is indicated as preoperative work-up of the superior mesenteric and splenic vein which are well seen and patent on this coronal oblique maximum intensity projection (MIP) image of the portal phase (c). Note in the axial plane (d) that the visibility of the portal vein confluence is hidden by the cavernoma. The wedge hepatic venography (e) with the catheter in the middle hepatic vein shows opacification of left and right portal vein branches that are thin but patent. A Rex shunt joining the splenic vein and the left portal vein branch via a venous graft was performed and the control US (f) shows good flow in the graft (in red) and normal inverted flow in the left portal branch (in blue). The portal confluence has returned to normal on US (g) 3 months later
The outcome of children with EHPVO depends on the control of variceal bleeding. The risk of bleeding is best evaluated by means of endoscopy and endoscopic treatment by sclerotherapy and/or band ligation can be performed at the same time. There has been a recent advance in the surgical treatment of EHPVO with the option of portal reperfusion by a Rex shunt joining an extrahepatic portal vein (splenic, superior mesenteric or collateral vein) to the intrahepatic left portal vein which is surgically accessible at the level of the Rex recess or umbilical segment [14]. The Rex shunt, which relieves portal hypertension and restores hepatopetal flow to the liver, also avoids the risk of pulmonary hypertension, hepatopulmonary syndrome, liver nodules and hepatic encephalopathy that have been reported as side-effects of porto-systemic shunts [15].
The preoperative work-up of children with EHPVO includes accurate evaluation of both sides of the shunt. The inflow that are the superior mesenteric and the splenic veins may be difficult to assess on US because of the small size of the liver and interposed intestinal gas. MDCT, in our opinion, is the best modality to display the complete portal vein system in the portal phase of intravenous contrast injection (Fig. 5). The intrahepatic portal veins are in most cases difficult to assess on US, CT or MRI because they are often hypoplastic and hidden by the portal cavernoma. They can be clearly identified on wedged hepatic venography (performed under general anaesthesia via an internal jugular vein approach) that provides retrograde opacification of the intrahepatic portal veins through the hepatic sinusoids (Fig. 5) [16]. Peroperative Doppler US can assess the patency of the shunt, showing reversed flow in the left portal vein toward the right portal vein. Very rapidly, within a few days, there is widening of the portal vein branches, decrease in the portal cavernoma and restoration of normal intrahepatic portal vein anatomy (Fig. 5).
Hepatoportal sclerosis (HPS)
HPS, also referred to as idiopathic portal hypertension, non-cirrhotic portal fibrosis, obliterative portal venopathy, Mikkelsen syndrome, incomplete septal cirrhosis, and nodular regenerative hyperplasia is diagnosed histologically [17]. It is characterized by periportal fibrosis and obliteration of small- or medium-size portal vein branches and a variable degree of nodular hyperplasia [18]. Major presenting findings are splenomegaly and pancytopenia. Liver function tests are usually slightly disturbed and complications include mainly gastrointestinal bleeding and rarely hepatopulmonary syndrome. Familial cases have been reported, as well as the association with Turner syndrome, Noonan syndrome, Adams-Oliver syndrome and also chemotherapy [19]. In half of the cases predisposing factors to thrombosis may be found.
US may be suggestive of the diagnosis when it shows hyperechoic bands along the portal tracks surrounded by hypoechoic stripes (Fig. 6) [20]. However, this finding is not always present and may be visible in other conditions with hypoplasia or thrombosis of small intrahepatic portal vein branches such as congenital portocaval fistula, CHF or biliary cirrhosis. In a few cases the presentation may be misleading with nodular appearance of the whole liver (Fig. 6). Portal vein thrombosis may complicate the course of the disease.
Imaging of hepatoportal sclerosis. Hepatic US (a) of a 7-year-old boy with Adams-Oliver syndrome (scalp and skull defects and abnormalities of terminal limbs) and portal hypertension. There are hyperechoic bands along the portal tracts (a) surrounded by hypoechoic stripes (arrows) suggestive of HPS, confirmed on liver biopsy. Transverse hepatic sonogram (b) through the portal confluence in a 6-month-old boy presenting with urinary tract infection and thrombocytopenia. Multiple hypoechoic nodules are well seen, without any sign of hypervascularity on colour Doppler and CT. The diagnosis of HPS was made later on, on liver biopsy. The father has the same disease. On a follow-up US at the age of 2 years (c) the nodules are less visible but the portal tracks are hyperechoic
The diagnosis is made on liver biopsy which often has to be performed via a transjugular approach because of the associated thrombocytopenia.
Therapeutic options are endoscopic treatment of variceal bleeding or surgical porto-systemic shunt or TIPS. Liver transplantation is indicated only in cases of associated hepatopulmonary syndrome. MDCT angiography in the portal phase has to be performed when a surgical or radiological shunt is considered.
CHF
CHF is a rare autosomal-recessive disease consisting of, in virtually all children, the association of three types of lesions: portal fibrosis without disruption of the architecture of the lobule; biliary dysplasia; and polycystic kidney disease that may vary from purely histological abnormalities to severe renal failure presenting early in infancy. The genetic basis of the disease has been found to be due to a mutation in the PKHD1 gene coding for the protein fibrocystin that functions on the primary cilia of renal and biliary epithelial cells [21]. The hepatic lesion is thought to be a ductal plate malformation of small interlobular bile ducts [22]. The biliary dysplasia that is a constant histological finding described as irregularly shaped proliferating small bile ducts in the portal tracts, may be associated in some cases with Caroli syndrome that consists of non-obstructive cystic dilatations of the peripheral bile ducts. Clinical manifestations depend on the degree of hepatic and renal involvement and include portal hypertension with gastrointestinal bleeding, rarely cholangitis, arterial hypertension and renal failure. Exceptionally cirrhosis may occur and HCC as well as cholangiocarcinoma has been reported.
US findings include hepatomegaly, predominantly of the left lobe, splenomegaly and presence of hepatofugal derivations, extra- or intrahepatic. There is often mild dilatation of hilar bile ducts and in cases of associated Caroli syndrome, multifocal cystic dilatations of peripheral intrahepatic bile ducts can be seen on US with the typical “central dot sign” corresponding to a portal vein branch protruding into the lumen of a dilated bile duct (Fig. 7) [23]. The main portal vein is usually patent, but it may be surrounded by a portal cavernoma of hepatopetal collateral veins, probably secondary to hypoplasia or thrombosis of the small intrahepatic portal branches and shunting of the presinusoidal block present in CHF (Fig. 7). This finding was seen in one third of our cases [24]. US changes of the kidneys are variable, ranging from markedly enlarged and diffusely hyperechogenic kidneys in severe forms to hyperechogenicity involving the medulla and part of the cortex or only the medulla (Fig. 7) [21]. The association of cystic bile duct dilatation and hyperechoic enlarged kidneys allows diagnosis of the disease.
Imaging findings of CHF. a–d Sonograms of a 10-year-old girl with CHF. a Central dot sign corresponding to a portal vein branch protruding into the lumen of a bile duct with cystic dilatation. b–c There is evidence of portal vein cavernoma along the portal vein and its confluence. Note the heterogeneous liver pattern on the US using the high-frequency transducer (c) and the cystic dilatation of the collecting tubules well seen in the renal medulla of the kidney (d). MRCP of a 15-year-old boy with CHF (e) demonstrates the typical cystic dilatation of the peripheral bile ducts
MRI may be more accurate than US to demonstrate mild cystic dilatation of the bile duct (Fig. 7). MDCT is only indicated as preoperative work-up of the portal vein system if surgery for relief of portal hypertension is considered.
Cirrhosis
Cirrhosis is an advanced stage of liver fibrosis that is accompanied by distortion of the lobular architecture and hepatic vasculature. The major clinical consequences of cirrhosis are impaired hepatocyte function, increased intrahepatic resistance leading to portal hypertension and the risk of development of HCC [3]. In contrast to adults, in whom cirrhosis is roughly estimated to be histologically present in 1% of the population, it is infrequent in children. The cause of the cirrhosis may influence its severity and its course and some forms may remain compensated for a long time if the underlying cause can be eliminated. Thus it is important to identify the aetiology and the major categories in children are biliary and post-necrotic cirrhosis (Table 1). The role of imaging is firstly to provide the diagnosis of cirrhosis in cases presenting acutely and secondly to follow the evolution of the disease, detect complications and provide vascular evaluation before liver transplantation that represents the only curative option.
US, MDCT and MRI are not very sensitive in the detection of cirrhosis and normal examinations do not exclude compensated cirrhosis, however their specificity is high when there are obvious abnormal findings. The main imaging findings consist of modifications of the liver morphology, contours, structure, and vascular changes and US can provide all these findings especially in children [25]. The parenchymal modifications are dysmorphology of the liver with atrophy of one lobe and hypertrophy of the other, irregular contours of the liver with micro or macronodules (well seen on the inferior surface or on the anterior surface when there is ascites), heterogeneous or coarsened echostructure with decreased beam penetration and hyperechoic parenchyma and portal tracts. Use of a high-frequency linear-array transducer is often contributory to the detection of nodules that may be overlooked with curved probes (Fig. 1). Modifications in the vascular anatomy involve both extra- and intrahepatic vessels. Hepatofugal veins, diagnostic of portal hypertension, have to be searched for in the lesser omentum, around the spleen, in the ligamentum teres presenting as a patent para umbilical vein and also in the ligamentum venosus presenting as a patent ductus venosus (Fig. 8) [26]. Decrease in the portal flow and increase in hepatic arterial flow are known hallmarks of cirrhosis. These changes are believed to be due to intrahepatic resistance and vascular distortion with abnormal connections between branches of the portal vein, hepatic artery and hepatic vein [27]. In most of cases of cirrhosis secondary to biliary atresia there is progressive and rapid portal vein hypoplasia with slow flow or even reverse flow (Fig. 2) [28]. As a buffer response, there is dilatation and acceleration of hepatic artery flow but often with a decrease in diastolic flow that can be quantified by a high resistance index of 0.8 or more. Both changes have been described as poor prognostic factors of survival in biliary atresia [29]. Moreover, because of precarious blood supply in these children, acute episodes of focal liver ischaemic necrosis may occur, leading to rapid progression to liver failure [30]. MDCT or MRI can demonstrate these parenchymal changes which are often overlooked by US.
Patent ductus venosus as a sign of portal hypertension. Eighteen-month-old infant with biliary atresia and cirrhosis. On pre-transplant contrast-enhanced MDCT there is evidence of a patent ductus venosus (arrow) joining the left portal branch and the IVC. This intrahepatic hepatofugal collateral is favourable as the portal vein remains large
In some cases of biliary atresia and in other causes of cirrhosis the diameter and velocity of the portal vein are variable and seem to be related to the type and location of intra or extrahepatic hepatofugal portosystemic collaterals. Presence of a paraumbilical vein or a patent ductus venosus is associated with a large portal vein that facilitates liver transplantation anastomosis (Fig. 8). Other US findings of cirrhosis concern hepatic vein flow which becomes flattened with loss of the normal triphasic waveform as a consequence of compression.
Assessment of portal vein, hepatic artery and IVC before liver transplantation is at best obtained with MDCT with arterial and portal phases (Fig. 2) [31]. In contrast to adults, portal vein thrombosis very rarely complicates the evolution of cirrhosis in children and in this circumstance HCC should be suspected.
Nodules and HCC in childhood cirrhosis
The main causes of nodules in children with chronic liver diseases include focal steatosis, regenerative nodule and dysplastic or neoplastic lesion. MR imaging is the best technique to help differentiate and follow these lesions (Table 3).
Regenerative nodules may be classified according to size as either micronodules (<3 mm) or macronodules (≥3 mm). They may progress along a well-described carcinogenic pathway to become dysplastic nodules or HCC [32, 33]. Nodule characterization is based on size and vascularity. As lesions grow, the likelihood of high grade dysplasia or HCC increases and as dedifferentiation progresses there is predominant arterial perfusion. Therefore the typical appearance of HCC on MRI is hypointense on T1-W images, hyperintense on T2-W, arterial enhancement and rapid porto-venous wash-out (Fig. 9). Although HCC is rare in young children, it has been reported in ten children younger than 5 years of age with progressive familial intrahepatic cholestasis (PFIC) and bile salt export pump (BSEP) deficiency, once known as Byler disease [34]. Additionally early occurrence of HCC has also been reported in an 8-month-old infant with biliary atresia [35]. Our paediatric protocol for follow-up of nodules associated with chronic liver disease includes periodic US examination and alpha fetoprotein level assay. If one of these tests is abnormal then MRI with gadolinium contrast injection is performed. If there is doubt on the benign nature of a nodule equal to or >2 cm in diameter on MRI, then a guided biopsy is advised [36].
Typical appearance of a HCC nodule on MRI. Eight-year-old boy presenting with tyrosinaemia under medical treatment from the age of 1 year. Increase in the level of alpha fetoprotein with US detection of a 15-mm nodule in segment 5. MRI was performed to characterize this nodule and also to search for other nodules. The single nodule (arrows) is hypointense on T1-W images (a), hyperintense on T2-W images (b), shows marked arterial enhancement (c) and rapid portal wash-out (d). Surgical resection was the chosen treatment
Anomalies of bile ducts in cirrhosis
Careful US analysis of the bile ducts is mandatory when an infant or a child presents with suspicion of chronic liver disease. The appearance of the bile ducts may be one of the clues to the diagnosis. As said earlier cystic dilatation of peripheral bile ducts with the central dot sign is very suggestive of CHF (Fig. 7). Marked regular dilatation of the intrahepatic bile ducts may be the sign of an undiagnosed congenital malformation such as choledochal cyst or an obstruction such as spontaneous or traumatic perforation of the bile duct or stenosis of a surgical anastomosis. Beaded cystic cavities along the porta hepatis may be found in the evolution of biliary atresia and may be complicated by cholangitis [31]. In some cases of biliary atresia these cystic cavities may be echogenic due to sludge or lithiasis (Fig. 10). Irregular segmental dilatation of bile ducts with thickening of the wall is suggestive of sclerosing cholangitis. In all these cases further evaluation with MRCP is recommended, to obtain complete assessment of the biliary tract for diagnostic or prognostic purpose (Fig. 11).
Cystic cavities of biliary atresia. Four-year-old girl with biliary cirrhosis of unknown aetiology. US (a) shows multiple hyperechoic nodules typical of cavities filled with sludge and calculi very suggestive of biliary atresia. Pre-transplant contrast-enhanced MDCT (b) confirms the findings and also displays a large portal vein with a patent paraumbilical vein
Sclerosing cholangitis. Four-year-old girl with chronic inflammatory bowel disease and elevated liver enzymes. MRCP [3-D FSE with thin slices and maximum intensity projection (MIP) reconstruction] depicts irregularities of bile ducts with segmental dilatation (arrows) and short stenoses typical of sclerosing cholangitis
Budd-Chiari syndrome
Suprahepatic causes of portal hypertension include venoocclusive disease which is mainly encountered after radiation therapy or intensive chemotherapy, Budd-Chiari syndrome and cardiac causes such as constrictive pericarditis or exceptional cor triatrium dextrum [1].
Budd-Chiari syndrome is a rare and severe liver disorder characterized by hepatic venous flow obstruction which in most cases is due to thrombosis of the hepatic veins and/or the inferior vena cava (IVC). Several prothrombotic conditions have been identified in adults and also in children [37].
Imaging plays a major role in the diagnosis and also for the treatment. The imaging findings depend on the stage (acute or chronic) of the disease. There is usually evidence of hepatomegaly and ascites. In the acute stage thrombus may be seen, enlarging one or more hepatic vein or the IVC. In chronic stage hepatic veins may be not visible or appearing as an echogenic line or dilated above a stenosis or thin and tortuous, corresponding to a collateral vein. Doppler is very useful to show slow or inverted flow in hepatic veins and presence of collaterals joining one hepatic vein to another, often present in an enlarged caudate lobe (Fig. 12). Reversed flow may be seen in the portal vein corresponding to severe portal hypertension. MDCT with multiplanar and MIP reconstruction is indicated for complete evaluation of the vessels before treatment. It also displays a typical heterogeneous contrast enhancement pattern of the liver, characteristic of the disease (Fig. 12) [38].
Budd-Chiari syndrome. Four-year-old boy with hepatomegaly and ascites 2 years after a severe acute myocarditis with shock and seizures. US shows thrombosis of the IVC and anomalies of the hepatic veins. The right is replaced by an echogenic line (a), the left is very slow (blue) and the middle has inverted flow (red) (b). On contrast-enhanced MDCT in the portal phase (c) there is very heterogeneous contrast enhancement typical of Budd-Chiari syndrome. After an attempt at percutaneous angioplasty on the left hepatic vein, a TIPS was successfully placed with very good evolution over a follow-up period of 3 years
Until recently the preferred treatment was surgical, including portosystemic shunt or liver transplantation for the most severe cases. However a therapeutic strategy aimed at minimal invasiveness has been reported in adults and can be also applied to children, consisting of anticoagulation in case of acute thrombosis, angioplasty in case of localized hepatic vein stenosis or TIPS and liver transplantation only in cases of failure of previous therapeutic options [37, 39].
Elasticity imaging techniques
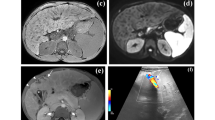
Liver biopsy is currently considered the gold standard for assessing hepatic fibrosis. However it is an invasive procedure, performed under general anaesthesia in children and with rare but potential life-threatening complications. In addition, the accuracy of liver biopsy may be questioned because of sampling error as the biopsy specimen represents only a very limited part of the whole liver and fibrosis is heterogeneously distributed. Thus it is not an ideal tool for screening, longitudinal monitoring and assessing therapeutic response. Several biological tests have been proposed in adults and children using blood marker ratio [aspartate aminotransferase-platelet ratio index (APRI)] or a score based on serum markers combined with the age and sex of the patient (Fibrotest) or combined with the height, weight, triglycerides and cholesterol for the non-alcoholic steatotic hepatitis (NASH) test. These tests are useful for identifying those patients with no fibrosis or with extreme levels of fibrosis; however the intermediate range of liver fibrosis cannot be satisfactorily predicted by any of the available tests [27]. Recently several elasticity imaging techniques have been developed for the assessment of the mechanical properties of liver tissues and fibrosis level staging using different imaging modalities. 2-D real-time US elastography involves a manual compression of the liver and does not allow quantitative estimation of the liver stiffness [40]. MR elastography is performed with a mechanical driver device placed in contact with the patient’s body wall adjacent to the liver generating shear waves within the abdomen during a gradient-echo sequence. It allows a 2-D stiffness mapping of the entire liver, but it is expensive, time-consuming, needs corrections for breathing movements and thus does not seem easily applicable and has not been yet evaluated in children [41]. Transient elastography (Fibroscan, Echosens, Paris, France) is a rapid, painless, bedside method, performed with a dedicated machine that evaluates the liver elasticity by creating an elastic shear wave within the liver, measuring its speed of propagation and calculating the corresponding stiffness expressed in kPa [42]. It has been shown to be reproducible and non-operator dependant. The operator places the probe in an intercostal space at the level of the right lobe of the liver and the vibrator gives a painless push to the tissue, creating the elastic shear wave. Ten measurements can be made and the final liver stiffness is the median value with standard deviation [43]. A special paediatric probe has been developed and tested for small children [44]. Good correlation has been obtained with liver biopsy, although the evaluation is only made along a single line and the measurement is impossible in patients with ascites. Acoustic radiation force impulse imaging (ARFI) technology is based on the same technology but integrated into a conventional US device and one can target an anatomic region to be interrogated for elastic properties. The shear-wave velocity is measured within a defined small region of interest of 5 mm axial by 4 mm width. Diagnostic accuracy of this technique has been shown to be comparable to that of transient elastography [45]. Supersonic shear imaging (SSI) is a new very promising technique which is based on the combination of a radiation force induced in tissues by focused ultrasonic beams (ARFI technique) and a very high frame rate US imaging sequence capable of catching in real-time the transient propagation of resulting shear waves [46]. This technique is performed with a special machine which allows complete US examination and as a complementary tool, liver elasticity mapping in a large and deep 2-D area (Fig. 13). Evaluation is yet to be done in adults and children. MR diffusion and perfusion imaging may also play a role in the future to noninvasively assess liver fibrosis [47, 48].
Supersonic shear imaging (SSI). One-year-old girl with biliary atresia after a successful Kasai procedure. Liver US using a high-frequency linear probe on a special machine allowing US examination and simultaneous elasticity mapping in a large 2-D area. The liver appears fairly homogeneous but the elasticity measurements in kPa are high. This rapid and easy-to-do technique is to be evaluated in children
Conclusion
Recent advances in the technology of all imaging modalities are helpful in the management of chronic liver diseases in children. US plays a key role in the initial diagnosis and also in the follow-up, as long as the examination is complete, analyzing both morphological and haemodynamic aspects of the liver. It has also become very useful to guide interventional radiology. MDCT has made great progress as it allows (even in children) rapid, high-resolution and multiplanar imaging, and has now replaced angiography in presurgical mapping of the hepatic vasculature, except in the assessment of hypoplastic intrahepatic portal veins in portal vein obstruction. MRI of the liver has increasing indications in children especially for parenchyma and nodule characterization and also for biliary tract evaluation. Although liver biopsy has become safer with US guidance there is a need for noninvasive quantification of liver fibrosis, longitudinal monitoring and assessment of therapeutic response. Several technical solutions are already available and have to be evaluated for accuracy and applicability in children.
References
Bernard O, Alvarez F, Brunelle F et al (1985) Portal hypertension in children. Clin Gastroenterol 14:33–55
Leonis MA, Balistreri WF (2008) Evaluation and management of end-stage liver disease in children. Gastroenterology 134:1741–1751
Schuppan D, Afdhal NH (2008) Liver cirrhosis. Lancet 371:838–851
Kruskal JB, Newman PA, Sammons LG et al (2004) Optimizing Doppler and color flow US: application to hepatic sonography. Radiographics 24:657–675
Patriquin H, Tessier G, Grignon A et al (1985) Lesser omental thickness in normal children: baseline for detection of portal hypertension. AJR 145:693–696
Chan FP, Rubin GD (2005) MDCT angiography of pediatric vascular diseases of the abdomen, pelvis, and extremities. Pediatr Radiol 35:40–53
Mendichovszky IA, Marks SD, Simcock CM et al (2008) Gadolinium and nephrogenic systemic fibrosis: time to tighten practice. Pediatr Radiol 38:489–496, quiz 602–483
Chavhan GB, Babyn PS, Manson D et al (2008) Pediatric MR cholangiopancreatography: principles, technique, and clinical applications. Radiographics 28:1951–1962
Chavhan GB, Babyn PS, Jankharia BG et al (2008) Steady-state MR imaging sequences: physics, classification, and clinical applications. Radiographics 28:1147–1160
Habdank K, Restrepo R, Ng V et al (2003) Combined sonographic and fluoroscopic guidance during transjugular hepatic biopsies performed in children: a retrospective study of 74 biopsies. AJR 180:1393–1398
Hoffer FA (2000) Liver biopsy methods for pediatric oncology patients. Pediatr Radiol 30:481–488
Abd El-Hamid N, Taylor RM, Marinello D et al (2008) Aetiology and management of extrahepatic portal vein obstruction in children: King’s College Hospital experience. J Pediatr Gastroenterol Nutr 47:630–634
Gauthier-Villars M, Franchi S, Gauthier F et al (2005) Cholestasis in children with portal vein obstruction. J Pediatr 146:568–573
Gauthier F (2005) Recent concepts regarding extra-hepatic portal hypertension. Semin Pediatr Surg 14:216–225
Guerin F, Porras J, Fabre M et al (2009) Liver nodules after portal systemic shunt surgery for extrahepatic portal vein obstruction in children. J Pediatr Surg 44:1337–1343
Puppala S, Patel J, Woodley H et al (2009) Preoperative imaging of left portal vein at the Rex recess for Rex shunt formation using wedged hepatic vein carbon dioxide portography. J Pediatr Surg 44:2043–2047
Sarin SK, Kumar A, Chawla YK et al (2007) Noncirrhotic portal fibrosis/idiopathic portal hypertension: APASL recommendations for diagnosis and treatment. Hepatol Int 1:398–413
Bernard PH, Le Bail B, Balabaud C et al (1996) Hepatoportal sclerosis, nodular regenerative hyperplasia, incomplete septal cirrhosis: lesions of hepatic microcirculation? Gastroenterol Clin Biol 20:335–338
Girard M, Amiel J, Fabre M et al (2005) Adams-Oliver syndrome and hepatoportal sclerosis: occasional association or common mechanism? Am J Med Genet A 135:186–189
Gurkaynak G, Yildirim B, Aksoy F et al (1998) Sonographic findings in noncirrhotic portal fibrosis. J Clin Ultrasound 26:309–313
Turkbey B, Ocak I, Daryanani K et al (2009) Autosomal recessive polycystic kidney disease and congenital hepatic fibrosis (ARPKD/CHF). Pediatr Radiol 39:100–111
Veigel MC, Prescott-Focht J, Rodriguez MG et al (2009) Fibropolycystic liver disease in children. Pediatr Radiol 39:317–327
Brancatelli G, Federle MP, Vilgrain V et al (2005) Fibropolycystic liver disease: CT and MR imaging findings. Radiographics 25:659–670
Besnard M, Pariente D, Hadchouel M et al (1994) Portal cavernoma in congenital hepatic fibrosis. Angiographic reports of 10 pediatric cases. Pediatr Radiol 24:61–65
Tuney D, Aribal ME, Ertem D et al (1998) Diagnosis of liver cirrhosis in children based on colour Doppler ultrasonography with histopathological correlation. Pediatr Radiol 28:859–864
Tsai A, Paltiel HJ, Sena LM et al (2009) Neonatal hemochromatosis and patent ductus venosus: clinical course and diagnostic pitfalls. Pediatr Radiol 39:823–827
Shiha G, Sarin SK, Ibrahim AE et al (2009) Liver fibrosis: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL). Hepatol Int 3:323–333
Khong PL, Ooi CG, Saing H et al (2002) Portal venous velocity in the follow-up of patients with biliary atresia after Kasai portoenterostomy. J Pediatr Surg 37:873–876
Kardorff R, Klotz M, Melter M et al (1999) Prediction of survival in extrahepatic biliary atresia by hepatic duplex sonography. J Pediatr Gastroenterol Nutr 28:411–417
Wildhaber BE, Rubbia-Brandt L, Majno P et al (2008) Focal ischemic necrosis in advanced biliary atresia cirrhosis. Pediatr Transplant 12:487–491
Caruso S, Miraglia R, Milazzo M et al (2009) Multidetector computed tomography hepatic findings in children with end-stage biliary atresia. Eur Radiol Dec 17 [Epub ahead of print]
Hanna RF, Aguirre DA, Kased N et al (2008) Cirrhosis-associated hepatocellular nodules: correlation of histopathologic and MR imaging features. Radiographics 28:747–769
Hussain SM, Reinhold C, Mitchell DG (2009) Cirrhosis and lesion characterization at MR imaging. Radiographics 29:1637–1652
Knisely AS, Strautnieks SS, Meier Y et al (2006) Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 44:478–486
Brunati A, Feruzi Z, Sokal E et al (2007) Early occurrence of hepatocellular carcinoma in biliary atresia treated by liver transplantation. Pediatr Transplant 11:117–119
Liang JL, Cheng YF, Concejero AM et al (2008) Macro-regenerative nodules in biliary atresia: CT/MRI findings and their pathological relations. World J Gastroenterol 14:4529–4534
Hermeziu B, Franchi-Abella S, Plessier A et al (2008) Budd-Chiari syndrome and essential thrombocythemia in a child: favorable outcome after transjugular intrahepatic portosystemic shunt. J Pediatr Gastroenterol Nutr 46:334–337
Brancatelli G, Vilgrain V, Federle MP et al (2007) Budd-Chiari syndrome: spectrum of imaging findings. AJR 188:W168–176
Cauchi JA, Oliff S, Baumann U et al (2006) The Budd-Chiari syndrome in children: the spectrum of management. J Pediatr Surg 41:1919–1923
Friedrich-Rust M, Ong MF, Herrmann E et al (2007) Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR 188:758–764
Faria SC, Ganesan K, Mwangi I et al (2009) MR imaging of liver fibrosis: current state of the art. Radiographics 29:1615–1635
Foucher J, Chanteloup E, Vergniol J et al (2006) Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 55:403–408
de Ledinghen V, Vergniol J (2008) Transient elastography (FibroScan). Gastroenterol Clin Biol 32:58–67
de Ledinghen V, Le Bail B, Rebouissoux L et al (2007) Liver stiffness measurement in children using FibroScan: feasibility study and comparison with Fibrotest, aspartate transaminase to platelets ratio index, and liver biopsy. J Pediatr Gastroenterol Nutr 45:443–450
Friedrich-Rust M, Wunder K, Kriener S et al (2009) Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 252:595–604
Muller M, Gennisson JL, Deffieux T et al (2009) Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: preliminary in vivo feasibility study. Ultrasound Med Biol 35:219–229
Razek AA, Abdalla A, Omran E et al (2009) Diagnosis and quantification of hepatic fibrosis in children with diffusion weighted MR imaging. Eur J Radiol Nov 17 [Epub ahead of print]
Sandrasegaran K, Akisik FM, Lin C et al (2009) Value of diffusion-weighted MRI for assessing liver fibrosis and cirrhosis. AJR 193:1556–1560
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pariente, D., Franchi-Abella, S. Paediatric chronic liver diseases: how to investigate and follow up? Role of imaging in the diagnosis of fibrosis. Pediatr Radiol 40, 906–919 (2010). https://doi.org/10.1007/s00247-010-1600-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-010-1600-3