Abstract
The Asian Pacific Association for the Study of the Liver (APASL) Working Party on Portal Hypertension has developed consensus guidelines on the disease profile, diagnosis, and management of noncirrhotic portal fibrosis and idiopathic portal hypertension. The consensus statements, prepared and deliberated at length by the experts in this field, were presented at the annual meeting of the APASL at Kyoto in March 2007. This article includes the statements approved by the APASL along with brief backgrounds of various aspects of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Asian Pacific Association for the Study of the Liver (APASL) set up a Working Party on Portal Hypertension in 2002 with a mandate to develop a consensus on the various clinical aspects of portal hypertension. The Asian region is fully aware and acknowledges the significant contributions made by the four Baveno consensus conferences on portal hypertension, the most recent being in 2005 [1]. However, the etiology, profile, and management options of diseases causing portal hypertension are quite different in the Asian region. We, therefore, present in this review consensus guidelines on noncirrhotic portal fibrosis (NCPF) and idiopathic portal hypertension (IPH). Extrahepatic portal vein obstruction (EHPVO) has been recently covered [2].
Experts predominantly from the Asia-Pacific region were requested to identify the different aspects of NCPF/IPH and prepare the consensus statements. We standardized the process for the development of these consensus statements with the following steps: review of published literature, a survey of the current approaches for diagnosis and management in Asia, discussion on contentious issues and deliberations to prepare the consensus statement by a core group of experts. A 2-day meeting was held on January 12 and 13, 2007, at New Delhi, to discuss and finalize the consensus statements. Only those statements that were unanimously approved by the experts are presented here. The working party adopted the Oxford System [3] for developing an evidence-based approach. The group assessed the level of existing evidence and accordingly ranked the recommendations (i.e., level of evidence from 1 [highest] to 5 [lowest]; grade of recommendation from A [strongest] to D [weakest]). A brief background note has been added to help us understand the genesis of the consensus statements.
Definition
NCPF/IPH is one of the important disease entities comprising noncirrhotic portal hypertension, a group of diseases that are characterized by an increase in portal pressure, due to intrahepatic or prehepatic lesions, in the absence of cirrhosis of the liver. Till now there is no universally accepted definition of this entity and even the nomenclature is confusing. In the Indian subcontinent, it is known as noncirrhotic portal fibrosis, while in Japan and other Asian countries, it is referred to as idiopathic portal hypertension. In other parts of the world it is variously named, as hepatoportal sclerosis [4, 5], noncirrhotic intrahepatic portal hypertension [6], and idiopathic noncirrhotic intrahepatic portal hypertension [7].
While there are subtle differences between NCPF and IPH as mentioned below, for the purposes of this review, both are considered together.
Consensus statement
(1) NCPF/IPH is a disease of uncertain etiology characterized by periportal fibrosis and involvement of small and medium branches of the portal vein, resulting in the development of portal hypertension. The liver functions and structure primarily remain normal. (5, D)
Epidemiology of NCPF/IPH
NCPF has been reported from all over the world; however, the condition is more common in the developing [8–10] than in the developed countries [4–7]. The reasons for the marked regional differences in prevalence are not clear, but differences in socioeconomic status, living conditions, average lifespan, and ethnic background may be responsible. NCPF and IPH have been commonly seen in people, who are socioeconomically disadvantaged [8–10].
Epidemiology of IPH
The incidence of IPH has declined in Japan [11]. Two large series of patients with portal hypertension in Japan before 1975 [12, 13] had shown that about one-third of all patients with portal hypertension could be classified as IPH. Imanaga et al. [12] analyzed 205 patients of portal hypertension, surgically treated before 1961 at Nagoya University Hospital and found 70 (31.1%) cases of IPH, while in another series at Kyushu University Hospital [13], there were 225 (29.2%) cases of IPH among 771 cases of portal hypertension in the period 1955–1975. The incidence of IPH appears to have declined in Japan since 1970 [11]. A government-aided survey was conducted throughout the country by sending out questionnaires to all hospitals, with more than 200 beds. It was estimated that about 80% of patients with IPH were compiled with the committee. It was then calculated that there were 1,376 patients with IPH living in Japan in 1985 (incidence rate of 0.75 per 100,000 population, with an average duration of morbidity of 12.5 years) [14]. This survey continued and disclosed that the yearly number of new cases ranged from 8 to 20, averaging 11 per year up to 1994 [15]. These figures were much lower compared with the past.
Epidemiology of NCPF
Contrary to Japan, in India, there are no national registry data of patients with NCPF and there has been no nationwide study to determine the trend in the incidence of NCPF. Thus, the Indian data are based on studies performed in the 1970s and 1980s, the data presented by the National Collaborative Study on Noncirrhotic Portal Hypertension [9], and the data collected through personal communication with investigators having interest in this disease.
Studies conducted in the 1980s or earlier found an incidence of approximately 23.3% (range 7.9–46.7%; Table 1) [9, 16, 17]. The majority of the studies found the peak age of incidence to be the third and fourth decades of life, and this is one to two decades younger than in patients with IPH from Japan [8, 16, 18, 19]. Several investigators from India have found a male dominance or no sex predilection (Table 2) in contrast to a female dominance (M:F = 1:3) in Japan [20]. A study from a large tertiary care center at New Delhi has shown that 98% of patients with NCPF were from the low-socioeconomic strata of society [10].
The incidence of NCPF has probably declined in India after 1990. There is also a concomitant decrease in the incidence of EHPVO (Table 1). It has been speculated that umbilical sepsis and/or repeated diarrheal episodes during infancy or in early childhood may be responsible for both diseases. Over the years, there has been an improvement in the living standards. The changed scenario of seroepidemiology of hepatitis A virus infection also confirms the improvement in sanitation and hygiene [21, 22]. Prenatal practices have also changed in India, resulting in a reduction in the frequency of umbilical sepsis. Only a large multicenter study could substantiate the decline in the incidence of NCPF and verify the above-mentioned observations.
Consensus statements
-
(2 .1) NCPF/IPH is an important cause of portal hypertension. (4)
-
(2.2.1) Incidence of IPH has declined in Japan after the 1970s. (2b)
-
(2.2.2) There is some indication that the incidence of NCPF is on the decline in India after 1990. (5, D)
-
(2.2.3) The current incidence of these diseases in Asia-Pacific needs to be studied. (5, D)
-
(2.3.1) The patients of NCPF are generally young adults in the third and fourth decade of life; IPH generally presents in the fourth and fifth decade of life. (3b)
-
(2.3.2) In NCPF, there is no sex predilection. IPH is more common in females. (3b)
-
(2.4) NCPF/IPH is linked to low socioeconomic status. (4)
Etiopathogenesis of NCPF/IPH
A number of hypotheses have been proposed, signifying limited understanding of the disease process.
Infections
Bacterial infection from the gut with repeated septic embolization of the portal circulation has been proposed as a possible etiology. Histopathological studies in NCPF also revealed the presence of mural thrombi and intimal sclerosis in the portal vein and its medium-sized branches. Thrombin is known to activate the stellate cells [23]. Stellate cell activation by various endogenous factors, including cytokines, and activated coagulation factors, might prove important for the formation of perisinusoidal fibrosis in NCPF/IPH. Boyer [24] and Wanless [25] also suggested that IPH and NCPF are caused by the thrombosis of large intrahepatic portal veins. However, the Japanese Research Committee on IPH has not endorsed the portal vein thrombosis theory.
Xenobiotic exposure
Prolonged exposure to xenobiotics is thought to predispose individuals to the development of NCPF. Among the suspected xenobiotics, exposure to inorganic arsenic has been investigated to be the most important [26]. Drinking of arsenic-contaminated water is suspected to cause NCPF, according to data from Chandigarh, India [27], and subsequently from Eastern India [28, 29]. Liver histology of chronic arsenicosis patients with liver dysfunctions showed high arsenic content and revealed periportal fibrosis and multiple vascular channels in the expanded portal zones, as seen in NCPF [29]. Similar observations have been made by other workers in the past [30, 31]. The mechanisms related to arsenic-induced NCPF are poorly known. Immunologic mechanisms might play a role in the endothelial cell damage from arsenic [32]; development of arsenic-induced hepatic fibrosis was found to be related to high hepatic oxidative stress and IL-6 and TNF-alpha levels [33–36]. However, other workers did not find high arsenic content in the liver tissue of patients with NCPF [9].
Immunologic abnormalities
There is some evidence to support immunologic abnormalities in NCPF [37]. Nayyar et al. found that the population of total peripheral T lymphocytes (T1) and suppressor/cytotoxic phenotype (T8) was significantly decreased in patients with NCPF compared with controls. While the subpopulations of helper/inducer lymphocytes (T4) and total B lymphocytes were comparable in size, the ratio of T4 to T8 lymphocytes was significantly increased in patients with NCPF in comparison with controls [38, 39]. There is evidence of increased vascular cell adhesion molecule-1 (VCAM-1) [40] and increased soluble TNF-receptor I and II without any significant increase of TNF in the blood in IPH [41, 42]. As TNF is involved in the induction and maintenance of fibrotic reaction [42], fibrosis around the portal vein as observed in patients with IPH could be explained. Furthermore, TNF causes upregulation of VCAM-1, which is also observed in patients with IPH. Heightened Th1 response has been noted in NCPF/IPH; however, cellular infiltration is not so remarkable in these patients [41, 42]. The role of NK cells, the TH1/TH2 responses and reciprocity, various cytokines involved, and the role of B-cells need to be studied further. It remains to be established, whether these immunological anomalies are a result or the cause of NCPF/IPH.
Consensus statements
-
(3 .1) NCPF/IPH is a heterogeneous group of diseases, which could be a result of the varied degree of portal venous injury.
-
(3.2) Injury predominantly manifests in the presinusoidal region.
-
(3.3) The factors/agents that have been reported to be associated with NCPF/IPH include umbilical/portal pyemia, diarrheal diseases, or bacterial infections in infancy; autoimmune disorders; prothrombotic states; chronic exposure to arsenic, vinyl chloride monomers, or copper sulfate (vineyard sprayers); prolonged treatment with methotrexate; hypervitaminosis A; and renal allograft recipients under treatment of 6-mercaptopurine, azathioprine, and corticosteroids. However, the exact etiology in the majority of cases remains unknown. (4)
Animal models of NCPF/IPH
The etiopathogenesis of NCPF/IPH is still obscure, as patients generally present with late bleeding from varices. Animal models help us in exploring the pathophysiology of NCPF/IPH. The most common models are given below.
Prolonged sensitization with albumin
Prolonged sensitization of rabbits with bovine serum albumin [43] was seen to result in splenomegaly. Later, the experiment was conducted on dogs as rabbits do not tolerate more than three intraportal injections. The histological changes that occurred in these animals were characterized by early portal inflammation immediately followed by portal fibrosis, aberrant vasculature, and disappearance of portal venules and were very similar to those in human IPH.
Prolonged sensitization with nonpathogenic Escherichia coli
In rabbits, killed nonpathogenic E. coli were administered intraportally. The animals that received an intraportal mixture of killed E. coli and rabbit antiserum (aggregated E. coli) developed histologic changes in the liver and portal hypertension [44]. However, these investigators had used repeated cannulation of the portal vein, which may itself cause damage to the portal vein intima, portal pyemia, and an altered hemodynamic and histological picture in the animal. Accordingly, alternative routes of introducing E. coli into the portal circulation have been proposed [44].
Schistosomiasis japonica models
Schistosomiasis japonica and IPH share some histological features of liver injury. Shekhar et al. [45] produced a rabbit model by infecting with 250 Schistosoma cercariae percutaneously and subcutaneously. The angioarchitecture of chronic schistosomiasis japonica is characterized by narrowing, obstruction, and obtuse angles of bifurcation of the peripheral portal veins, and this disease is quite similar to IPH in both histology and angioarchitecture, strongly suggesting that portal change is the primary lesion of the hepatic disorder in IPH. However, splenomegaly invariably noted in IPH is not necessarily observed in chronic schistosomiasis japonica, suggesting that portal system involvement may be more extensive in IPH [46].
Chronic arsenic ingestion
Chronic arsenic toxicity is a form of hepatic fibrosis that causes portal hypertension, but does not progress to cirrhosis. Hepatotoxic effects of arsenic in humans have been reported earlier [31, 47–49]. Injury to the intrahepatic portal vein and even development of cirrhosis has been alleged to occur with the prolonged use of Fowler’s solution containing sodium arsenite [49]. Sarin et al. in 1999 [50] produced a reproducible and homogenous murine model of hepatic fibrogenesis with increased hydroxyproline and collagen levels, without significant hepatocellular necrosis and inflammation through chronic arsenic feeding. Development of portal hypertension was not observed. Guha Mazumder and Santra [28, 29] demonstrated hepatic fibrosis due to arsenic toxicity in mice receiving arsenic (3.2 mg/l) in drinking water for at least 15 months. Arsenic feeding for 6 months showed a significant decrease in hepatic glutathione and the enzymes glucose-6-phosphate dehydrogenase and glutathione peroxidase in association with a significant increase of lipid peroxidation compared with a control group [34]. Increasing dose and duration of arsenic exposure in mice further showed progressive increase of oxystress and elevation of cytokines TNF-alpha and IL-6, which are associated with an increasing level of collagen in the liver [35].
Repeated immunosensitization by rabbit splenic extract
Repeated intramuscular injection of splenic extract was shown [51] to produce significant splenomegaly and rise in portal pressure at 1, 3, and 6 months without hepatic parenchymal injury, quite akin to NCPF seen in humans.
Repeated low-dose endotoxemia of portal circulation
Portal pyelephlebitis, due to repeated abdominal infections and thrombosis in the portal circulation could lead to the obstruction of small and middle branches of the portal vein and development of NCPF. On the basis of this hypothesis, repeated low-dose portal endotoxemia was produced by injecting E. coli (heat-killed) through an indwelling cannula (placed in the gastrosplenic vein) [52]. Splenomegaly and rise in portal pressure was noted at 1 and 3 months, which persisted up to 6 months. Absence of hepatic parenchymal injury and persistently elevated portal pressure makes this model ideal for investigating the vascular reactivity to various agents.
Consensus statements
-
(4 .1) Current animal models of NCPF and IPH do not accurately mimic human disease. (5)
-
(4.2) Some of the pathophysiological features of this disease such as hepatic fibrosis or vascular changes have been reproduced in different animal models. (2c)
-
(4.3) Combination of systemic and direct portal antigen delivery should be evaluated further. (5, D)
Clinical presentation of NCPF/IPH
NCPF
These patients present with well-tolerated episodes of gastrointestinal hemorrhage, a longstanding mass in the left upper quadrant (splenomegaly), anemia, and consequences of hypersplenism. Development of ascites, jaundice, and hepatic encephalopathy is uncommon and may be seen only after an episode of gastrointestinal hemorrhage. Of all the causes of portal hypertension, a massive and disproportionately large spleen is seen most commonly in NCPF. Left upper quadrant pain due to perisplenitis and splenic infarction is not uncommon [53]. Like cirrhosis, NCPF also may have odd presentations, such as glomerulonephritis [54, 55], hypoxemia [56], or autonomic dysfunction [57]. Over a 24-year-period (1983–2006), Sarin et al. [10] saw 366 patients of NCPF; the clinical presentation profile of these patients is shown in Table 3. Qureshi from Pakistan [58, 59] found 73 cases in 20 years (1981–2001) with similar clinical features.
IPH
Major presenting symptoms in Japanese patients with IPH [60] were splenomegaly (88%), hepatomegaly (44%), gastrointestinal (GI) bleeding (35%), and ascites (12%). These figures are different from NCPF, where the majority present with upper GI bleeding, splenomegaly, and anemia. Hematological studies confirmed more severe anemia and thrombocytopenia in NCPF than in IPH [60].
Consensus statements
-
(5 .1) Patients of NCPF/IPH usually have a longstanding history of mass in the left hypochondrium (enlarged spleen). (2b)
-
(5.2) Most cases of NCPF/IPH present with enlarged spleen and GI bleeding (hematemesis) and some have features of anemia. (2b)
-
(5.3) Signs of chronic liver disease like palmar erythema, spider angioma, testicular atrophy in abdominal wall veins, and gynecomastia are rare. (3b)
-
(5.4) Jaundice, ascites/edema, and signs of liver failure are uncommon. (3b)
-
(5.5) Ascites is transient and usually seen soon after a variceal bleed. (2b)
Natural history of NCPF/IPH
In the clinical courses of patients with IPH, the liver slowly undergoes atrophy, which is not necessarily progressive, and the liver functional reserve is well maintained. Although mortality from variceal rupture is generally lower in NCPF/IPH, because of better liver functions compared with cirrhosis, the major cause of death is variceal bleeding. Repeated uncontrollable bleeding may induce hepatic insufficiency. The survival curve for patients with NCPF/IPH is somewhat between that for those with cirrhosis and for a healthy population of comparable age [11, 60–62]. Good prognostic features in patients with NCPF, a 2- and 5-year survival of nearly 100% after successful eradication of esophagogastric varices, have been described [63].
Hillaire et al. reported death of 4 out of 28 patients with IPH owing to terminal liver failure [7]. According to a clinical study in Japan, 4 out of the 22 patients with IPH with portal vein thrombosis (PVT) died and all patients without PVT were alive during the mean clinical course of 12 years [64]. The causes of death were systemic exhaustion as a result of chronic ascites in three patients and infection in one patient.
The incidence of PVT is higher in patients with IPH than in those with cirrhosis, and ascites is not uncommon [7, 64]. Hillaire et al. examined the outcome of patients with IPH having PVT, and found it in 3 of 4 patients who died and in only 10 out of 24 patients who survived [7]. They also mentioned that ascites developed in 14 out of the 28 patients with IPH and in 11 with GI bleeding or severe concurrent diseases, and that ascites was transient in 10 patients and constant in 4 patients. The other study shows that patients without PVT had less clinical problems after long-term follow-up [64]. However, marked hypersplenism and low serum albumin were significantly frequent in patients with PVT than in patients without PVT. Moreover, ascites was present only in patients with PVT (seven of nine) and four patients with PVT died. Development of PVT in a patient with IPH may be a significant factor for poor prognosis, and ascites may indicate a deterioration of the condition in patients with IPH. Furthermore, PVT and ascites may be mutually related in this disease.
Consensus statements
-
(6 .1.1) Bleeding rate from gastroesophageal varices is high in patients with NCPF/IPH (32–95%). (2b)
-
(6.1.2) Mortality from variceal rupture is generally lower in NCPF/IPH, because of better liver functions compared with cirrhosis. (2b)
-
(6.1.3) Management of gastroesophageal varices to prevent the rupture should be a priority in the care of patients with NCPF/IPH. (5, D)
-
(6.2.1) The incidence of PV thrombosis is more frequent in patients with IPH than in patients with liver cirrhosis. The same should also be studied in NCPF. (3b, D)
-
(6.2.2) Development of portal vein thrombosis in patients with IPH may be a significant factor for poor prognosis. The practical benefits of the management of portal vein thrombosis to improve the clinical course of IPH should be elucidated in future studies. (4, C)
-
(6.3.1) Ascites is not rare in patients with IPH in spite of preserved liver functions. It occurs in association with PVT. (4, C)
-
(6.3.2) However, clinical ascites is rare in patients with NCPF and it is transient after a bleed. (4)
-
(6.3.3) Ascites is considered to be a sign for deterioration of the condition in patients with IPH. (5, D)
-
(6.4.1) In NCPF/IPH, the liver probably undergoes atrophy, owing to reduced blood supply to the periphery. It is not necessarily progressive, and the liver functional reserve is well maintained. (4, C)
-
(6.4.2) The survival rate for patients with NCPF/IPH is much better than that for patients with cirrhosis. (4, C)
-
(6.4.3) PV thrombosis and ascites may indicate the deterioration of the condition in certain cases of IPH, and thrombosis and ascites may be mutually related in this disease. (5, D)
Histopathology of NCPF/IPH
The histopathology of NCPF/IPH has been described in studies from India and Japan, including a few cases in Western literature. These studies include the description of autopsy livers and wedge and needle biopsies.
Autopsy liver
Gross examination may reveal a normal, enlarged, or even shrunken liver. The surface is smooth, wrinkled, or even show some nodularity resembling cirrhosis. Fibrous thickening of the capsule of the liver with increased vascularity and some inflammation may be seen. Subcapsular septation can be noted, while deeper parenchyma shows normal architecture. Sclerosis of large to small intrahepatic portal vein branches and approximation of portal tracts to surface has been documented [65, 66]. This has been confirmed by histomorphometry by Kage et al. [67]. Histological features noted in autopsies include increased portal collagenous connective tissue and sclerosis and obliteration of small branches of portal veins in most cases. This histological hallmark of NCPF was termed obliterative portal venopathy by Nayak and Ramalingaswami [68]. Intimal fibrosis and elastosis is also common, leading to subendothelial thickening of the wall of large- and medium-sized portal vein branches even compromising the lumen. Veins may be thickened to the extent that they resemble an artery. Furthermore, aberrant vasculatures characterized by thin-walled vessels mainly located adjacent to the portal tracts and at times in the hepatic lobules have been described. Although some of them are morphologically very similar to hepatic vein branches, they are portal in nature [69]. Recanalized thrombi are sometimes seen. The inconspicuous branches of the terminal portal vein may be replaced by small vascular channels. Mild inflammation is seen in a few cases. Incomplete portal pseudolobule and scattered regenerative nodules may be noted in a few cases.
Hepatic vein may show sclerosis or small branches may show slight dilatation. Collagen deposition in the space of Disse has been observed by electron microscopy [70]. The collagen and elastin deposition in IPH may be a result of increased connective tissue growth factor expression and decreased MMP-9 expression in portal tracts of IPH as demonstrated by immunohistochemistry in a study by Tsuneyama et al. [71].
Needle biopsies
Biopsy material may show only mild and subtle changes from normal. These changes include inconspicuous portal tracts and obliterated veins, or fibrous expansion of portal tracts. Alternatively there may be dilatation of vessels in or near portal tracts, with vessel-like dilatation of sinusoids. Distortion of portal–central relation may be noted. Ludwig et al. [72] studied the changes in 25 liver biopsies. Changes in the portal tract included capillary dilatation, phlebosclerosis, and fibroelastosis of the stroma. Portal vein dilatation with herniation into the surrounding hepatic parenchyma was also noted. Portal vein obliteration and loss of bile ducts were rare in their study. The acinar architecture showed capillary and necroinflammatory bridging between portal tracts and terminal hepatic veins, isolated megasinusoids in a random distribution, displaced, and abnormally large hepatic vein branches with or without phlebosclerosis, and slender, curved fibrous septa.
Wedge biopsies
Wedge biopsies show changes similar to autopsy material, but changes in medium and large portal vein branches may not be seen if not sampled. The changes in pre- or intraoperative biopsy specimens are subtle, and may be missed by the casual observer because of the absence of significant fibrosis. Nodular regenerative hyperplasia, focal nodular hyperplasia, and incomplete septal cirrhosis have all been described with NCPF [73]. Association of NCPF with hepatocellular carcinoma has also been described [74, 75].
A deep-core wedge biopsy (not broad-based wedge) along with a needle biopsy should be taken, as they would complement each other in the information provided. This material may be valuable in looking for clues to the etiopathogenesis of NCPF.
Early changes and staging
Early hepatic changes in NCPF were observed incidentally in a patient of cervical cancer [76]. These include lymphoid cell infiltration of the portal tract and of subendothelial regions of portal vein branches, and nonspecific lobular hepatitis.
Nakanuma et al. [77] proposed a staging of IPH with a combination of hepatic parenchymal atrophy and portal venous thrombosis. Stage I is nonatrophic liver without subcapsular parenchymal atrophy, stage II is nonatrophic liver with subcapsular parenchymal atrophy, stage III is atrophic liver with subcapsular parenchymal atrophy, and stage IV is portal venous occlusive thrombosis. IPH livers can progress from stage I to stage III, while stage IV occurs relatively late.
Consensus statements
-
(7 .1) Diagnostic criteria of NCPF/IPH on needle liver biopsy are as follows:
-
Essential for diagnosis:
-
Absence of regenerative nodules with features of possible or definite cirrhosis in an adequate-sized liver biopsy. (1a, A)
-
Features usually seen (however, these may not be seen in all):
-
Small portal vein obliteration; aberrant vasculature; portal tract fibrosis, rounded or streaky; absence of significant hepatocellular injury. (2b, B)
Laboratory studies in NCPF/IPH
The laboratory evaluation in NCPF/IPH reveals only mild and subtle abnormalities predominantly related to hypersplenism.
Liver function tests
The parenchymal damage manifest by increased aminotransferase levels is very minimal in NCPF. Results of conventional tests of liver function are normal or near normal (Table 4) [8, 61]. Semiquantitative tests of liver function in these patients, such as monoethylglycinexylidide extraction, also yield results comparable to those of healthy individuals [78]. The frequency of hepatitis B and C in nontransfused patients with NCPF is comparable to that found in the general population, excluding a role for these viruses in the causation of NCPF.
Hematologic tests
Pancytopenia is found in the majority of patients with NCPF. Anemia may be microcytic, hypochromic (due to gastrointestinal blood loss) or normocytic, normochromic (due to hypersplenism). Leucopenia (<4,000 mm−3) and thrombocytopenia (platelets <50,000 mm−3) may also be present and are due to hypersplenism. Whether the leucopenia in NCPF increases susceptibility to infections, and whether splenectomy is required in such cases remain debatable. The bone marrow is hypercellular.
Coagulation factors
A state of mild, compensated, and disseminated intravascular coagulation secondary to endotoxemia or portosystemic collaterals has been reported in some cases of NCPF. In a study by Bajaj et al. [79], both EHPVO (83%) and NCPF (78%) patients had a significantly increased international normalized ratio (INR) and a decrease in fibrinogen and platelet aggregation. Although the EHPVO patients had a significant prolongation in partial thromboplastin time (67%), with increased levels of fibrinogen degradation products, this was normal in patients with NCPF. This suggests a mild-disseminated intravascular coagulation disorder in these diseases. However, in a previous study, only low platelet aggregatability was reported [80]. Deficiency of proteins C and S has been proposed along with mutations in Factor V Leiden; however, a cause-and-effect hypothesis remains to be confirmed [64, 81]. Moreover, these abnormalities are more prevalent in EHPVO. Sarin et al. found significant derangements in the INR and fibrinogen levels in their series of patients with NCPF (Table 5) [63].
Tests for immunological derangements and autoimmunity
There are several studies and individual case reports of association of IPH with autoimmune disorders like progressive systemic sclerosis, systemic lupus erythematosus, rheumatoid arthritis, mixed connective tissue disease, etc. Positive antinuclear antibodies and high titers of anti-Scl-70, anti-SS-A, anticentromere, etc. have been reported. Positive antimitochondrial (M2) antibodies in the absence of any histological features of primary biliary cirrhosis have also been reported. Nearly two-thirds of Japanese female patients with IPH were found to test positive for anti-double-stranded DNA and one-fourth tested positive for antinuclear antibody and had hypergammaglobulinemia [82, 83].
Such a high prevalence of associated autoimmune conditions has not been the experience in the Indian subcontinent. However, familial aggregation and a high frequency of HLA-DR3 have been found in NCPF [84]. Terada et al. [85] have studied the expression of HLA-DR on endothelial cells in IPH and found increased expression in the smaller venous radicles, raising the possibility that the smaller venous radicles in the small and medium-sized portal tracts are targets of immunologic attack. There is some evidence supporting immunological derangements/deficiency in the causation of NCPF [38, 86]. In patients with IPH, a poor autologous mixed lymphocyte reaction has been reported [87, 88]. More recently, studies have suggested that the imbalance of Th1 and Th2 CD4+ T-cells and TNF may be associated with the pathogenesis of IPH [89].
Consensus statements
-
(8 .1) Results of liver function tests are normal or near normal in patients of NCPF/IPH. (2b)
-
(8.2) Hypersplenism is common but usually asymptomatic. (2b)
-
(8.3) The frequency and characteristics of the coagulation abnormalities in these patients need to be investigated further. (5, D)
Features at endoscopy in NCPF/IPH
The risk of bleeding in NCPF/IPH has not been independently investigated, but is believed to be similar to the risk in patients with varices due to other causes. In contrast to patients with cirrhosis who bleed from varices, patients with NCPF tolerate variceal bleeding relatively well, probably because of well-preserved hepatic synthetic functions. The GI-bleed-related mortality rate varies. In the West, patients with noncirrhotic etiologies have mortality ranging from 7 to 31% for a single bleeding episode [90]. The mortality rate is higher in Japan with IPH [62].
Prevalence of varices
Esophagogastric varices have been reported in 85–95% of patients with NCPF [78]. Gastric varices are more common in NCPF than in cirrhosis and are reported in up to 44% of cases. Gastric varices are usually associated with large esophageal varices [91]. Antral varices are uncommon in NCPF and may develop after eradication of esophageal varices in up to 3.8% of patients on follow-up. [92]. Portal hypertensive gastropathy is relatively less common in NCPF [57] than in cirrhosis and manifests mainly after variceal obliteration. Anorectal varices are more common in NCPF than in cirrhosis. In one study, significantly more patients with NCPF had anorectal varices than did patients with cirrhosis (89 vs. 56%) [93].
Bleeding risk from varices in NCPF
Approximately 70% of patients with NCPF present with a history of major variceal bleeding. NCPF is an important cause of upper gastrointestinal bleeding, constituting 15% of the cases of variceal bleed in patients with portal hypertension [94]. Antral varices rarely bleed, and if they are not the source of bleeding they can be managed conservatively [92].
Consensus statements
-
(9 .1) Esophageal varices are seen in 85–90% of patients. (2b)
-
(9.2) These varices are generally large at the time of diagnosis. (2b)
-
(9.3) If esophageal varices are small, investigations for the presence of gastric varices and spontaneous shunts are required. (5, D)
-
(9.4) Gastric varices are seen in about 25% of patients with NCPF/IPH. (2b)
-
(9.5) Portal hypertensive gastropathy is uncommon in these patients. (2b)
-
(9.6.1) Anorectal varices are common in NCPF. (2b)
-
(9.6.2) Prevalence of anorectal varices in IPH is not known. (5)
Hemodynamics in NCPF/IPH
The portal vein pressures are elevated markedly in patients who have NCPF. Two pathoanatomic sites of obstruction have been identified. A pressure gradient exists between the spleen and the liver (intrasplenic pressure – intrahepatic pressure [IHP]) and another exists between the IHP and the wedged hepatic venous pressure (WHVP) (IHP – WHVP) [95]. Generally, the WHVP is normal or only slightly elevated in NCPF. Variceal pressure also has been studied in these patients and is comparable to that in cirrhotic portal hypertension [95, 96]. Intravariceal pressure closely reflects portal pressure in patients who have NCPF and is the investigation of choice for measurement of portal pressure. Splenic and portal vein blood flow are known to be increased markedly in Japanese patients with IPH, which is suggestive of a hyperdynamic circulatory state.
Consensus statements
-
(10 .1) Hepatic venous pressure gradient (HVPG) is normal or near normal in NCPF/IPH. (2b)
-
(10.2) Hemodynamic studies indicate site of resistance as predominantly presinusoidal. (3b)
-
(10.3) Whether HVPG increases on long-term follow-up needs to be studied. (5, D)
-
(10.4) Portal venous blood flow is significantly increased. (2b)
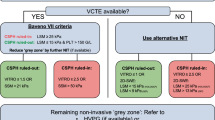
Diagnosis of NCPF/IPH
The diagnosis of NCPF is comparatively easy in developing countries, where the disease is common. Clinical presentation of variceal bleeding, moderate to massive splenomegaly without features of chronic liver disease, and growth retardation would make one suspect NCPF. In Japan and in the West, such patients are a decade or so older and often present with anemia, splenomegaly, and variceal bleeding. NCPF and IPH are not merely diagnoses of exclusion, and need well-defined and agreed-upon criteria for diagnosis.
Consensus statements
-
(11 .1) Diagnostic features of NCPF/IPH:
-
Presence of moderate to massive splenomegaly
-
Evidence of portal hypertension, varices, and/or collaterals
-
Patent spleno-portal axis and hepatic veins on ultrasound Doppler,
-
Test results indicating normal or near-normal liver functions,
-
Normal or near-normal hepatic venous pressure gradient, and
-
Liver histology—no evidence of cirrhosis or parenchymal injury. (2b)
-
(11.2) Other features:
-
Absence of signs of chronic liver disease,
-
No decompensation after variceal bleed except occasional transient ascites,
-
Absence of serum markers of hepatitis B or C virus infection,
-
No known etiology of liver disease, and
-
Imaging with ultrasound or other imaging techniques showing dilated and thickened portal vein with peripheral pruning and periportal hyperechoic areas. (2b)
Management of acute bleeding in NCPF/IPH
Variceal bleeding is a common and life-threatening complication of portal hypertension due to NCPF. There is paucity of data on the management of acute variceal bleeding in NCPF; however, the principles and modes of management remain the same as those for patients with cirrhosis.
General management
Blood transfusion, intravenous fluids, and standard ICU care are provided [97–99]. Placement of nasogastric tube is optional, especially if the bleeding has taken place more than 12 hours ago [100].
Bacterial infections are more common in patients with cirrhosis having variceal bleeding (35–66%) than in noncirrhotic patients (5–7%) [101]. It has been shown that infected cirrhotic patients have a higher rate of variceal rebleeding (43%) than noninfected patients (10%) [98, 102]. In patients with cirrhosis and variceal bleeding, the prophylactic antibiotics reduce variceal rebleeding and improve survival [103, 104]. In NCPF, however, there is no study on the use of prophylactic antibiotics.
Balloon tamponade
Balloon tamponade using Sengstaken Blakemore tube enables temporary control of bleeding, by direct compression of varices at the esophagogastric junction, in 40–90% of cases [97, 98]. Owing to high rates of complications and rebleeding, balloon tamponade is not used routinely as the first-line treatment for control of acute variceal bleeding.
Pharmacological therapy
Vasoactive drugs, such as somatostatin, octreotide, or terlipressin, have been used in the treatment of acute variceal bleeding while endoscopic therapy is being arranged. The vasoactive drugs lead to reduction in portal pressure, which is associated with a better control of variceal bleeding [105–108]. However, there are no data on the efficacy of vasoactive drugs in patients with NCPF with acute variceal bleeding.
Endoscopic therapy
Endoscopic sclerotherapy and band ligation are effective in 80–90% of patients in controlling acute bleeding from esophageal varices and preventing rebleeding [109, 110]. At present, band ligation is preferred owing to lower complication rates.
Combined pharmacological and endoscopic therapy
Combination treatment with drugs plus endoscopic therapy is more effective than endoscopic therapy or drug therapy alone in controlling acute bleeding (88% vs. 76%) and preventing rebleeding for 5 days (77% vs. 58%), while there is no difference in mortality [109, 110]. There is, however, paucity of data for NCPF.
Failure of endoscopic therapy is defined, as further variceal bleeding after two endoscopic treatments during a single hospital admission for acute bleeding. The current therapies fail to control bleeding or prevent early rebleeding in 8–12% of patients [109], who should be treated by alternative modes of treatment like surgery or transjugular intrahepatic portosystemic shunt (TIPS).
Consensus statements
-
(12 .1) General measures for the control of acute bleeding are same as for cirrhosis. (5, D)
-
(12.2) Endoscopic therapy is effective for the control of acute variceal bleed in NCPF/IPH. (4, C)
-
(12.3) Role of vasoactive drugs alone or in combination with endoscopic therapy needs to be evaluated. (5, D)
-
(12.4) If a diagnosis of NCPF/IPH is unlikely, the condition should be treated as cirrhosis. (5, D)
-
(12.5) In case of failure of medical management (as in Baveno IV), decompressive surgery or TIPS is useful and should be used on the basis of available expertise. (5, D)
-
(12.6) Patients with transient ascites should undergo devascularization procedure. (5, D)
Prevention of variceal bleeding in NCPF/IPH
Primary prophylaxis
The natural history of esophageal varices in NCPF is not known. Progression of variceal size occurs at a rate of 10–15% per year in patients with cirrhosis, mostly dependent on liver dysfunction. Such a progression of varices in NCPF is less likely to occur, as the liver function continues to be normal. Similarly, a decrease in the size of esophageal varices, as seen in patients with cirrhosis with an improvement in liver functions is unlikely in NCPF, unless interventions like endoscopic sclerotherapy are applied, which after variceal obliteration results in the development of spontaneous splenorenal shunts [111–114].
Endoscopic variceal ligation (EVL) and beta blockers are the common modes of therapy for the primary prophylaxis of large esophageal varices in cirrhosis [115]. There are no randomized controlled trials on primary prophylaxis in NCPF. Since patients with NCPF are all in Child’s A class, the results of EVL and beta blockers could be extrapolated to NCPF.
In a study by Sarin et al. [116], 8 patients with NCPH and 18 with Child’s A cirrhosis were given a combination of EVL and propranolol and compared with 9 and 15 patients with NCPH and cirrhosis, respectively, given EVL only. None of the NCPH or cirrhotic patients bled on a follow-up of an year, indicating that both EVL and drug therapies are effective in preventing the first bleed. EVL is the preferred mode of therapy because it is difficult to assess the response to beta blockers in patients with NCPF as HVPG is near normal. Hence, to assess the efficacy of beta blocker therapy, measurements of splenic pulp pressure or direct portal pressure would need to be taken. Moreover, since the lifespan of patients with NCPF is normal, compliance of drug therapy is not likely to be good.
Shunt surgery for primary prophylaxis is likely to be indicated if the patient of NCPF has large esophageal varices with a symptomatic large splenomegaly, a very low platelet count (<20,000), stays far away from a good medical center where an upper GI bleed can be tackled, or has a rare blood group. A study from India on 45 patients with NCPF [117]—41 of whom were treated with a prophylactic proximal lienorenal shunt, 2 with splenectomy, and 2 with devascularization—showed no operative mortality. Over a follow-up period of 49 months, three patients bled and two late deaths unrelated to surgery occurred. There was delayed morbidity in 47%, including seven patients, who developed partial splenic embolization; four, glomerulonephritis; two, pulmonary AV fistulae; and five, ascites requiring diuretics. It was thus considered safe, but at the cost of high, delayed morbidity [117]. Patients with gastric varices of more than 2 cm could be taken up for surgical shunt or balloon-occluded retrograde transvenous obliteration (BRTO) if a splenorenal shunt is present, although studies are lacking.
Secondary prophylaxis
Literature on secondary prophylaxis is scanty. One study on 72 patients with NCPF with recurrent bleeding [118] showed that endoscopic sclerotherapy significantly reduced the bleeding rate over a follow-up period of 21.4 ± 20.4 months; rebleed after obliteration occurred in seven patients (9.2%). Recurrence occurred in nine (13.9%) patients. Similarly, Bhargava et al. [119] and Kochhar et al. [120] have shown sclerotherapy to be effective in managing patients with NCPF. EVL has been shown to be better than EST in almost all the studies; hence, it could be recommended in NCPF [121]. There is an isolated study using nonselective beta blockers in NCPF [122]. There is also no information on the role of TIPS in NCPF except for a case report [123].
Shunt surgery is effective in NCPF. One study involving 30 patients with NCPF reported a significant decrease in splenic pulp pressure (44.3 ± 13.5 vs. 33.8 ± 7.6 cm of saline) and splenic size from 9.1 ± 3.3 to 6.8 ± 4.6 cm in 28 patients after successful patent shunt surgery [124]. Shunt occlusion, overt chronic portosystemic encephalopathy, and rebleeding after elective shunt surgery were seen in approximately 10% of patients [125]. A recent trial comparing shunt surgery with TIPS in patients with cirrhosis has shown the two to be equally effective in treating variceal rebleeding and encephalopathy in Child’s A patients and ensuring survival [126].
Role of image-guided interventions in preventing variceal bleeding
Image-guided interventions (IGI) are a relatively recent means of treating and preventing variceal bleed. These include partial splenic embolization, BRTO, percutaneous transhepatic obliteration (PTO), and TIPS [63, 127]. The concept behind these modalities is again occlusion of varices and treatment of splenic lump, in an otherwise well-preserved patient with a significant lifespan ahead. An approach combining medical, endoscopic, and/or surgical management with an IGI may be beneficial in certain situations [127].
Transjugular intrahepatic portosystemic shunt
TIPS creation is indicated for uncontrollable variceal hemorrhage, recurrent variceal hemorrhage despite endoscopic therapy, and portal hypertensive gastropathy bleed [63, 128].
Surgical shunt procedures continue to be a safe, highly effective, and durable treatment for variceal bleeding in patients with low operative risk and good liver function [129, 130]. For patients with NCPH, in particular with EHPVO, portosystemic shunt surgery represents an effective therapy that leads to freedom from recurrent bleeding and repeated endoscopies for many years, and improves hypersplenism without worsening liver function or encephalopathy [129].
Primary long-term shunt patency was not as expected. Wolff et al. [129] found shunt stenosis or occlusion rates to be about 29% at 6 months, 42% at 1 year, and 51% at 2 years. Balloon angioplasty, new stent placement, or both can re-establish shunt patency in most patients. In view of good overall prognosis in patients having NCPF, a routine follow-up is mandatory; the number of repeat procedures one might have to undertake in such patients seems to be higher with the use of presently popular bare stents [129]. PTFE-covered stents (stent-grafts) rather than bare, fenestrated stents can help prevent TIPS stenosis and occlusions in the parenchymal tract as well as the hepatic venous end [131].
TIPS is still a multistage procedure requiring close surveillance and frequent maintenance, with interventions for re-establishing shunt function [132], especially in the third world countries because of the high cost of a stent-graft [129]. No published data are available on the rate of complications of TIPS in patients with NCPF; however, owing to the good hepatic functions, it might be logical to conclude that such complications would be uncommon in NCPF [133]. The role of prophylactic IGI/TIPS in patients with NCPF has not been evaluated [134].
Balloon-occluded retrograde transvenous obliteration and percutaneous transhepatic obliteration
These modalities were introduced as less invasive alternatives to surgical shunt and TIPS [135–137]. Specific situations where they may be attempted include poor hepatic functional reserve, isolated/predominant gastric cardiac and fundal varices, and gastric fundal varices with active bleeding (spurting or oozing), signs of recent bleeding, or in danger of rupturing [136]. The criteria mentioned above have been derived from studies not dealing directly with patients with NCPF. This is because of the scant citation of interventional experiences with patients with NCPF.
Image-guided interventions: Is there a role?
Image-guided intervention is largely considered a secondary tool for managing portal hypertension after conservative and/or endoscopic management have been explored. The role of IGI in the treatment of NCPF is still not clear. We would like to submit that IGI can replace surgical options in most cases and complement endoscopic management in managing patients with NCPF.
Consensus statements
Screening
-
(13 .1) All patients of moderate to massive splenomegaly with suspected NCPF should have screening endoscopy. (5, D)
Preprimary and early-primary prophylaxis
-
(13 .2) There is insufficient data to recommend it at present. (5, D)
Primary prophylaxis
-
(13 .3.1) EVL is recommended for large varices. There is no consensus on the use of beta blockers in such patients. (5, D)
-
(13.3.2) BRTO may be an option in patients with large gastric varices. (5, D)
-
(13.3.3) Decompressive shunt surgery is not recommended for primary prophylaxis. (3b, C)
-
Secondary prophylaxis
-
(13.4.1) Endoscopic therapy and elective decompressive surgery are effective and safe. (2b)
-
(13.4.2) There should be head-to-head comparison between these two modalities. (5, D)
-
(13.4.3) There is insufficient data on the role of TIPS in secondary prophylaxis. (5)
-
(13.4.4) BRTO is effective in patients with NCPF/IPH with gastric variceal bleed if gastrorenal shunt is present. (5)
Differences between NCPF and IPH
Although, the terms NCPF and IPH often have been used interchangeably, there are subtle differences between the two. NCPF is more common among men. This is in contrast to IPH, which is more common among women in Japan, Europe, and the United States. The mean age of patients who have NCPF varies from 25 to 35 years, which is much lower than for patients who have IPH. There are also distinct differences in the prevalence of autoantibodies and histopathology between the two diseases [138–140]
Consensus statements
-
(14 .1) While NCPF and IPH represent the same disease entity, there are some differences. (1a)
-
(14.2) In NCPF, there is no sex predilection and the mean age of presentation is about 30 years. This is in contrast to IPH, which is more common among middle-aged females in Japan, Europe, and the United States. (1a)
-
(14.3) Autoimmune features are common in IPH while rare in NCPF. (1a)
-
(14.4) Irregular parenchymal nodules and bile duct proliferation are more common in NCPF than IPH. (1a)
-
(14.5) Wedged hepatic venous pressure is almost normal in NCPF, while it is moderately raised in IPH. (1a)
Noncirrhotic portal fibrosis and idiopathic portal hypertension have withstood scientific indifference, since their description. It is hoped that the current short review and guidelines would be seen as an endeavor to enhance the clinician’s desire and pursuit for this seemingly oriental fibrosis. These diseases, however, provide unique opportunities to understand the genesis and pathophysiology of portal hypertension in the presence of near-normal liver function.
References
de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005;43:167–76.
Sarin SK, Sollano JD, Chawla YK, Amarapurkar D, Hamid S, Hashizume M et al. Members of the APASL Working Party on Portal Hypertension. Consensus on extra-hepatic portal vein obstruction. Liver Int 2006;26:512–9.
Centre for Evidence Based Medicine. Department of Primary Care, Old Road Campus, Headington, Oxford, OX3 7LF, UK. http://www.cebm.net/index.aspx?o=1025.
Mikkelsen WP, Edmondson HA, Peters RL, Redeker AG, Reynolds TB. Extra- and intra-hepatic portal hypertension without cirrhosis (hepatoportal sclerosis). Ann Surg 1965;162:602–20.
Bioulac-Sage, Le Bail B, Bernard PH, Balabaud C. Hepatoportal sclerosis. Semin Liver Dis 1995;15:329–39.
Kingham JGC, Levison DA, Stansfeld AG, Dawson AM. Non-cirrhotic intrahepatic portal hypertension: a long term follow-up study. Q J Med 1981;50:259–68.
Hillaire S, Bonte E, Denninger MH, Casadevall N, Cadranel JF, Lebrec D et al. Idiopathic non-cirrhotic intrahepatic portal hypertension in the West: a re-evaluation in 28 patients. Gut 2002;51:275–80.
Dhiman RK, Chawla Y, Vasishta RK, Kakkar N, Dilawari JB, Trehan MS et al. Non-cirrhotic portal fibrosis (idiopathic portal hypertension): experience with 151 patients and a review of the literature. J Gastroenterol Hepatol 2002;17:6–16.
Sarin SK. Non-cirrhotic portal hypertension. In: Advances in therapeutic hepatology: a world view. Postgraduate Course, AASLD 1998.
Pande C, Kumar A, Sarin SK. Non-cirrhotic portal fibrosis: a clinical profile of 366 patients [abstract]. Am J Gastroenterol 2006;101:S191.
Okuda K. Non-cirrhotic portal hypertension: why is it so common in India? J Gastroenterol Hepatol 2002;17:1–5.
Imanaga H, Yamamoto S, Kuroyanagi Y. Surgical treatment of portal hypertension according to state of intrahepatic circulation. Ann Surg 1962;155:43–50.
Kobayashi Y, Inokuchi K, Saku M et al. Epidemiology and clinical features of idiopathic portal hypertension. 1975 Report of the Ministry of Health and Welfare Research Committee on Idiopathic Portal Hypertension. Tokyo: Ministry of Health and Welfare, 1975:10–13.
Iwata H, Nishikawa A, Tanaka H et al. National survey on idiopathic portal hypertension. In: Kameda H, editor. 1985 Report of the Research Committee on Aberrant Portal Hemodynamics. Tokyo: Ministry of Health and Welfare; 1986. p. 117–29.
Imai F, Kuga K, Komaba M et al. Interim report on IPH survey. In: Futagawa S, editor. 1992 Report of the Research Committee on Aberrant Portal Hemodynamics. Tokyo: Ministry of health and Welfare; 1993. p. 107–10.
Bhargava DK, Dasarathy S, Sundaram KR, Ahuja RK. Efficacy of endoscopic sclerotherapy on long-term management of esophageal varices: a comparative study of results in patients with cirrhosis of the liver, non-cirrhotic portal fibrosis (NCPF) and extra hepatic portal venous obstruction (EHO). J Gastroenterol Hepatol 1991;6:471–5.
Banghar PK, Abraham P, Mistry FP, Bhatia SJ, Modi A. Profile of portal hypertension in Bombay. Indian J Gastroenterol 1992;11(Suppl 1):A25.
Abraham P, Malkan GH, Bhatia SJ, Parenjape AY, Nagral A, Mistry FP. Non-cirrhotic portal fibrosis in Bombay. Indian J Gastroenterol 1995;14(Suppl 1):A97.
Habibullah CM, Rao GN, Murthy DK, Padmanabhan CG, Kumar N, Chandra V et al. Non-cirrhotic portal fibrosis in Andhra Pradesh. (Clinical, radiological, biochemical, hemodynamic, and histological aspects). J Assoc Physicians India 1978;26:379–82.
Okudaira M, Ohbu M, Okuda K. Idiopathic portal hypertension and its pathology. Semin Liver Dis 2002;22:59–72.
Dhawan PS, Shah SS, Alvares JF, Kher A, Shankaran, Kandoth PW et al. Seroprevalence of hepatitis A virus in Mumbai, and immunogenicity and safety of hepatitis A vaccine. Indian J Gastroenterol 1998;17:16–8.
Mall ML, Rai RR, Philip M, Naik G, Parekh P, Bhawnani SC et al. Seroepidemiology of hepatitis A infection in India: changing pattern. Indian J Gastroenterol 2001;20(4):132–5.
Marra F, Valente AJ, Grandaliano G, Abboud HE. Thrombin stimulates proliferation of fat storing cells and expression of monocyte chemotactic protein-1. Hepatology 1995;22:780–7.
Boyer JL. Non cirrhotic portal hypertension. In: Hoofnagle JH, Goodman editors. Liver biopsy. Interpretation for the 1990s; Clinicopathologic correlation in liver disease. Thorofare, NJ: Slack/AASLD; 1991. p. 428–39.
Wanless IR. Non-cirrhotic portal hypertension and nodular transformation (nodular regenerative hyperplasia). In: Hoofnagle JH, Goodman editors Liver Biopsy. Interpretation for the 1990s; Clinicopathologic correlation in liver disease. Thorofare, NJ: Slack/AASLD; 1991. p. 440–55.
Abernathy C, Liu YP, Longfellow D, Aposhian HV, Beck B, Fowler B et al. Arsenic: health effects, mechanisms of actions and research issues. Environ Health Perspective 1999;107:593–7.
Dutta DV, Mitra SK, Chhuttani PN, Chakravarti RN. Chronic oral arsenic intoxication as a possible aetiological factor in idiopathic portal hypertension (non cirrhotic portal fibrosis) in India. Gut 1979;20:378–84.
Guha Mazumder DN, Das Gupta J, Santra A, Pal A, Ghose A, Sarkar S. Chronic arsenic toxicity in West Bengal – the worst calamity in the world. J Indian Med Assoc 1998;96:4–7.
Santra A, Das Gupta JD, De B, Roy B, Guha Mazumder DN. Hepatic manifestations in chronic arsenic toxicity. Indian J Gastroenterol 1999;18:152–5.
Morris JS, Schmid M, Newman S, Scheuer PJ, Sherlock S. Arsenic and non cirrhotic portal hypertension. Gastroenterology 1974;66:86–94.
Huet PM, Guillaume E, Cote J, Legare A, Lavoie P, Viallet A. Noncirrhotic presinusoidal portal hypertension associated with chronic arsenical intoxication. Gastroenterology 1975;68:1270–7.
Straub AC, Stolz DB, Ross MA, Hernandez-Zavala A, Soucy NV, Klei LR. Arsenic stimulates sinusoidal endothelial cell capillarization and vessel remodeling in mouse liver. Hepatology 2007;45:205–12.
Nevens F, Fevery J, Steenbergen WV, Sciot R, Desmet V, De Groote J. Arsenic and non-cirrhotic portal hypertension. J Hepatol 1990;11:80–85.
Santra A, Maiti A, Das S, Lahiri S, Charkaborty SK, Mazumder DN. Hepatic damage caused by arsenic (As) toxicity in experimental animals. Clinical Toxicology 2000;38:395–405.
Das S, Santra A, Lahiri S, Guha Mazumder DN. Implications of oxidative stress and hepatic cytokine (TNF-a and IL-6) response in the pathogenesis of hepatic collagenesis in chronic arsenic toxicity. Toxicol Appl Pharmacol 2005;204:18–26.
Sakurai T, Kaise T, Matsubara C. Inorganic and methylated arsenic compounds induced cell death in murine macrophages via different mechanisms. Chem Res Toxicol 1998;11:273–83.
Guha Mazumder DN, Ghose S, Das K, Ghosh A, Ghose KK, Nag S. Immunological studies in cirrhotic and non-cirrhotic portal fibrosis. Indian J Med Res 1986;84:59–61.
Nayyar AK, Sharma BK, Sarin SK, Malhotra P, Broor SL, Sachdev G. Characterization of peripheral blood lymphocytes in patients with non-cirrhotic portal fibrosis: A comparison with cirrhotic and healthy controls. J Gastroenterol Hepatol 1990;5:554–9.
Tokushige K, Yamauchi K, Komatsu T, Takasaki K, Hayashi N. Predominant T helper 1 cells in patients with idiopathic portal hypertension. J Gastroenterol Hepatol 2000;15:1312–7.
Yamaguchi N, Tokushige K, Haruta I, Yamauchi K, Hayashi N. Analysis of adhesion molecules in patients with idiopathic portal hypertension. J Gastroenterol Hepatol 1999;14:364–9.
Keane HM, Sheron N, Goka J, Hughes RD, Williams R. Plasma inhibitory activity against tumor necrosis factor in fulminant failure. Clin Sci 1996;90:77–80.
Miyazaki Y, Araki C, Vesin I, Garcia I, Kapanci Y, Whitsett JA et al. Expression of a tumor necrosis factor alpha transgene in murine lung causes lymphocytic and fibrosing alveolitis: A mouse model of progressive pulmonary fibrosis. J Clin Invest 1996;96:250–9.
Albini B, Ito S, Brentjens J, Andres G. Splenomegaly and immune complex splenitis in rabbits with experimentally induced chronic serum sickness: immunopathological findings. J Reticuloendothel Soc 1983;34:485–500.
Kono K, Ohnishi K, Omata M, Saito M, Nakayama T, Hatano H et al. Experimental portal fibrosis produced by intraportal injection of killed nonpathogenic Escherichia coli in rabbits. Gastroenterology 1988;94:787–96.
Shekhar KC, Pathmanathan R. Hepatosplenic lesions induced by experimental Schistosoma malayensis in rabbits. Southeast Asian J Trop Med Public Health 1993;24:333–9.
Cheever AW, Duvall RH, Minker RG, Nash TE. Hepatic fibrosis in rabbits infected with Japanese and Philippine strains of Schistosoma japonicum. Am J Trop Med Hyg 1980;29:1327–39.
Villeneuve JP, Huet PM, Joly JG, Marleau D, Cote J, Legare A et al. Idiopathic portal hypertension. Am J Med 1976;61:459–64.
Narang AP. Arsenicosis in India. J Toxicol Clin Toxicol 1987;25:287–95.
Nevens F, Fevery J, Van Steenbergen W, Sciot R, Desmet V, De Groote J. Arsenic and non-cirrhotic portal hypertension. A report of eight cases. J Hepatol 1990;11:80–5.
Sarin SK, Sharma G, Banerjee S, Kathayat R, Malhotra V. Hepatic fibrogenesis using chronic arsenic ingestion: studies in a murine model. Indian J Exp Biol 1999;37:147–51.
Kathayat R, Pandey GK, Malhotra V, Omanwar S, Sharma BK, Sarin SK. Rabbit model of non-cirrhotic portal fibrosis with repeated immunosensitization by rabbit splenic extract. J Gastroenterol Hepatol 2002;17:1312–6.
Omanwar S, Rizvi MR, Kathayat R, Sharma BK, Pandey GK, Alam MA et al. A rabbit model of non-cirrhotic portal hypertension by repeated injections of E. coli through indwelling cannulation of the gastrosplenic vein. Hepatobiliary Pancreat Dis Int 2004;3:417–22.
Vasia M, Curciorello JO, Corron SF, Viola M, Zamboni E, Castagno M et al. Idiopathic portal hypertension with splenic infarct. An unreported complication. Acta Gastroenterol Latinoam 2001;31:27–30.
Soma J, Saieto T, Sato H, Ootaka T, Abe Ke. Membranoproliferative glomerulonephritis induced by portosystemic shunt surgery for non-cirrhotic portal hypertension. Clin Nephrol 1997;48:274–81.
Kumar A, Bhuyan UN, Nundy S. Glomerulonephritis complicating non-cirrhotic portal fibrosis. J Gastroenterol Hepatol 1998;13(Suppl 1):271–5.
Babbs C, Warnes TW, Haboubi NY. Non-cirrhotic portal hypertension with hypoxaemia. Gut 1998;29:129–31.
Rangari M, Sinha S, Kapoor D, Mohan JC, Sarin SK. Prevalence of autonomic dysfunction in cirrhotic and noncirrhotic portal hypertension. Am J Gastroenterol 2002;97:707–13.
Qureshi H, Zuberi SJ, Maher M. Idiopathic portal hypertension an overlooked entity. Hepatol Res 1998; 12:169–76.
Qureshi H. Diagnosis and management of portal hypertension [thesis]. Pakistan: Karachi University; 1996.
Okuda K, Nakshima T, Kameda H, Sugirua M, Ohinshi K, Kobyashi M. Idiopathic noncirrhotic portal hypertension: a national study, Ministry of Health and Welfare Research Committee on Idiopathic Portal Hypertension. In: Brunner H, Thaler H, editors. Hepatology: a festschrift for Hans Popper. New York: Raven; 1985.
Okuda K, Nakashima T, Kameda H et al., for the Japan Ministry of Heath and Welfare Research Committee on Idiopathic Portal Hypertension. Idiopathic noncirrhotic portal hypertension: a national study. In: Brunner H, Thaler H, editors. Hepatology: a festschrift for Hans Popper. New York: Raven; 1985. p. 95–108.
Okuda K, Obata H. Idiopathic portal hypertension (hepatoportal sclerosis). In: Okuda K, Benhamou JP editors. Portal hypertension: clinical and physiological aspects. Tokyo: Springer-Verlag; 1991. p. 271–87.
Sarin SK, Kapoor D. Non-cirrhotic portal fibrosis: Current concept and management. J Gastroenterol Hepatol 2002;17:526–34.
Ishii M, Katada Y. Idiopathic portal hypertension in a systemic sclerosis patient heterozygous for factor V Leiden mutation. Rheumatol Int 2003;23:44–6.
Okuda K, Nakashima T, Okudaira M, Kage M, Aida Y, Omata M et al. Liver pathology of idiopathic portal hypertension. Comparison with non-cirrhotic portal fibrosis of India. The Japan idiopathic portal hypertension study. Liver 1982;2:176–92.
Okudaira M, Ohbu M, Okuda K. Idiopathic portal hypertension and its pathology. Semin Liver Dis 2002;22:59–72.
Kage M, Arakawa M, Fukuda K, Kojiro M. Pathomorphologic study on the extrahepatic portal vein in idiopathic portal hypertension. Liver 1990;10:209–16.
Nayak NC, Ramalingaswamy V. Obliterative portal venopathy of the liver. Arch Pathol 1979;87:359–69.
Fukuda K, Kage M, Arakawa M, Nakashima T. Portal vein or hepatic vein? A curious aberrant vasculature in the liver with idiopathic portal hypertension. Acta Pathol Jpn 1985;35:885–97.
Bredfeldt JE, Enriquez RE, Groszmann RJ. Idiopathic portal hypertension in a renal transplant recipient. J Clin Gastroenterol 1982;4:157–61.
Tsuneyama K, Kouda W, Nakanuma Y. Portal and parenchymal alterations of the liver in idiopathic portal hypertension: a histological and immunochemical study. Pathol Res Pract 2002;198:597–603.
Ludwig J, Hashimoto E, Obata H, Baldus WP. Idiopathic portal hypertension; a histopathological study of 26 Japanese cases. Histopathology 1993;22:227–34.
Sciot R, Staessen D, Van Damme B, Van Steenbergen W, Fevery J, De Groote J et al. Incomplete septal cirrhosis: histopathological aspects. Histopathology 1988;13:593–603.
Okuda K, Nakashima T, Kojiro M, Kondo Y, Wada K. Hepatocellular carcinoma without cirrhosis in Japanese patients. Gastroenterology 1989;97:140–6.
Hidaka H, Ohbu M, Kokubu S, Shibuya A, Saigenji K, Okayasu I. Hepatocellular carcinoma associated with idiopathic portal hypertension: review of large nodules in seven non-cirrhotic portal hypertensive livers. J Gastroenterol Hepatol 2005;20:493–4.
Nakanuma Y, Kouda W, Nakano T, Uneno K, Tachibana S, Araki I. A case report of early idiopathic portal hypertension. Pathol Res Pract 2001;197:759–63.
Nakanuma Y, Tsuneyama K, Ohbu M, Katayanagi K. Pathology and pathogenesis of idiopathic portal hypertension with an emphasis on the liver. Pathol Res Pract 2001;197:65–76.
Sarin SK, Aggarwal SR. Idiopathic portal hypertension. Digestion 1998;59:420–3.
Bajaj JS, Bhattacharjee J, Sarin SK. Coagulation profile and platelet function in patients with extrahepatic portal vein obstruction and non-cirrhotic portal fibrosis. J Gastroenterol Hepatol 2001;16:641–6.
Prasad CV, Kaur U, Marwaha N, Ghosh K, Chawla YK, Dilawari JB. Hemostatic alterations in non-cirrhotic portal fibrosis, extrahepatic portal venous obstruction and Budd–Chiari syndrome. Indian J Gastroenterol 1990;9:57–60.
Matsutani S, Maruyama H, Akiike T, Kobayashi S, Yoshizumi H, Okugawa H et al. Study of portal vein thrombosis in patients with idiopathic portal hypertension in Japan. Liver Int 2005;25:978–83.
Nakanuma Y, Nonomura A, Hayashi M, Doishita K, Takayanagi N, Uchida T et al. Pathology of the liver in “idiopathic portal hypertension” associated with autoimmune disease. The Ministry of Health and Welfare Disorders of Portal Circulation Research Committee. Acta Pathol Jpn 1989;39:586–92.
Saito K, Nakanuma Y, Takegoshi K, Ohta G, Obata Y, Okuda K et al. Non-specific immunological abnormalities and association of autoimmune diseases in idiopathic portal hypertension. A study by questionnaire. Hepatogastroenterology 1993;40:163–6.
Taneja V, Mehra NK, Sarin SK, Sharma BK, Vaidya MC. Possible HLA influence in governing susceptibility to non-cirrhotic portal fibrosis. Tissue Antigens 1987;30:184–7.
Terada T, Nakanuma Y, Obata H. HLA-DR expression on the microvasculature of portal tracts in idiopathic portal hypertension. Immunohistochemical characteristics and relation to portal phlebosclerosis. Arch Pathol Lab Med 1991;115:993–7.
Sarin SK, Nayyar AK, Malhotra P, Sharma BK, Kumar R, Broor SL. Immunological profile of patients with non-cirrhotic portal fibrosis. Gastroenterol Hepatol 1990;5:425–31.
Yamaue H, Tanimura H, Iwahashi M, Tsunoda T, Tani M, Tamai M et al. Impairment of autologous mixed lymphocyte reaction in the spleen and peripheral blood lymphocytes of patients with idiopathic portal hypertension. Gastroenterol Jpn 1990;25:193–8.
Tokushige K, Komatsu T, Ohzu K, Yamauchi K, Obata H. A defective autologous mixed lymphocyte reaction in patients with idiopathic portal hypertension. J Gastroenterol Hepatol 1992;7:270–3.
Tokushige K, Yamauchi K, Komatsu T, Takasaki K, Hayashi N. Predominant T helper 1 cells in patients with idiopathic portal hypertension. J Gastroenterol Hepatol 2000;15:1312–7.
Walker RM. Treatment of portal hypertension in children. Proc R Soc Med 1962;55:770–2.
Amarapurkar DN, Dhawan PS, Chopra K, Shankaran K, Kalro RH. Stomach in portal hypertension. J Assoc Physicians India 1993;41:638–40.
Shah SR, Desai CS, Mathur SK. Incidence and fate of antral varices. Eur J Gastroenterol Hepatol 1999;11:1041–3.
Chawla Y, Dilawari JB. Anorectal varices—their frequency in cirrhotic and non-cirrhotic portal hypertension. Gut 1991;32:309–11.
Dilawari JB, Kaur U, Narayanan VA, Augustine P, Das J, Ali H, Bambery P. Pattern of upper gastrointestinal hemorrhage in northern India: an endoscopic study of 316 patients. J Gastroenterol Hepatol 1987;2:443–449.
Sarin SK, Sethi KK, Nanda R. Measurement and correlation of wedged hepatic, intrahepatic, intrasplenic and intravariceal pressure in patients with cirrhosis of liver and non-cirrhotic portal fibrosis. Gut 1987;28:260–6.
El Atti EA, Nevens F, Bogaerts K, Verbeke G, Fevery J. Variceal pressure is a strong predictor of variceal hemorrhage in patients with cirrhosis as well as in patients with non-cirrhotic portal hypertension. Gut 1999;45:618–21.
Gow PJ, Chapman RW. Modern management of esophageal varices. Postgrad Med J 2001;77:75–81.
Zaman A, Chalasani N. Bleeding caused by portal hypertension. Gastroenterol Clin North Am 2005;34:623–42.
Grace ND, Groszmann RJ, Garcia-Tsao G, Burroughs AK, Pagliaro L, Makuch RW. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology 1998;28:868–80.
Laine L, Planas R, Nevens F, Banares R, Patch D, Bosch J. Treatment of the acute bleeding episode. In: de Franchis R (editor) Portal hypertension IV. Blackwell Publishing, 2006. p. 217–42.
Goulis J, Patch D, Burroughs AK. Bacterial infection in the pathogenesis of variceal hemorrhage. Lancet 1999;353:139–42.
Bernard B, Cadranell JF, Valla D, Escolano S, Jarlier V, Opolon P. Prognostic significance of bacterial infection in bleeding cirrhotic patients: a prospective study. Gastroenterology 1995;108:1828–34.
Bernard B, Nguyen KE, Opolon P, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis (ABP) for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding (GB): a meta-analysis. Hepatology 1999;29:1655–61.
Hou MC, Lin HC, Liu TT, Kuo BI, Lee FY, Chang FY et al. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial. Hepatology 2004;39:746–53.
D’Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology 1995;22(1):332–54.
McCormack G, McCormick PA. A practical guide to the management of esophageal varices. Drug 1999;57:327–35.
Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology 2001;120:726–48.
Moitinho E, Planas R, Banares R, Albillos A, Ruiz-del-Arbol L, Galvez C et al. Multicenter randomized controlled trial comparing different schedules of somatostatin in the treatment of acute variceal bleeding. J Hepatol 2001;35:712–8.
de Franchis R, Dell’Era A, Iannuzzi F. Diagnosis and treatment of portal hypertension. Dig Liver Dis 2004;36:787–98.
Dagher L, Burroughs A. Variceal bleeding and portal hypertensive gastropathy. Eur J Gastroenterol Hepatol 2001;13:81–8.
Sarin SK, Shastri HM, Jain M Jain AK, Issar SK, Murthy NS. The natural history of portal hypertensive gastropathy. Influence of variceal eradication. Am J Gastro 2000;95:2888–93.
Sarin SK, Govil A, Jain AK, Guptan RC, Issar SK, Jain M. Prospective trial of endoscopic sclerotherapy versus variceal band ligation for esophageal varices, influence on gastric varices and variceal recurrence. J Hepatol 1994;26:826–32.
De Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis 2001;5:645.
Chawla YK, Bhushnurmath SR, Dilawari JB. Cruveilhier Baumgarten syndrome in idiopathic portal hypertension. Am J Gastro. 1987;82:12.
Lay CS, Tsai Y Te, Lee FY, Lai YL, Yu CJ, Chen CB et al. Endoscopic variceal ligation versus propranolol in prophylaxis of first variceal bleeding in patients with cirrhosis. Ann Gastro Hepatol J 2006;21:413–9.
Sarin SK, Wadhawan M, Agrawal SR, Tyagi P, Sharma BC. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastro 2005;100:797–804.
Pal S, Radhakrishna P, Sahni P, Pande GK, Nundy S, Chattopadhyay TK. Prophylactic surgery in non-cirrhotic portal fibrosis: is it worthwhile? Indian J Gastroenterol 2005;24:239–42.
Chawla YK, Dilawari JB, Dhiman RK, Goenka MK, Bhasin DK, Kochhar R. Sclerotherapy in noncirrhotic portal fibrosis. Dig Dis Sci 1997;42:1449–53.
Bhargav DK. Dasarathy S, Sunderam KR, Ahuja RK. Efficacy of endoscopic sclerotherapy on long term management of esophageal varices: a comparative study of results in patients with cirrhosis beta, NCPF and EHPVO. J Gastroenterol Hepatol 1991;6:471–5.
Kochhar R, Goenka MK, Mehta SK. Outcome of infection sclerotherapy using absolute alcohol in patients with cirrhosis, NCPF and EHPVO. Gastrointest Endosc 1991;37:460–4.
Fakrly S, Omar M, Gannam AI. EST versus EVL in management of bleeding esophageal varices: a prospective randomized study in schiolosomal hepatic fibrosis. Endoscopic Arch Ed 2000;1:39–44.
Kire CF. Controlled trial of propranolol to prevent recurrent variceal bleeding in patients with non cirrhotic portal fibrosis. BMJ 1989;298:1363–5.
Jacobi D, de Muret A, Asbertle B, Perarnan TA. TIPS for treatment of portal hypertension secondary to noncirrhotic perisinusoidal hepatic fibrosis. Eur J Gastrohepatol 2006;18:549–51.
Sharma BC, Singh RP Chawla YK, Narasimhan KL, Rao KL, Mitra SK. Effect of shunt surgery on spleen size, portal pressure and esophageal varices in non cirrhotic portal fibrosis. J Gastroenterol Hepatol 1997;12:582–4.
Mathur SK, Shah SR, Nagral SS, Soonawala ZF. Transabdominal extensive esophagogastric devascularization with gastroesophageal stapling for management of noncirrhotic portal hypertension: long-term results. World J Surg 1999;23:1168–74.
Rosemurgy AS, Serafini FM, Ziverbel BR Black TJ, Kudryk BT, Nord HJ. TIPS vs small diameter porta caval shunt. Extended follow up of our expanded randomized prospective trial. J Gastrointest Surg 2000;4:589–97.
Shozo H, Satoshi I, Shinichi M, Tomofumi M, Tetsuya F, Takeshi Y. Interventional radiologic treatment for idiopathic portal hypertension. Cardiovasc Intervent Radiol 1999;22:311–4.
Haskal ZJ, Louis M, Cardella JF, Cole PJ, Drooz A, Grassi CJ et al. Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol 2003;14:S265–S270.
Wolff M, Hirner A. Current state of portosystemic shunt surgery. Langenbeck’s Arch Surg 2003;388:141–9.
Rösch J, Keller FS. Transjugular intrahepatic portosystemic shunt: present status, comparison with endoscopic therapy and shunt surgery, and future perspectives. World J Surg 2001;25:337–46.
Hausegger KA, Karnel F, Georgieva B, Tauss J, Portugaller H, Deutschmann H et al. Transjugular intrahepatic porto systemic shunt creation with the Viatorr expanded polytetrafluoroethylene-covered stent-graft. J Vasc Interv Radiol 2004;15:239–48.
Freedman AM, Sanyal AJ, Tisnado J, Cole PE, Sbiffman M, Luketic VA et al. Complications of transjugular intrahepatic portosystemic shunt: a comprehensive review. Radiographics 1993;13:1185–210.
Barton RE, Rösch J, Saxon RR, Lakin PC, Petersen BD, Keller FS. TIPS: short and long-term results: a survey of 1750 patients. Semin Interv Radiol 1995;12:364.
Orloff MJ. Prophylactic portasystemic shunt in non-cirrhotic portal fibrosis: is it worthwhile? Nobody knows. Indian J Gastroenterol 2005;24:233–5.
Kanagawa H, Mima S, Kouyama H, Gotoh K, Uchida T, Okuda K. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol 1996;11:51–8.
Hirotaka A, Takehiko A, Hitoshi T, Masatomo M. Efficacy of balloon-occluded retrograde transvenous obliteration, percutaneous transhepatic obliteration and combined techniques for the management of gastric fundal varices. World J Gastroenterol 2006;12:3866–73.
Shimoda R, Horiuchi K, Hagiwara S, Suzuki H, Yamazaki Y, Kosone T et al. Short-term complications of retrograde transvenous obliteration of gastric varices in patients with portal hypertension: effects of obliteration of major portosystemic shunts. Abdom Imaging 2005;30:306–13.
Sama SK, Bhargawa S, Nath NG, Talwar JR, Nayak NC, Tandon BN et al. Non-cirrhotic portal fibrosis. Am J Med 1971;51:160–9.
Sarin SK. Non-cirrhotic portal fibrosis. Gut 1989;5:336–51.
Sarin SK, Kumar A. Non cirrhotic portal hypertension. Clin Liver Dis 2006;10:627–51.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Sarin, S.K., Kumar, A., Chawla, Y.K. et al. Noncirrhotic portal fibrosis/idiopathic portal hypertension: APASL recommendations for diagnosis and treatment. Hepatol Int 1, 398–413 (2007). https://doi.org/10.1007/s12072-007-9010-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-007-9010-9