Abstract
Background
Despite wide use as a diagnostic tool in medical and dental practice, radiography can induce cytotoxic effects and genetic damage.
Objective
To evaluate DNA damage (micronucleus) and cellular death (pyknosis, karyolysis and karyorrhexis) in exfoliated buccal mucosa cells taken from healthy children following exposure to radiation during dental radiography.
Materials and methods
A total of 17 children who had undergone panoramic dental radiography were included.
Results
We found no statistically significant differences (P > 0.05) between micronucleated oral mucosa cells in children before and after exposure to radiation. On the other hand, radiation did cause other nuclear alterations closely related to cytotoxicity including karyorrhexis, pyknosis and karyolysis.
Conclusion
Taken together, these results indicate that panoramic dental radiography might not induce chromosomal damage, but may be cytotoxic. Overall, the results reinforce the importance of evaluating the health side effects of radiography and contribute to the micronucleus database, which will improve our understanding and practice of this methodology in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, considerable progress has been made in imaging science, and this has led to substantial improvements in the field of medical and dental care. In this context, radiography plays an important role in diagnosis in medical and dental practice. However, it is well known that X-rays can induce cytotoxic effects and DNA damage [1]. Considering the strong evidence for a relationship between genetic damage and carcinogenesis [2], the elucidation of the genotoxic effects induced by X-rays is relevant to the identification of the degree of cancer risk and to the minimization of the potential risks to patients and clinicians.
Relevant genetic parameters for human biomonitoring studies include whether the exposure results in the induction of DNA damage, accumulation of damage with repeated exposure, and persistence of damage after exposure to elucidate the kinetics of the recovery process, which includes DNA repair and tissue regeneration [3]. Cytogenetic methods have been used longest and most extensively for the biological monitoring of populations exposed to known mutagens and carcinogens [4]. A great deal of enthusiasm was raised by the application for this purpose of the micronucleus test to uncultured exfoliated cells [5]. A micronucleus arises from acentric fragments or whole chromosomes that are not included in the main nuclei of the daughter cells. Therefore, the formation of micronuclei can be induced by substances that cause chromosome breakage (clastogens) as well as by agents that affect the spindle apparatus (aneugens) [6]. Recently, by means of the micronucleus assay we have encountered cytogenetic damage in buccal mucosa cells of patients with head-and-neck cancer undergoing radiotherapy [7]. In order to contribute to a better understanding of the effects of X-rays upon the cellular system in developing children, in the present study we investigated the frequencies of micronucleated cells in the oral mucosa cells of children who had undergone panoramic dental radiography. To monitor the cytotoxic effects, pyknosis, karyolysis and karyorrhexis were also evaluated in this setting.
Material and methods
Subjects
The subjects of this study comprised 17 healthy children (8 boys and 9 girls) with a mean age of 7.70 ± 1.50 years who had undergone panoramic dental radiography as outpatients at the Department of Pediatrics, São Paulo Metodista University, UMESP, SP, Brazil. Information gathered on the subjects included gender, age, and exposure to genotoxic agents. All panoramic dental radiography examinations were requested by the dentist and were performed using Siemens Orthophos equipment (Erlangen, Germany) with the following settings: 250–71 kV, 15 mA 14 s, 110 mGycm2. The entrance dose was 0.08 R. The study was approved by the Human Ethics Committee of UMESP. Informed consent was obtained from the individuals included in the study or from their parents.
Micronucleus test in oral mucosa cells
Exfoliated oral mucosa cells were collected immediately before the X-ray exposure and after 10 days. After the children had rinsed their mouths with tap water, cells were obtained by scraping the right/left cheek mucosa with a moist wooden spatula. Cells were transferred to a tube containing saline solution, centrifuged (800 rpm) for 5 min, fixed in 3:1 methanol/acetic acid, and dropped onto precleaned slides. Later, the air-dried slides were stained using the Feulgen/Fast-Green method and examined under a light microscope at ×400 magnification to determine the frequency of micronucleated cells. For each sampling time (before and after X-ray exposure), 2,000 cells were scored from each patient.
Data analysis
Micronuclei were scored according to the criteria described by Sarto et al. [8] as a measure of DNA damage (mutagenicity). For cytotoxicity, the following nuclear alterations were considered as described elsewhere [9]: pyknosis, karyolysis and karyorrhexis. The results are expressed as percentages.
Statistical methods
The Wilcoxon rank sum test for dependent samples was used to compare the frequencies of micronuclei and other cellular alterations among the samples before and after X-ray exposure. P < 0.05 was considered significant.
Results
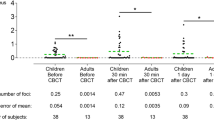
Table 1 shows the frequency of micronucleated cells in children undergoing panoramic dental radiography. Before X-ray exposure, the mean frequency of micronucleated cells was 0.04%. No statistically significant differences (P > 0.05) were noted after X-ray exposure. On the other hand, we observed an increase in other nuclear alterations after dental radiography, specifically karyorrhexis, pyknosis and karyolysis (Fig. 1). These data are summarized in Table 1. None of the children evaluated was exposed to other known genotoxic agents.
Discussion
Industrialization and urbanization has led to the production of genotoxic chemicals that frequently endanger children’s health. This is because children, who are still developing, express increased susceptibility to environmental hazards because of differences in the uptake, metabolism, distribution and excretion of DNA-damaging agents [10]. For example, some studies have shown an increased risk of tumors in children as a result of exposure to genotoxic carcinogens such as pesticides [11, 12]. It is possible that the occurrence of tumors is associated with a reduced ability to metabolize the pesticides. Furthermore, a higher capacity for DNA damage and a decreased capacity for DNA repair have been found in children suffering from malnutrition, a common problem in developing countries [13]. Because micronuclei are assumed to be expressed in dividing cells that contain chromosome fragments and/or whole chromosomes that are unable to migrate to the spindle poles during mitosis, we considered the micronucleus test appropriate for assessing chromosome damage in children following exposure to X-rays. The micronucleus assay of exfoliated buccal mucosa cells has been systematically used in genetic biomonitoring of populations exposed to genotoxic chemicals such as tobacco products and alcohol [14, 15]. The usefulness of the micronucleus assay for biomonitoring of children has also been confirmed by a growing number of studies [16]. The key advantage of the micronucleus assay is the relative ease of scoring, the limited cost and person-time required, and the precision obtained from scoring larger numbers of cells. However, the approach has not been addressed in the literature because the most common reason for studying micronuclei in children is exposure to ionizing radiation as a consequence of accidents [17, 18].
Damage that leads to the formation of micronuclei takes place in the basal layer of the epithelial tissue, where cells undergo mitosis. Rapid turnover of epithelial tissues brings the cells to the surface where they exfoliate. As a result, the maximal rate of micronuclei formation in exfoliated cells is seen 1–3 weeks after exposure to the genotoxic agent [19, 20]. For this reason, we studied cells 10 days after X-ray exposure.
Micronucleated cell indexes might reflect genomic instability [21, 22]. The detection of an elevated frequency of micronuclei in a given population indicates an increased risk of cancer [23]. We found that the micronucleus frequencies were not significantly different before and after X-ray exposure. Such findings are in line with those of many other studies [24]. There have been some reports of higher rates of cytogenetic damage induced by X-rays [25]. Biomonitoring studies of populations exposed to X-rays are quite difficult and rather specific because each population is exposed to different doses of radiation. This could explain why some studies have shown an increase in genetic damage in populations exposed to X-rays. Taken together, we assumed a lack of clastogenic and/or aneugenic effects related to panoramic dental radiography in children.
To monitor cytotoxic effects, the frequencies of karyorrhexis, karyolysis and pyknosis were evaluated in this experimental design. Despite the lack of cytogenetic damage, our results demonstrated that panoramic dental radiography induced cellular death as indicated by statistically significant differences (P < 0.05) between values before and after X-ray exposure. Similar results have been reported by others [24, 26]. Taken as a whole, such results support the idea that X-rays are a cytotoxic agent. It is important to stress that cytotoxicity interferes with micronucleus induction because some micronucleated cells are inevitably lost after a cytotoxic insult, confirming the lack of a mutagenic effect of X-rays. Nevertheless, it has been postulated that repeated exposure to cytotoxic agents can result in chronic cell injury, compensatory cell proliferation, hyperplasia and ultimately tumor development [27]. In fact, a correlation between cell proliferation and induction of cancer can be assumed [28]. Proliferation likely increases the risk of mutations within target cells and is important in selective clonal expansion of (exogenously or endogenously) initiated cells from preneoplastic foci and eventually tumors [27].
In human cytogenetic studies, some confounding factors must be considered. Viruses, alterations in the immune system, failures in the DNA repair system, and interindividual variations have been associated with increased frequencies of chromosome aberrations [29]. Furthermore, an age-related increase in micronuclei has been postulated [16]. Because of heterogeneity in the casuistics, it was not possible to correlate the frequency of micronucleated cells with the age in this setting. The influence of tobacco smoke and alcohol are usually considered relevant confounding factors [14]. Obviously, biomonitoring studies in children are less affected by confounders such as cigarette smoke and drinking habits, occupational exposure and diet, which are of great concern in adults.
Conclusion
The results of the present study suggest that X-rays can induce cytotoxic effects in oral mucosa cells. Thus, we cannot assert that X-rays did not induce any such effects in the specific group of children included in this study. In this regard, panoramic dental radiography should be used only when necessary because it can induce cellular death. This study also confirms the usefulness of the micronucleus assay in biomonitoring studies conducted in children, emphasizing their great sensitivity even to exposure to low doses of environmental agents. Further studies in children would be beneficial.
References
Koturbash I, Rugo RE, Hendricks CA et al (2006) Irradiation induces DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene 20:4267–4275
Ribeiro DA, Marques ME, de Assis GF et al (2004) No relationship between subchronic fluoride intake and DNA damage in Wistar rats. Caries Res 38:576–599
Collins AR (1998) Molecular epidemiology in cancer research. Mol Aspects Med 19:359–432
Sari-Minodier I, Orsiere T, Bellon L et al (2002) Cytogenetic monitoring of industrial radiographers using the micronucleus assay. Mutat Res 521:37–46
Stich HF, Parida BB, Brunnemann KD (1992) Localized formation of micronuclei in the oral mucosa and tobacco-specific nitrosamines in the saliva of “reverse” smokers, Khaini-tobacco chewers and gudakhu users. Int J Cancer 21:172–176
Belien JA, Copper MP, Braakhuis BJ et al (1995) Standardization of counting micronuclei: definition of a protocol to measure genotoxic damage in human exfoliated cells. Carcinogenesis 16:2395–2400
Minicucci EM, Kowalski LP, Maia MA et al (2005) Cytogenetic damage in circulating lymphocytes and buccal mucosa cells of head-and-neck cancer patients undergoing radiotherapy. J Radiat Res (Tokyo) 46:135–142
Sarto F, Finotto S, Giacomelli L et al (1987) The micronucleus assay in exfoliated cells of the human buccal mucosa. Mutagenesis 2:11–17
Tolbert PE, Shy CM, Allen JW (1992) Micronuclei and other nuclear anomalies in buccal smears: methods development. Mutat Res 271:69–77
Suk WA, Murray K, Avakian MD (2003) Environmental hazards to children’s health in the modern world. Mutat Res 544:235–242
Cordier S, Mandereau L, Preston-Martin S et al (2001) Parental occupations and childhood brain tumors: results of an international case-control study. Cancer Causes Control 12:865–874
Efird JT, Holly EA, Preston-Martin S et al (2003) Farm-related exposures and childhood brain tumours in seven countries: results from the SEARCH international brain tumour study. Paediatr Perinat Epidemiol 17:201–211
Gonzalez C, Najera O, Cortes E et al (2002) Hydrogen peroxide-induced DNA damage and DNA repair in lymphocytes from malnourished children. Environ Mol Mutagen 39:33–42
Bloching M, Hofmann A, Lautenschlager C et al (2000) Exfoliative cytology of normal buccal mucosa to predict the relative risk of cancer in the upper aerodigestive tract using the MN-assay. Oral Oncol 36:550–555
Nersesyan AK (2006) Does cigarette smoking induce micronuclei in buccal cells? Am J Clin Nutr 84:946–947
Xu GL, Bestor HH, Bourc’his D et al (1999) Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature 402:187–191
da Cruz AD, McArthur AG, Silva CC et al (1994) Human micronucleus counts are correlated with age, smoking, and cesium-137 dose in the Goiania (Brazil) radiological accident. Mutat Res 313:57–68
Livingston GK, Jensen RH, Silberstein EB et al (1997) Radiobiological evaluation of immigrants from the vicinity of Chernobyl. Int J Radiat Biol 72:703–713
Fenech M, Holland N, Chang WP et al (1999) The human micronucleus project-an international collaborative study on the use of the micronucleus technique for measuring DNA damage in humans. Mutat Res 428:271–283
Majer BJ, Laky B, Knasmuller S et al (2001) Use of the micronucleus assay with exfoliated epithelial cells as a biomarker for monitoring individuals at elevated risk of genetic damage and in chemoprevention trials. Mutat Res 489:147–172
Maluf SW, Erdtmann B (2001) Genomic instability in Down syndrome and Fanconi anemia assessed by micronucleus analysis and single cell-gel electrophoresis. Cancer Genet Cytogenet 124:71–75
Neri M, Fucic A, Knudsen LE et al (2003) Micronuclei frequency in children exposed to environmental mutagens: a review. Mutat Res 544:243–254
Hagmar L, Bonassi S, Stomberg U et al (1998) Chromosomal aberrations in lymphocytes predict human cancer: a report from the European Study Group on Cytogenetic Biomarkers and Health (ESCH). Cancer Res 58:4117–4121
Cerqueira EM, Gomes-Filho IS, Trindade S et al (2004) Genetic damage in exfoliated cells from oral mucosa of individuals exposed to X-rays during panoramic dental radiographies. Mutat Res 562:111–117
He JL, Chen LF, Jin LF et al (2000) Comparative evaluation of the in vitro micronucleus test and the comet assay for the detection of genotoxic effects of X-ray radiation. Mutat Res 469:223–231
Torres-Bugárin O, Ventura-Aguilar A, Zamora-Perez A et al (2003) Evaluation of cisplatin+5-FU, carboplatin+5-FU, and ifosfamide+epirubicine regimens using the micronuclei test and nuclear abnormalities in buccal mucosa. Mutat Res 539:177–186
Mally A, Jagetia JK (2002) Non-genotoxic carcinogens: early effects on gap junctions, cell proliferation and apoptosis in the rat. Toxicology 180:233–248
Swenberg JA (1993) Cell proliferation and chemical carcinogenesis: conferences summary and future directions. Environ Health Perspect 101:153–158
Jagetia GC, Jayakrishnan A, Fernandes D et al (2001) Evaluation of micronuclei frequency in the cultured peripheral blood lymphocytes of cancer patients before and after radiation treatment. Mutat Res 491:9–16
Acknowledgements
G.R. de Oliveira is recipient of CNPq student’s fellowship (PIBIC). The authors are thankful to Maria Luisa Falaguera Ardanaz for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Angelieri, F., de Oliveira, G.R., Sannomiya, E.K. et al. DNA damage and cellular death in oral mucosa cells of children who have undergone panoramic dental radiography. Pediatr Radiol 37, 561–565 (2007). https://doi.org/10.1007/s00247-007-0478-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-007-0478-1