Abstract
Device closure of atrial septal defect (ASD) is commonly performed in older children and adults. Infants and toddlers (age <4 years) are seldom referred for ASD closure due to size constraints. However, in many cases device ASD closure can be performed in this population. Between 2002 and 2012, 61 infants and toddlers were taken to the catheterization laboratory at our institution for ASD closure. Precatheterization transthoracic echocardiograms, intracatheterization transesophageal echocardiograms, and catheterization reports were reviewed. Fifty-three infants and toddlers presented for percutaneous ASD occlusion. Forty-eight (79 %) underwent successful closure, and 13 were referred for surgery without device attempt (n = 8) or after unsuccessful device occlusion (n = 4). Median age and weight at time of ASD closure were 2.99 years (range 0.3–3.8) and 11.7 kg (range 3.7–16.5). The device-to-septal length ratio was 0.81 (range 0.44–1.03). The 12 unsuccessful cases occurred in patients with larger defects (ASD diameter 17.5 ± 6.1 vs. 12.1 ± 4.2, p < 0.01). Deficient rims (absent or ≤4 mm) were seen in 9 of 12 (75 %) unsuccessful cases and in 19 of 41 (46 %) successful cases (p = 0.12). Multivariate analysis showed that patient size and ASD size were not independently associated with procedural success but that ASD size-to-patient weight ratio <1.2 (hazard ratio 9.5 [range1.7–17]) was associated with successful ASD closure. ASD device occlusion can be safely achieved in small children. An ASD size-to-patient weight ratio >1.2, not absolute patient weight or age, is associated with failure of the percutaneous approach. The midterm outcomes in these young patients are excellent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Device occlusion of ostium secundum atrial septal defect (ASD) has been successfully undertaken since King and Mills first described their approach in 1976 [13]. Refinements and miniaturization of devices and delivery systems have made ASD closure by way of device a routine procedure in older patients. Normally, device closure of ASD is reserved for older children, adolescents, and adults for both anatomic and physiologic reasons. Larger patients with centrally located defects represent the typical population presenting for device closure. Infants and toddlers (age <4 years) with ASDs are often observed until they have grown to acceptable size to allow for device closure.
There are occasions, however, where device closure of ASD in infants and toddlers is contemplated [11]. Young patients are rarely symptomatic from isolated ASD, yet infants with left heart obstruction may present with significant pulmonary overcirculation and congestive heart failure [19]. Infants with significant lung disease, e.g., former premature infants with chronic lung disease, may benefit from early elimination of left-to-right shunt [14, 16]. Finally, infants who undergo cardiac catheterization for other interventions may be considered for concomitant ASD device closure. Limited data exist regarding the feasibility of transcatheter ASD closure in infants and toddlers, and there are no data assessing factors associated with inability to close ASDs in this population [7, 24]. We evaluated the outcomes from our institutional experience with transcatheter ASD closure in patients <4 years of age. We focused not only on cases of successful closure of the ASD but also on cases in which ASD closure was attempted or contemplated but found to be imprudent or impossible.
Methods
Patient Population
A retrospective review was performed that included all patients <4 years of age with ostium secundum ASD referred to the cardiac catheterization laboratory between January 1, 2002, and March 31, 2012. Primary indication for ASD closure was reviewed for each patient. Transthoracic echocardiograms immediately preceding catheterization were retrospectively reviewed (R. H. P.) for measurement of total septal length (TSL) as measured by apical four-chamber view. Patient characteristics at the time of catheterization (age, sex, weight, and body surface area [BSA]) were recorded. All patients underwent transesophageal echocardiogram (TEE) after induction of general endotracheal anesthesia but before placement of intravascular sheaths. TEE assessment was used for ASD size, which was defined as the maximal dimension of the ASD in any plane. Deficient atrial septal rim was defined as absence of any rim or an atrial rim <4 mm in length. Balloon-sized measurement of the ASD was also recorded for patients who underwent catheterization.
Procedural details from the catheterization, including sheath size, procedural complications, and success of ASD device–occlusion, were recorded. Concomitant interventions at the time of catheterization were noted. Type of ASD occlusion device was also noted. At our institution during the study period, only the Amplatzer Septal Occluder (ASO; Medtronic, St. Paul, MN) and the Helex device (Gore, Flagstaff AZ) were used for ASD closure. For patients who underwent device closure of the ASD, late outcomes, including length of follow-up, late complications (erosion, embolization, etc.), need for cardiac surgery, and survival, were reviewed.
Statistical Analysis
Patients were divided into two groups: Group 1 comprised patients who underwent device-closure of the ASD and were discharged from the catheterization laboratory with a closed ASD, and group 2 comprised patients who, after TEE, were referred to surgery after coming to the catheterization laboratory. Group 2 patients underwent TEE ± catheterization and attempted device placement. Patients with unfavorable anatomy (e.g., absent posterior septal rim), in whom no sheaths were placed, were also included in group 2. The anatomic and patient-specific characteristics of the two groups were compared. For continuous variables, Student t test was performed; for categorical variables, Chi-square test was performed. Binary logistic regression was performed for multivariate analysis of factors and variables that showed p < 0.15 on univariate analysis. A p value <0.05 was considered statistically significant. SPSS (IBM, Chicago, IL) version 19.0 was used for analysis. The Institutional Review Board of Baylor College of Medicine approved this study.
Results
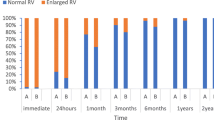
A total of 61 children <4 years of age presented to our institution for possible ASD closure between 2002 and 2012. The median age and weight of the patients were 2.9 years (range 0.3–3.8) and 11.8 kg (range 3.5–16.5), respectively (Table 1). Of these 61 patients, 22 (36 %) presented for other interventions primarily. The primary indication for catheterization was right-ventricular (RV) enlargement in 21 patients, failure to thrive in 4, chronic lung disease or frequent pulmonary exacerbations in 16, and other cardiac intervention in 21 patients (Table 1). TEE was performed in all patients. The median ASD dimension was 13.0 mm (range 4–26), whereas the median balloon-stretched dimension was 14.8 mm (range 5.7–28). The median pulmonary-to-systemic flow (Qp:Qs) ratio was 1.9 (1.1–4.3) among the entire cohort.
A total of 48 patients (79 %) underwent successful occlusion of the ASD after TEE and hemodynamic catheterization. Nine patients had their ASD closed with a Helex device (Gore Medical, Flagstaff, AZ) device; one patient underwent occlusion with a CardioSEAL device (NMT Medical, Boston, MA); and the remaining 38 patients underwent closure with an ASO device (Medtronic, St. Paul, MN). Table 1 lists procedural details for the 48 patients who underwent device closure of their ASD. After ASD closure, these 48 patients were followed-up for 1.7 (range 0.1–7.1) years. In a small infant with pulmonary hypertension, the ASD device embolized to the left ventricle within 24 h. This device was surgically retrieved and the ASD closed surgically. The remaining 47 patients had uncomplicated postcatheterization courses. On latest echocardiogram, no residual shunting was noted across the ASD in all patients. There have been no instances of erosions or other complications after ASD closure. No patient required cardiac surgery after successful ASD closure.
After TEE, 13 patients were referred for surgical closure of the ASD; 5 patients were referred after TEE alone; and 8 patients underwent TEE and catheterization for balloon sizing of the ASD. Five of the 13 patients had an attempt at ASD device closure; in all 5 cases, the device was withdrawn due to unfavorable appearance on TEE. Ten of the 13 patients underwent surgical closure 82 days (range 15–369) after catheterization. Three patients await surgical closure.
The group who underwent successful ASD closure (group 1) was compared with the group (n = 13) who did not undergo device ASD closure (group 2). Table 2 lists the anatomic characteristics of the patients, including comparison of the 48 successful ASD-closure cases with the 13 unsuccessful-closure cases. Although the patients in both groups were of similar age, weight, and BSA, the ASD was significantly larger in the unsuccessful cases at 16.2 ± 5.6 mm compared with 12.8 ± 4.1 mm in the successful cases (p < 0.01). Furthermore, although TSL was similar between groups, the ASD-to-TSL ratio was significantly higher in the unsuccessful group at 0.53 ± 0.14 compared with 0.33 ± 0.11 for the successful group (p = 0.001) (Fig. 1). ASD size relative to BSA and patient weight was also greater in unsuccessful cases (Fig. 2). Of the group 1 patients, 88 % had an ASD-to-weight ratio <1.2 compared with only 17 % of the group 2 patients (p < 0.01). Multivariate analysis (Table 3) showed that ASD-to-weight ratio <1.2 was associated with significantly higher rate of successful device closure (hazard ratio [HR] 9.5, p < 0.01). Reflecting the difference in defect dimensions, for group 2 patients the anticipated device size (left atrial disc dimension) was larger than TSL, whereas for group 1 patients, the maximal device dimension (LA disc dimension) was less than or equal to TSL (Fig. 3).
Scatterplots show the correlation between relative size of the ASD and success of ASD device closure. a Scatterplot of ASD (mm)-to-weight (kg) ratio There is an inverse relationship between ASD-to-weight ratio and probability of successful device closure. The dotted line indicates a ratio of 1.2. The majority of unsuccessful cases (10 of 12) had a ratio >1.2. b Scatterplot of ASD-to-BSA ratio, which shows that the probability of successful device closure is higher in patients with a lower ratio. The patients referred for surgical ASD closure (group 2) had a median ASD-to-BSA ratio of 32.6
There were 8 patients who weighed <10 kg in the entire cohort. Of these patients, 6 (75 %) underwent successful closure compared with 79 % of patients who weighed >10 kg at the time of catheterization (p = 0.785). Similarly, there were 9 patients <2 years of age at the time of ASD closure attempt, and 7 (78 %) had successful ASD device closure compared with 41 of 52 (79 %) patients >2 years of age (p = 0.942).
Discussion
Device closure of ASDs was initially attempted in 1976 when the King–Mills device was described [13]. Due to the size of delivery systems inherent to early devices, percutaneous closure of ASDs was reserved for older children and adults in the early experience. The King–Mills device used a 23F delivery “baton,” whereas the first Rashkind ASD device used a 16F delivery system [18, 20]. After the ASO device was approved by the United States Food and Drug Administration, an ASD device was now available that could be delivered through a 6F to 11F delivery system [6]. This miniaturization has helped make commonplace transcatheter ASD closure in children [12, 22].
With broader application of this procedure, there has been a natural trend toward closing defects in more challenging clinical scenarios, including both larger defects and defects in increasingly smaller patients. Lim et al. reported successful device closure in a 2.8-kg premature infant with bronchopulmonary dysplasia [16]. There have been a number of other reports of ASD closure in young children and infants [3, 5, 10, 24]. Our study adds to the collective experience that documents the feasibility of device closure of ASDs in small children. During follow-up, we found no late complications despite the fact that the device-to-TSL ratio approached and exceeded 1.0 in some cases. We also reviewed the instances in which ASDs were not device-closed in this population. We found that patient age and weight are not important factors in determining probability of success of device closure. Rather, the size of the ASD relative to patient weight and TSL are the most important factors when considering the likelihood of successful device implantation.
The majority of infants and toddlers are asymptomatic when an isolated moderate-sized ASD is present [1, 4]. In this case, the cardiologist may allow these patients to grow until later childhood, when cardiac catheterization and ASD closure can be performed with fewer challenges. There are cases, nonetheless, in which early closure of ASD may be indicated. Infants with isolated secundum ASDs may experience congestive heart failure and fail to thrive [2, 11]. Particularly in the setting of left heart obstruction, medical management of ASD in infants may be insufficient to relieve heart failure [17, 19]. In these patients, the decrease in RV volumes is accompanied by improved left-ventricular volumes [8, 11].
Nearly 20 % of our cohort had a history of prematurity and chronic lung disease as the primary indication for ASD closure. A number of studies have shown that elimination of atrial-level shunting improves lung function [15, 25]. Zaqout et al. showed that forced vital capacity (FVC) and forced expired volumes (FEV1) improve early after ASD closure and that this improvement is more rapid in patients undergoing percutaneous compared with surgical closure [25]. Lee et al. reported that among a cohort of 55 children with mean age 9.5 years, FVC and FEV1 improved within 3 days of device closure, and improvements in pulmonary function continued 6 months after the procedure [15]. In this cohort, patients with pulmonary hypertension were less likely to exhibit significant improvement. Eerola et al. found that hemodynamic benefits of ASD closure occurred more quickly in children undergoing percutaneous occlusion [9].
Premature infants with chronic lung disease may have significant benefit from ASD closure. Lammers et al. showed that surgical repair of isolated ASDs improved clinical scores in small infants with median weight <5 kg having severe bronchopulmonary dysplasia, prematurity, and pulmonary hypertension [14]. In that cohort, all infants were in congestive heart failure, and the majority were long-term inpatients due to respiratory disease. After surgical closure, all but one patient were discharged.
Among our cohort, 34 % of our patients were considered for ASD closure as part of another primary cardiac intervention. Combining multiple interventions in a single catheterization affords these patients benefits [23]. For these patients, device occlusion of the ASD obviated the need for a second interventional procedure. Interestingly, we were able to achieve technical success in patients with deficient or absent rims. Rastogi et al. reviewed factors associated with successful device implantation for children and adults with ASDs and found that deficient rims and device-to-ASD ratio <1.125 or >1.33 were risk factors for complications in that group [21]. In our cohort, device success was achieved among a wide range of device-to-ASD ratios. The critical factor associated with success of device closure was relative ASD size to patient weight and TSL. Possible explanations for the difference in factors include the different populations—our study involved only young children <4 years of age, whereas the Rastogi cohort mean age was 23 years—and the fact that our practice rarely brings patients to the laboratory for possible closure when two or more septal rims appear deficient or absent.
Our study is limited by its retrospective nature and relatively small cohort. The follow-up available was likewise limited. Nevertheless, our study shows that device closure is feasible in this population of young children. The operator should be aware of the size of the ASD in relation to TSL and the weight of the patient when counseling families anticipating possible device closure. When the ASD size is >1.2 times the patient’s weight (in kilograms), the likelihood of success of device closure decreases significantly. With careful preselection, this procedure offers significant benefit to infants and toddlers with important lung disease or who are undergoing other catheter-based interventions.
References
Allen H, Gutgesell H, Clark E, Driscoll D (2003) Moss and Adams’ heart disease in infants, children, and adolescents, 6th edn. Lippincott Williams & Wilkins, Philadelphia
Bull C, Deanfield J, de Leval M, Stark J, Taylor JF, Macartney FJ (1981) Correction of isolated secundum atrial septal defect in infancy. Arch Dis Child 56(10):784–786
Butera G, De Rosa G, Chessa M, Rosti L, Negura DG, Luciane P et al (2003) Transcatheter closure of atrial septal defect in young children: results and follow-up. J Am Coll Cardiol 42(2):241–245
Campbell M (1970) Natural history of atrial septal defect. Br Heart J 32(6):820–826
Cardenas L, Panzer J, Boshoff D, Malekzadeh-Milani S, Ovaert C (2007) Transcatheter closure of secundum atrial defect in small children. Catheter Cardiovasc Interv 69(3):44–452
Chan KC, Godman MJ, Walsh K, Wilson N, Redington A, Gibbs JL (1999) Transcatheter closure of atrial septal defect and interatrial communications with a new self expanding nitinol double disc device (Amplatzer septal occluder): multicentre UK experience. Heart 82(3):300–306
Diab KA, Cao QL, Bacha EA, Hijazi ZM (2007) Device closure of atrial septal defects with the Amplatzer septal occluder: safety and outcome in infants. J Thorac Cardiovasc Surg 134(4):960–966
Ding J, Ma G, Huang Y, Wang C, Zhang X, Zhu J et al (2009) Right ventricular remodeling after transcatheter closure of atrial septal defect. Echocardiography 26(10):1146–1152
Eerola A, Pihkala JI, Boldt T, Mattila IP, Poutanen T, Jokinen E (2007) Hemodynamic improvement is faster after percutaneous ASD closure than after surgery. Catheter Cardiovasc Interv 69(3):432–441 discussion 442
Fischer G, Smevik B, Kramer HH, Bjornstad PG (2009) Catheter-based closure of atrial septal defects in the oval fossa with the Amplatzer device in patients in their first or second year of life. Catheter Cardiovasc Interv 73(7):949–955
Hunt CE, Lucas RV Jr (1973) Symptomatic atrial septal defect in infancy. Circulation 47(5):1042–1048
Karamlou T, Diggs BS, Ungerleider RM, McCrindle BW, Welke KF (2008) The rush to atrial septal defect closure: is the introduction of percutaneous closure driving utilization? Ann Thorac Surg 86(5):1584–1590 discussion 1590–1581
King TD, Thompson SL, Steiner C, Mills NL (1976) Secundum atrial septal defect, Nonoperative closure during cardiac catheterization. AMA 235(23):2506–2509
Lammers A, Hager A, Eicken A, Lange R, Hauser M, Hess J (2005) Need for closure of secundum atrial septal defect in infancy. J Thorac Cardiovasc Surg 129(6):1353–1357
Lee YS, Jeng MJ, Tsao PC, Yang CF, Soong WJ, Hwang B et al (2009) Pulmonary function changes in children after transcatheter closure of atrial septal defect. Pediatr Pulmonol 44(10):1025–1032
Lim DS, Matherne GP (2007) Percutaneous device closure of atrial septal defect in a premature infant with rapid improvement in pulmonary status. Pediatrics 119(2):398–400
Manning PB, Mayer JE Jr, Sanders SP, Coleman EA, Jonas RA, Keane JF et al (1994) Unique features and prognosis of primum ASD presenting in the first year of life. Circulation 90(5 Pt 2):II30–II35
Mills NL, King TD (2003) Late follow-up of nonoperative closure of secundum atrial septal defects using the King-Mills double-umbrella device. Am J Cardiol 92(3):353–355
Petit C, Justino H, Fraser C (2012) Percutaneous closure of an atrial septal defect in an infant with Shone’s syndrome. Catheter Cardiovasc Interv 80:880–883
Rashkind WJ (1983) Transcatheter treatment of congenital heart disease. Circulation 67(4):711–716
Rastogi N, Smeeton NC, Qureshi SA (2009) Factors related to successful transcatheter closure of atrial septal defects using the Amplatzer septal occluder. Pediatr Cardiol 30(7):888–892
Rossi RI, Cardoso Cde O, Machado PR, Francois LG, Horowitz ES, Sarmento-Leite R (2008) Transcatheter closure of atrial septal defect with Amplatzer device in children aged less than 10 years old: immediate and late follow-up. Catheter Cardiovasc Interv 71(2):231–236
Turkay S, Abdullah E, Celal A, Cenap Z, Nurdan E, Fadli D et al (2010) Multiple transcatheter interventions in the same session in congenital cardiopathies. J Cardiovasc Dis Res 1(4):181–190
Wood AM, Holzer RJ, Texter KM, Hill SL, Gest AL, Welty SE et al (2011) Transcatheter elimination of left-to-right shunts in infants with bronchopulmonary dysplasia is feasible and safe. Congenit Heart Dis 6(4):330–337
Zaqout M, De Baets F, Schelstraete P, Suys B, Panzer J, Francois K et al (2010) Pulmonary function in children after surgical and percutaneous closure of atrial septal defect. Pediatr Cardiol 31(8):1171–1175
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petit, C.J., Justino, H., Pignatelli, R.H. et al. Percutaneous Atrial Septal Defect Closure in Infants and Toddlers: Predictors of Success. Pediatr Cardiol 34, 220–225 (2013). https://doi.org/10.1007/s00246-012-0413-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-012-0413-6