Abstract
Atrial septal defect (ASD) is one of the most common congenital heart defects. Transcatheter device closure of ASDs is safe and effective with most of the reported data being described from developed countries. To evaluate the short and mid-term results and experience of device closure of ASDs at a tertiary center in a developing country and compare it to that from developed countries. Retrospective study based on data collection from all patients who have undergone transcatheter percutaneous device closure for ASD from January 2005 until December 2017 at the Children’s Heart Center at the American University of Beirut, Medical Center, Lebanon. During the study period, a total of 254 cardiac catheterizations were performed for device closure of ASDs. The mean age of the patients was 18 ± 17.9 years with 37% being less than 6 years of age. Females were 54%. Defect size ranged from 7 to 37 mm and device size ranged from 8 to 40 mm. The procedure was executed with a success rate of 96%. Five patients had device embolization (2%); in one patient the device was snared and for the remainder the devices were removed surgically. None of the study patients had thrombus formation, neurological complications, bacterial endocarditis, or cardiac erosions. There was no mortality. Device closure of ASDs at our tertiary center in a developing country has an effective and safe profile with excellent results and low complications rates, which compare favorably to those reported from centers in developed countries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atrial septal defect (ASD) is the third most common type of congenital heart disease (CHD); It generally accounts for 7–10% of all congenital heart anomalies in children with an incidence of 1/1500 live births, and represents up to one-third of cardiac defects in adults with CHD [1]. ASD represents the first entity to be successfully closed via catheter-employed device. The historical background of closure was initiated by King and Mills in 1976 who described the transcatheter delivery approach [2]. Several modifications of the procedure were subsequently reported with two devices, the Amplatzer™ Septal Occluder (ASO) (AGA Medical Corp, GoldenValley, MN, USA) and the Helex Septal Occluder (HSO), eventually being approved by FDA for clinical use in 2001 and 2006, respectively [3, 4]. Recently, the Figulla ® ASD Occluder (FSO; Occlutech GmbH, Jena, Germany) was introduced and is now an alternative device for ASD closure and is widely used outside the United States [4,5,6,7].
As per the 2008 ACC/AHA Guidelines and 2010 ESC Guidelines [8, 9], device closure is the method of choice for secundum ASD [10,11,12,13] when applicable (Class I C) and closure of ASDs is indicated in the presence of significant shunt, showing signs of RV volume overload and with PVR < 5 WU regardless of symptoms (Class IB). Patient selection for device therapy depends on the age and weight of the patient, as well as the defect properties including its position, size, and adequacy of the surrounding rims of atrial septal tissue.
Extensive data have described the results of catheter closure of ASD in developed countries, including those from large multicenter studies [14,15,16,17,18]. However, the experience and outcome of device closure of ASD in developing countries remains scarce and limited [19,20,21].
Hence, the primary objective of this study was to retrospectively evaluate and review the safety, feasibility, complications, and outcome in Atrial Septal Defect (ASD) device closure at the Children’s Heart Center at the American University of Beirut—Medical Center (AUBMC), a tertiary referral center in a developing country, Lebanon; and compare it to that reported from developed countries.
Materials and Methods
Study Population and Data Collection
We retrospectively reviewed the in-hospital documentations of all patients who underwent cardiac catheterizations for ASD device closure at the Children’s Heart Center at AUBMC from January 2005 through December 2017.
Patients’ data were collected from the AUBMC medical records. The documents reviewed included pre- and post-procedural echocardiography reports, catheterization reports, physician progress notes, and discharge summaries. Gathered information included patient demographic data, defect properties (size, presence or absence of sufficient rims of atrial tissue, device to defect ratio), size and type of device used, procedural success, and complications at the time of intervention or during the follow-up period. Potential complications included device embolization, transient arrhythmia, need for surgical intervention, residual significant shunt, cerebrovascular accidents, permanent heart block, pericardial effusion, cardiac erosions, bleeding from the catheterization access site, infection, and death.
All procedures were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the AUBMC Institutional Review Board.
Devices
The two devices used at our center are the Amplatzer septal occluder (ASO) and the Occlutech Figulla (Occlutech GmbH., Jena, Germany) [4,5,6]. The Occlutech is very similar to the ASO, made of recapturable double round disc of Nitinol wire mesh, but with only one central pin in the right atrial disc [4,5,6].
Procedure
The procedures were performed under general anesthesia, in the presence of the anesthesia team. Patients were given prophylactic antibiotics (Cefazolin) prior to the procedure and continued every 8 h, for additional two doses. Patients underwent complete transesophageal echocardiography (TEE) studies, for better assessment of the defect including size, rims, and as a baseline assessment of all cardiac structures and function. Exclusion criteria for device closure based on TEE included a defect (> 38 mm), unfavorable anatomy (where there is deficient inferior/posterior rims), significant mitral valve prolapse and mitral regurgitation, or the presence of any lesion requiring surgical intervention. Absence of anterior rim was not considered as a contra indication for device closure of the defect.
After obtaining femoral venous access, heparin was administered at a dose of 100 units/Kg. Sizing of the defect was done mainly by TEE and/or in few cases by sizing balloon. The latter was used in cases of floppy or aneurysmal septum, multiple closely spaced defects, when questions arise regarding the defect size, or when the defect could not be well assessed by TEE. The ASD size was determined by noting the maximum width of color flow signal across the defect. The ASD device size is chosen to be 2–4 mm larger than the measured defect size or identical to or within 2 mm larger than the stretched diameter when sizing balloon was used.
Device implantation was performed using one of the several techniques; the most commonly performed classical approach was used for small-to-moderate size defects (< 25 mm) with adequate rims and it entailed exposing the left atrial (LA) disc from the long sheath, followed by withdrawal of the whole system until the LA disc touches the atrial septum, retracting then the long sheath to open the right atrial disc; the pulmonary vein delivery technique was utilized when the classical technique failed, the deployment of the LA disc in the right or left pulmonary veins was performed for better device alignment with the plane of the atrial septum, where the LA disc is parallel to the atrial septum. In few cases where the above techniques failed, we utilized the balloon-assisted technique to close the defect [22].
The position and stability of the device, the presence of residual leak, and interference with vital structures was evaluated using 2D and occasionally 3D TEE prior to its release. Device stability was rarely tested by the so-called “Minnesota wiggling maneuver.” Afterwards, the device is released from the carrier system. The sheaths were then withdrawn and hemostasis was achieved by manual compression in most of the cases; in some cases where large caliber sheaths were utilized, we used the temporary subcutaneous “Figure-of-Eight” suture technique for venous access site closure after removal of the large caliber sheaths [23].
All patients were monitored post procedure, and were assessed clinically by transthoracic echocardiography and ECG the following day. The device position, residual leakage, and its proximity to other structures were noted. The heart rhythm/rate and the presence of any ECG changes from the baseline were documented. Patients were discharged on Aspirin ® and were instructed about infective endocarditis prophylaxis regimen for the first 6 months. They were also counseled to seek medical advice in case of new symptoms, such as palpitations, chest pain, dizziness, severe headache, fever, abdominal pain, or bleeding.
Patients were followed up regularly by TTE and electrocardiograms after 1 week from the procedure date, 1 month, 6 months, 12 months, and yearly thereafter.
Statistical Analysis
Discrete variables are presented as absolute numbers and percentages of the total. Continuous variables are shown as means and standard deviation. The statistical analysis is conducted using IBMR SPSSR Statistics 24.
Results
Patient’s Characteristics
The mean age of the patients was 18 ± 17.9 years (range 2–73 years) with 37% being less than 6 years of age and 35% being more than 18 years (Table 1).
The female gender is slightly more predominant in our study population (54%).
Defect and Device
The defect size, as assessed by TEE, varied between 7 and 37 mm, whereas the device size ranged between 8 and 40 mm, with a mean of 21 ± 8 mm, (Tables 1, 2).
The ratio of the device size to the size of the ASD was 1.12 ± 0.10.
ASDs were occluded using the AmplatzerTM Septal Occluder (ASO) in 86% of the cases and the Figulla ® ASD Occluder in 14%.
Procedure Success
During the study period, a total of 254 cardiac catheterizations were performed with the intention of device closure of ASDs. In five patients, concomitant PDA closures were done and another pulmonary valvuloplasty was performed.
The procedure was executed with a success rate of 96.4%. Four patients attempted ASD device closure with no subsequent deployment. The device was unstable as demonstrated by TEE and fluoroscopy due to deficient or floppy rims and it was not released and these patients were referred for surgery.
Five patients had device embolization (2%) for which surgery was performed in four (device migrated into the right ventricle) for removal of the devices and closure of the ASDs. In one patient, embolization of the device into the descending aorta was noted the next day on follow-up and it was successfully retrieved and snared with no complications. Three patients had migration of the device on the table. The fourth patient had embolization that was noted 4 h post procedure and the fifth had embolization that was noted the next day. The device migration process occurred during the first 24 h of monitoring, with no sequelae. No late device migration was noted.
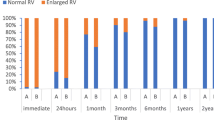
None of these patients had significant residual shunt (defined as > 3 mm) by transthoracic echocardiography (TTE) within 6 months after the procedure.
Complications
We used previously established and tested definitions for adverse events severity for this study that ranged from minor to high severity [24, 25]. The major complication that occurred was device embolization in 2% of the total cases. As discussed previously, these events were recognized and treated with no sequelae.
Transient complications including transient ST changes were noted in 1.3% and atrial arrhythmias (Atrial fibrillation/flutter) that required cardioversion occurred in another 1.5%. None of our patients developed heart block.
Patients were followed up for a mean period of 2 years. None of the patients developed thrombus formation, CNS accidents, hemodynamically significant pericardial effusions, cardiac erosions, pulmonary edema, endocarditis, or significant vascular complications. Moreover, surgery or additional device closure for significant residual shunt was not performed on any of our patients.
Discussion
Transcatheter closure of the secundum ASD is now commonly and widely used. It is considered a safe and effective procedure, with low rate of complications [10,11,12,13]. However, most of the reported data are described from developed countries with scarce data from developing countries.
To evaluate the experience of device closure of ASDs in a developing country, our study retrospectively examined 254 cases who underwent cardiac catheterizations with the intention to close the ASDs from 2005 to 2017. This study included a diverse population with 37% of the patients being less than 6 years of age and more than a third being more than 18 years of age. The measured mean ASD diameter was 18.6 (ranging from 7.0 to 37.0 mm). The device size ratio to the TEE size of the ASD defect was 1.12 ± 0.10 in our study, avoiding oversizing and commensurate with the reported norms.
In our patient population, the success rate was comparable to that reported from developed countries [4,5,6]. Our technical success rate was 96.4%; compared to 95.7% reported from the improving pediatric and adult congenital treatment (IMPACT) registry which provides the most comprehensive overview of congenital catheterization with 81 participating centers in 2013, 96%, by the MAGIC report, 95% by the C3PO report, and 95.7%, by the Amplatzer Septal Occluder (ASO) FDA study.
Four patients attempted ASD device closure with no subsequent deployment. The devices were unstable as demonstrated by TEE and fluoroscopy due to deficient or floppy rims and they were not released and these patients were referred for surgery. This subgroup of patients had large defects size and/or deficient rims [26].
We report a procedural complication rate of 4.8% which compares favorably to 5.7% in the IMPACT report, 5.9% in the MAGIC report, 11.5% in the C3PO report, and 7.2% in the ASO FDA study. In addition, the reported high severity adverse events, such as ASD device embolization, cardiac perforations, bacterial endocarditis, erosions, thromboembolic complications, or death, were acceptable at 2% in our study compared to the range of 1.6 and 2.2% reported in developed countries [14,15,16,17,18].
Five patients had device embolization (2%) for which surgery was performed on four for removal of the devices and closure of ASDs and these included 3 for large ASD measuring more than 34 mm in dimensions and one of relatively deficient SVC rim.
It is worth noting that three of the embolized devices included defects measuring more than 34 mm in diameter. The mean ASD diameter in our study was measured at 18.6 mm (7–37 mm), compared to a median diameter of 11 mm (2–32) in the C3PO study, 13.1 mm diameter in the IMPACT study and an ASD size ranging from 2 to 35 mm in the Magic study, illustrating a population with relatively larger defects in our study.
Although successful percutaneous retrieval of embolized devices is reported with success rates of 50–70%, we elected to use the surgical retrieval methods in 4 out of the 5 cases, since in those cases the defects were larger than 34 mm in dimensions to start with and thus the chance of another embolization was high, in addition to having an onsite surgeon available during the procedure. However, without surgical backup, the operator should be skilled in transcatheter device retrieval in the event of embolization. In fact, although rare, device embolization is a serious complication that must be highlighted.
The absence of major complications, other than device migration that occurred in 2% of our study population, ascertained the rarity of such events, and confirmed the high level of safety profile associated with the transcatheter closure of secundum ASD. Other major complications such as thrombus formation, recurrent transient ischemic attacks, cardiac erosions, or pericardial effusion were absent, similar to the results of other recently published reports.
Studies have reported that the Occlutech ® Figulla ® septal occluder closure outcome is very comparable to that of the ASO device and both devices are clinically safe and effective in ASD closure [5, 6]. In our study, ASDs were occluded using Amplatzer™ Septal Occluder (ASO) in 86% of the cases and Figulla(®) ASD Occluder in 14%. Comparing success rates and complications between Amplatzer and Occlutech groups in our study is not doable due to the small sample size and since selections were not randomized.
The main limitation of our study is inherent to the retrospective design, from a single institution, and the relatively short follow-up period. Finally, this study was not powered enough to evaluate the differences between the two device types with respect to anatomic subtypes. Follow-up data of 6 months or more were not available for all patients.
Conclusion
ASD device closure in a developing country with a well-trained team has excellent results and low complications rates which compare favorably to those reported from centers in developed countries.
References
Webb G, Gatzoulis MA (2006) Atrial septal defects in the adult: recent progress and overview. Circulation 114(15):1645–1653
King TD et al (1976) Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA 235(23):2506–2509
Moore J et al (2013) Transcatheter device closure of atrial septal defects: a safety review. JACC Cardiovasc Interv 6(5):433–442
Jones TK, Latson LA, Zahn E, Fleishman CE, Jacobson J, Vincent R et al (2007) Results of the US multicenter pivotal study of the HELEX septal occluder for percutaneous closure of secundum atrial septal defects. J Am Coll Cardiol 49:2215–2221
Pac A, Polat TB, Cetin I, Oflaz MB, Balli S (2009) Figulla ASD occluder versus Amplatzer Septal Occluder: a comparative study on validation of a novel device for percutaneous closure of atrial septal defects. J Interv Cardiol 22(6):489–495. https://doi.org/10.1111/j.1540-8183.2009.00497.x
Roymanee S et al (2015) Comparison of the Occlutech (R) Figulla (R) septal occluder and Amplatzer (R) septal occluder for atrial septal defect device closure. Pediatr Cardiol 36(5):935–941
Aytemir K et al (2012) Early-midterm follow-up results of percutaneous closure of the interatrial septal defects with occlutech figulla devices: a single center experience. J Interv Cardiol 25(4):375–381
Feltes TF, Bacha E, Beekmand RH 3rd, Cheatham JP, Feinstein JA, Gomes AS et al (2011) Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation 123:2607–2652
Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N et al (2010) ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 31:2915–2957
Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K, Amplatzer Investigators (2002) Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol 39(11):1836–1844
Moake L, Ndinjiakat SK (2011) Transcatheter device closure for atrial septal defects: safety, efficacy, complications, and costs. Crit Care Nurs Clin North Am 23(2):339–348
Kefer J et al (2012) Percutaneous transcatheter closure of interatrial septal defect in adults: procedural outcome and long-term results. Catheter Cardiovasc Interv 79(2):322–330
Fiarresga A et al (2010) Percutaneous closure of atrial septal defects: a decade of experience at a reference center. Rev Port Cardiol 29(5):767–780
El-Said H, Hegde S, Foerster S, Hellenbrand W, Kreutzer J, Trucco SM, Holzer R, Burch G, Mirani A, Nicolas R, Porras D, Bergersen L, Moore J (2015) Device therapy for atrial septal defects in a multicenter cohort: acute outcomes and adverse events. Catheter Cardiovasc Interv 85(2):227–233. https://doi.org/10.1002/ccd.25684
Everett AD, Jennings J, Sibinga E, Owada C, Lim DS, Cheatham J, Holzer R, Ringewald J, Bandisode R, Ringel R (2009) Community use of the amplatzer atrial septal defect occluder: results of the multicenter MAGIC atrial septal defect study. Pediatr Cardiol 30(3):240–247. https://doi.org/10.1007/s00246-008-9325-x
Moore JW, Vincent RN, Beekman RH 3rd, Benson L, Bergersen L, Holzer R, Jayaram N, Jenkins K, Li Y, Ringel R, Rome J, Martin GR, NCDR IMPACT Steering Committee (2014) Procedural results and safety of common interventional procedures in congenital heart disease: initial report from the National Cardiovascular Data Registry. J Am Coll Cardiol 64(23):2439–2451. https://doi.org/10.1016/j.jacc.2014.09.045
Clem A, Awadallah S, Amin Z (2017) Safety, Feasibility, results, and economic impact of common interventional procedures in a low-volume region of the United States. Pediatr Cardiol 38(7):1332–1336. https://doi.org/10.1007/s00246-017-1664-z
Chessa M et al (2002) Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol 39(6):1061–1065
Ali SH, El Sisi A, Raafat DM, Amry SE, Mahamoud SED (2018) Short-term outcomes of transcatheter closure of secundum atrial septal defect in children and adolescents: an experience of two centers in Upper Egypt. J Saudi Heart Assoc 30(1):14–20. https://doi.org/10.1016/j.jsha.2017.04.004
Putra ST, Djer MM, Idris NS, Samion H, Sastroasmoro S (2015) Transcatheter closure of atrial septal defects in a center with limited resources: outcomes and short term follow-up. Iran J Pediatr 25:e3906
Ali M, Salah El-Din H, Bakhoum S, El-Sisi A, Mahmood K, Farouk H, Kandil H (2018) Feasibility of percutaneous closure of atrial septal defects in adults under transthoracic echocardiography guidance using the Figulla atrial septal defect occluder device. J Saudi Heart Assoc 30(1):21–27. https://doi.org/10.1016/j.jsha.2017.04.002
Quek SC et al (2010) Transcatheter closure of atrial septal defects–is balloon sizing still necessary? Ann Acad Med Singapore 39(5):390–393
Cilingiroglu M, Salinger M, Zhao D, Feldman T (2011) Technique of temporary subcutaneous “Figure-of-Eight” sutures to achieve hemostasis after removal of large-caliber femoral venous sheaths. Catheter Cardiovasc Interv 78(1):155–160. https://doi.org/10.1002/ccd.22946
Bergersen L, Marshall A, Gauvreau K, Beekman R, Hirsch R, Foerster S et al (2010) Adverse event rates in congenital cardiac catheterization – a multi-center experience. Catheter Cardiovasc Interv 75:389–400
Bergersen L, Gauvreau K, Foerster SR, Marshall AC, McElhinney DB, Beekman RH et al (2011) Catheterization for Congenital Heart Disease Adjustment for Risk Method (CHARM). JACC Cardiovasc Interv 4:1037–1046
Mulukutla V, Qureshi AM, Pignatelli R, Ing FF (2017) Predictive factors for patients undergoing ASD device occlusion who “crossover” to surgery. Pediatr Cardiol. https://doi.org/10.1007/s00246-017-1771-x
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest related with the manuscript and the study is not supported by any grant or institution.
Ethical Approval
The study was performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments. The study was approved by the Institutional Review Board at the American University of Beirut – Medical Center.
Rights and permissions
About this article
Cite this article
Almanla, A., Charafeddine, F., Abutaqa, M. et al. Transcatheter Closure of Atrial Septal Defects: Comparable Experience and Outcomes Between Developing and Developed Countries. Pediatr Cardiol 40, 610–615 (2019). https://doi.org/10.1007/s00246-018-2034-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-018-2034-1