Abstract
Seven estrogenic compounds—estrone (E1), 17β-estradiol (E2), 17α-ethynylestradiol (EE2), diethylstilbestrol (DES), nonylphenol (NP), octylphenol (OP), and bisphenol A (BPA)—in sediments, surface water, pore water, and organisms were investigated and estrogenic activities were estimated by examining estradiol equivalent (EEQ) concentrations in Yundang Lagoon of Xiamen. The results showed that estrogenic compounds were present in all matrixes of interest: in surface water, ranging from 609.61 to 711.31 ng/l; in pore water, ranging from 562.12 to 1038.15 ng/l; in sediments, ranging from 1433.12 to 2060.41 ng/g; and in biota samples, ranging from 1373.76 to 3199.09 ng/g (lipid weight). NP was the predominant component in all collected samples and the highest concentration was 1964.80 ng/g in sediment. Total EEQ ranged from 4.56 to 13.79 ng/l in surface water, from 2.40 to 17.16 ng/l in pore water, and from 8.66 to 23.95 ng/g in sediments. However, major contributors to total EEQ concentrations were E2, E1, and DES. The EEQ concentrations in surface water samples were at a higher level in comparison to that reported in European countries. To biological sample, the highest level of total estrogenic compounds was found in the short-necked clam. Higher values of the biota–sediment accumulation factor (BSAF) were found in short-necked clam and black seabream, indicating that the living habits of organism and physical–chemical properties of estrogenic compounds might influence the bioavailability of estrogenic compounds in organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endocrine-disrupting compounds (EDCs) are compounds with the potential to elicit negative effects on endocrine systems of humans and wildlife. Estrogenic EDCs are a subclass of EDCs that, when organisms are exposed to them, function as estrogens. Estrogenic EDCs consisting of either natural or synthetic hormonal estrogens are capable of mimicking or inducing an estrogenlike response in an organism. Specific examples of estrogenic EDCs include the following: surfactants such a alkyphenol-ethoxalates (Folmar et al. 2002; Ying et al. 2002), natural hormones and pharmaceutical estrogens 17β-estradiol (E2) and 17a-ethynylestradiol (EE2) (Legler et al. 2002), phytoestrogens, including isoflavonoides and coumestrol (Bacaloni et al. 2005; Stopper et al. 2005), as well as other industrial compounds like bisphenol A (BPA). Various estrogenic EDCs have been concluded to be the cause of reproductive disturbance in humans and wildlife (Colborn et al. 1993). Human exposure to these compounds in food, water, and the environment is a critical concern with unknown long-term impacts.
Xiamen was one of the cities designated as a Special Economic Zone in China in the 1980s. Its rapid industrial, commercial, and urban developments have put enormous stress on the coastal environments fringing the harbor as well as a nearby lagoon (Yundang Lagoon). Yundang Lagoon is located at the north of the downtown area of Xiamen City and is surrounded by the newly developed urban area, which once served as a main municipal sewage-accepting lagoon and was seriously polluted. However, the Integrated Treatment Project was carried out in 1988 by the local government to improve the water and sediment quality. The treatments included sewage disposal, removing sludge and constructing banks, exchanging the water, and afforestation. Yundang Lagoon became one of the pilot bases for the Regional Programme on Prevention and Management of Marine Pollution in East Asian Seas sponsored by the United Nations Environment Programme (UNEP) for removing contaminates by tidal flow. However, until now, a small amount of sewage is still discharged into the lagoon, although most of it is intercepted into the urban sewage treatment system. In our previous study, we found that Xiamen Bay had serious pollution of estrogenic compounds mainly coming from Yundang Lagoon (Zhang et al. 2009). The purpose of this study is to investigate the distribution of estrogenic EDCs, such as estrone (E1), 17β-estradiol (E2), 17α-ethynylestradiol (EE2), diethylstilbestrol (DES), nonylphenol (NP), octylphenol (OP), and bisphenol A (BPA) in water, sediments, pore water, and organism from Yundang Lagoon and provide useful information for environmental management of this region.
Materials and Methods
Chemicals
The following standards and reagents of compounds were used in this study: 17β-Estradiol (98%), diethylstilbestrol (99%), 4-octylphenol (99%), 17α-ethynylestradiol (98.5%), and estrone (99%) were purchased from Acros Organics, Belgium. Bisphenol A (99%) and nonylphenol (99%) were from Aldrich (USA). Silylation reagent N-methyl-N-trimethylsilyl trifluoroacetamide (MSTFA) was purchased from Sigma(USA). 17β-Estradiol-2,4,16,16,-d4 (E4-d4, 99%) as the surrogate standard and BPA-d16 (99%) as the internal standard were from Cambridge (USA). All organic solvents were of pesticide residue-free grade. Hexane, acetone, methanol, and dichloromethane were from Fisher (USA). Silica (60 mesh), supplied by Fisher, was activated at 450°C for at least 6 h before use.
Sample Collection and Sample Treatment
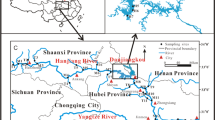
Sediment samples were collected from station 1 to station 4 in Yundang Lagoon were collected on Apirl 26, 2008 (Fig. 1). About 1000 g of surficial sediments were collected with a stainless-steel sediment grab sampler. All samples were transported to the laboratory. After returning to the laboratory, the sediments were centrifuged (3000 rpm) at 4°C for 30 min to obtain sediment pore water (200 ml). The sediment samples were stored at −80°C before their extraction and analysis. Water samples were collected in precleaned amber-glass bottles, refrigerated, and transported to the laboratory. Then water samples were filtered through glass microfiber filters (Cat. No. 10370105; Whatman, Maidstone, UK). During August 2008, organisms including the short-necked clam (Ruditapes philippinarum), yellow fin seabream (Sparus latus), black seabream (Acanthopagrus schlegel), and tilapia were collected from station 3 in Yundang Lagoon. The shell of the clam and crab was removed, and the soft tissues were washed to remove particles in the tissue. Then clean soft tissues and the filet of each fish were homogenized in a blender. The samples were dried using a freeze-dryer before extracting.
Samples of 150 ml pore water and 500 ml surface spiked with 200 ng E2-d4 as a surrogate standard were extracted with Oasis HLB solid-phase extraction (SPE) cartridges (Waters, USA), following established procedures (Zhang et al. 2009). Then 10 ng BPA-d16 was added to the extract as the internal standard. The extract was derived with 70 μl hexane and 30 μl MSTFA at 70°C for 100 min (Jin et al. 2008). The derivatives of target compounds were analyzed by gas chromatography/mass spectrometry (GC/MS).
Five grams of dried sediment were extracted according to the method of Zhang et al. (2009). A biological sample was extracted according to the method described by Hu et al. (2005) with minor modifications. One grams of a dried biological sample spiked with 200 ng E2-d4 as a surrogate standard were mixed with anhydrous sodium sulfate, placed in a cylindrical cell (33 ml), and extracted using a Dionex Accelerated Solvent Extractor (Model 100) with dichloromethane/methanol (7:3, v/v) mixture solution. The lipid content of biota was detected by gravimetric determination of (Borga et al. 2001). Purification of the sample extracts was accomplished by eluting the GPC column. The eluting solvent was collected and concentrated to 1 ml. The purified extracts were further purified and fractionated on a layered silica (H2O 5% deactivated silica gel) using 10 ml ethyl acetate as an eluent. Then the extract was evaporated just to dryness with a gentle stream of N2 for derivatization. The method of derivatization was the same as the extraction of pore water.
Quantification by GC/MS
Estrogenic compounds in sediment were identified and quantified by GC/MS (Agilent 7890A-5975C) according to the method of Zhang et al. (2009). Detailed information on the target compounds and analysis parameters are provides in Table 1.
Quality Assurance and Quality Control
A surrogate standard (E2-d4) was added to each sample before extraction. If the surrogate recoveries are beyond the range of 70–120%, the sample should be reanalyzed. No target analytes were detected in the blanks. The spiking recoveries in sediment samples were at 73.5–103%. The surrogate recoveries were between 82.6% and 104%.
Total Organic Carbon in Sediment
Total carbon (TC) and inorganic carbon (IC) were determined by a TOC-VCPH SSM 5000A elemental analyzer (Shimadzu, Japan) on freeze-dried powdered samples. Total organic carbon (TOC) is obtained by TC substrating IC. Phosphoric acid (25%, 0.45 ml) was added to detect inorganic carbon in the samples.
Estrogen Equivalent Concentration
Calculation of the estrogen equivalent concentration (EEQ) of a chemically determined mixture is based on all measured estrogens with a known estradiol equivalency factor (EEF) using the estrogen receptors (ERs) α according to Brigitte and Johannes (2001):
Biota-Sediment Accumulation Factor (BSAF)
In the study, the biota–sediment accumulation factor (BSAF) was taken as a measure of the biotic fate of estrogenic compounds and defined by the following equation:
where C b is the biota contaminant concentration (ng/g lipid weight), f l is the biota lipid concentration (fraction by weight), Cs is the sediment contaminant concentration (ng/g dry weight), and f oc is the organic carbon fraction of the sediment (fraction by weight).
Results and Discussion
This study reports the results obtained from a comprehensive survey of Yundang Lagoon for the levels of estrogenic compounds and represents an attempt to understand the current contamination status in the area. A summary of data for the levels of estrogenic compounds in water, sediment, pore water, and biological samples is shown in Tables 2 and 3 and estrogenic activities are present in Fig. 2.
Estrogenic Compounds and Estrogenic Activity (EEQ) in Surface Water, Pore Water, and Sediment
In Table 2, all target compounds were detected in station 4 and the concentrations were also highest among S1–S4. This is probably because station 4 is located at the effluent of the First Sewage Treatment Plant in Xiamen. Similarly, high concentration was also found at station 1, which is at the top of Yundang Lagoon, where urban runoffs and sewage were input. Alkylphenols were main pollutants among monitoring estrogenic compounds in all the samples. Although DES has been banned for use as an additive in animal feed in China, DES as a drug is still used in the treatment of gynecological diseases. In this study, DES was found to be present in all waters in this study, with the levels in the range of 2.54–6.75 ng/l. The reason for DES’s wide distribution needs further studies. On the other hand, E1 was detected in all stations of the analyzed surface water except station 2, with concentrations ranging from 1.75 to 5.34 ng/l. However, levels of E2 in surface water varied from <LOD (limit of detection) to 1.56 ng/l, and its concentrations were lower than those of E1. Additionally, it was found that the levels of EE2 was also lower than those of E1 and the levels varied from <LOD to 0.43 ng/l. It was reported that 95% of E2 transformed into E1 in 1–3 h in sludge (Ternes et al. 1999). If this transformation process also occurs in the natural aquatic environment, more widespread distribution of E1 compared with E2 is expected.
Due mainly to the discharge of effluents from wastewater treatment plants [both industrial and municipal sewerage treatment plants (STPs)], the occurrence of alkylphenols has been widely reported in surface waters (rivers, lakes, and coastal waters as well as aquatic biota) around the world. NP concentrations ranging from 0.1 to 2.6 μg/l and from 0.031 to 0.934 μg/l were found in UK estuaries (Blackburn et al. 1999) and Dutch estuaries (Jonkers et al. 2003), respectively. However, in Switzerland, levels of NP up to 2.25 μg/l in water and 3520 mg/kg in sediment were found in rivers (Ahel et al. 1996). Along the northern Mediterranean coast, NP concentrations ranging from 0.181 to 4.1 μg/l have been found (Arditsoglou and Voutsa 2008; Petrovic et al. 2002). NP concentrations up to 0.416 μg/l in surface water and 13,700 μg/kg in surface sediments were reported from Jamaica Bay (New York, USA) (Ferguson et al. 2001). In Asian areas, NP concentrations were found to range from 0.28 to 2.76 μg/l in the coastal area close to Singapore, whereas OP concentrations ranged from 0.001 to 0.8 μg/l (Basheer et al. 2004). NP was also found at levels that varied from 30 to 13,000 μg/kg in the Tokyo Bay in Japan (Isobe et al. 2001). In Masan Bay (Korea), NP concentrations ranged from 0.0097 to 0.928 μg/l (Li et al. 2008). In the Pearl River Delta and adjacent northern South China Sea, NP and OP levels ranged up to 0.628 μg/l and 0.068 μg/l, respectively. In the coast of Taiwan, alkylphenols were present, ranging from 61 to 370 ng/l in water (Cheng et al. 2006a, 2006b). In comparison with published data, Yundang Lagoon in Xiamen had mid-range water pollution in this study. However, the maximum NP concentration measured for estuaries and coastal areas (lagoon, bay) (e.g., 4.1 μg/l) was much lower than that of certain freshwater sites receiving sewage inputs. For example, NP concentrations of up to 644 μg/l were measured in a Spanish river (Sole et al. 2000). At a distance from the NP source, salinity could have an effect on the presence of NP in water (Li et al. 2005).
There are many reports on steroidal estrogen contamination in surface waters from other locations, as shown in Table 3. The levels of steroidal estrogens in this study were higher in comparison with those from European countries, where they received the treated wastewater. Some studies showed that treatment processes in the STP were sufficient for the removal of estrogenic compounds in wastewater. In Xiamen City, the percentage of the treated sewage was only 80.45% (data from government report in 2007). For Yundang Lagoon, it received not only treated wastewater but also untreated domestic sewage from Xiamen City with higher population density that is 9371 people/km). Hanselman et al. (2003) demonstrated that steroid hormones in surface waters mainly came from municipal wastewater.
The distribution characteristics in pore water were very similar to those in surface water and showed a marked predominance by alkylphenol compounds (e.g., NP, OP). The estrogenic compounds were present in higher levels in pore water than in surface water, due possibly to higher concentrations of dissolved organic carbon or colloids with which the hydrophobic pollutants were strongly associated. However, such a concentration difference between pore water and sediment might be due to the highly hydrophobic characteristics of estrogenic compounds and hence their strong tendency to become associated with organic colloids in sediments.
It is clear from Table 2 that in terms of individual estrogenic compounds composition in sediments, all target compounds were detected at station 1 and station 4. In terms of composition estrogenic compounds in sediments, it is dominated by alkylphenol along the contamination, which is similar to those in water. However, the levels of estrogenic compounds in sediments were high, which is probably because the hydrophobic character of estrogenic compounds makes them easily absorb to bed sediment in water (Campbell et al. 2006). In our previous study (Zhang et al. 2009), we have compared levels of estrogenic compounds in sediments in Xiamen Bay with those of other countries, where it was indicated that Xiamen Bay was seriously contaminated, especially by steroidal estrogens, whereas estrogenic compounds in Xaimen Bay mainly came from Yundang Lagoon.
The EEQ concentrations of estrogenic compounds might be varied according to the EEF obtained from different assays (Campbell et al. 2006; Chrzan and Bradford 2007; Jin et al. 2008). In this study, EEQ values were calculated using potencies derived from ER assay results of those studies (1.00, 1.16, 7.00 × 10−3, 1.75, 2.30 × 10−4, 1.75 × 10−4 and 7.00 × 10−4 for E2, EE2, E1, DES, BPA, NP and OP, respectively) using Equation 1. As shown in Fig. 2, the concentrations of estrogenic compounds [expressed as the estradiol equivalent (EEQ] in surface water, pore water, and sediments were estimated. The highest concentrations of EEQ were 13.79 ng/l in surface water, 23.95 ng/g in sediment, and 17.16 ng/g in pore water. For the EEQ in this study, major contributors were E2, EE2, and DES. Although the total concentrations of estrogenic compounds were not high in surface water, the concentrations of EEQ were higher than those in European countries, where the EEQ level was at less than 3 ng/l (Ying et al. 2002). In contrast to this study, EEQ was estimated as high as nearly 20 ng/l in surface water. Imai et al. (2007) found that the lowest observed effect concentration (LOEC) and no observed effect concentration (NOEC) for Java medaka (Oryzias javanicus) were 484 and 198 ng/l of E1, respectively. Those concentrations are approximately equal to3.34 and 1.39 ng/l of EEQ. However, in this study, EEQ values in surface water were higher than those of LOEC. Chronic exposure to estrogen compounds in the presented area might cause adverse effects to individual organism and the population of aquatic species. Therefore, it is suggested that Yundang Lagoon should be continuously monitored and controlled the introduction of estrogenic compounds and the occurrence of estrogenic activities in waters.
Estrogenic Compounds in the Biological Sample
The concentrations of estrogenic compounds in biota collected from Yundang Lagoon are reported in Table 4. The highest median concentration of estrogenic compounds was found in the short-necked clam (3199.09 ng/g lipid weight). Black seabream, yellow fin seabream, and tilapia showed mean concentrations of 3061.53, 2012.45, and 1373.76 ng/g lipid weight, respectively. As far as individual compound were concerned, the highest level was NP and 2724.56 ng/g lipid weight for the short-necked clam, followed by 2615.10 ng/g lipid weight for black seabream. Although a large variation of NP concentrations in different biological species was observed, high levels of NP in the biological sample might be correlated with the feeding habitat and biodegradation level of NP. The clam is a sediment-dwelling predator with the highest average concentration of estrogenic compounds (3199.09 ng/g). Black seabream is a carnivorous fish and feeds on bottom-dwelling invertebrates and crustaceans, whereas tilapia is a cyprinus fish. Thus, the level of estrogenic compounds in black seabream was higher than those in tilapia. Some studies have shown that environmental pollutants, likes dioxins and PCBs, in biota are correlated to their habitat and metabolic ability (Zhou and Wong 2000). In addition, biodegradation of estrogenic compounds in the sediment might also affect the bioaccumulation of estrogenic compounds in organisms.
Biota-Sediment Accumulation Factor
The BSAF is a valuable parameter for predicting bioaccumulation of lipophilic compounds, which is primarily associated with tissue lipid and sediment organic carbon (Hu et al. 2005). The mean organic carbon and lipid contents of sediment and biological samples were 0.039 (0.024–0.061) g TOC/g of sediment and 0.082 (0.043–0.160) g lipid/g of fish, respectively. Figure 3 shows the BSAF for estrogenic compounds in individual biological samples. The BSAFs of NP in the short-necked clam (0.4) and black seabream (0.38) were much higher than those in yellow fin seabream (0.07) and tilapia (0.07). The profile of the BSAF for estrogenic compounds in the short-necked clam and black seabream were similar to each other. It was possible that primary food sources of these organisms are benthic animals that live near the sediment. From our knowledge, few reports about BSAFs of estrogenic compounds were available. Figure 4 shows the relationship between the BSAF and log K ow of estrogenic compounds in the organism. It indicated that there was no relationship between log K ow and the BSAF. Although the physical–chemical properties of organic environmental pollutants can affect the absorption and elimination of these compounds in an organism an thereby might also affect the accumulation of estrogenic compounds in fish (Burkhard 2003; Maund et al. 2002), in this study there was no relation between log K ow and the BSAF. It was probably because metabolic ability was different to different organism or there existed disequilibria between in the sediment chemical and organism for chemical (Burkhard et al. 2004, 2005). Burkhard et al. (2004) reported that the rate of metabolism in the organism was important and contributed to the apparent differences in the BSAF. Therefore, organism species, trophic level, metabolic ability, and physical–chemical properties of estrogenic compounds might influence the BSAF of estrogenic compounds in biota (Burkhard et al. 2005).
Conclusions
This article has provided important data on estrogenic compounds levels in surface water, sediment, pore water, and organism of Yundang Lagoon in Xiamen, an area under rapid development in south China. All target compounds were detected in all samples, which showed that Yundang Lagoon was polluted by estrogenic compounds. Estrogenic compounds in Yundang mainly came from municipal treated and untreated sewage. The concentrations of contaminants in pore water were significantly higher than those in surface water, suggesting that such contaminants prefer to stay in a sedimentary rather than an aqueous environment. The difference of concentration between pore water and surface water implied that potential fluxes could be set up in transporting micropollutants from sediments to overlying water. The concentrations of EEQ were higher than those in European countries and should be controlled in the introduction of estrogenic compounds to Yundang Lagoon. In addition, the estrogenic compounds level in organisms was affected by feeding habits of organisms and physiochemical properties of estrogenic compounds.
References
Ahel M, Schaffner C, Giger W (1996) Behaviour of alkylphenol polyethoxylate surfactants in the aquatic environment: III. Occurrence and elimination of their persistent metabolites during infiltration of river water to groundwater. Water Res 30:37–46
Arditsoglou A, Voutsa D (2008) Passive sampling of selected endocrine disrupting compounds using polar organic chemical integrative samplers. Environ Pollut 156:316–324
Bacaloni A, Cavaliere C, Faberi A, Foglia P, Samperi R, Lagana A (2005) Determination of isoflavones and coumestrol in river water and domestic wastewater sewage treatment plants. Anal Chim Acta 531:229–237
Baronti C, Curini R, Dascenzo G, DI Corcia A, Gentili A, Samperi R (2000) Monitoring natural and synthetic estrogens at activated sludge sewagetreatment plants and in a receiving river water. Environ Sci Technol 34:5059–5066
Basheer C, Lee HK, Tan KS (2004) Endocrine disrupting alkylphenols and bisphenol-A in coastal waters and supermarket seafood from Singapore. Marine Pollut Bull 48:1161–1167
Beck I, Bruhn R, Gandrass J, Ruck W (2005) Liquid chromatography–tandem mass spectrometry analysis of estrogenic compounds in coastal surface water of the Baltic Sea. J Chromatogr A 1090:98–106
Belfroid AC, Van der Horst A, Vethaak AD, Schäfer AJ, Rijs GBJ, Wegener J, Cofino WP (1999) Analysis and occurrence of estrogenic hormones and theirglucuronides in surface water and waste water in The Netherlands. Sci Total Environ 225:101–108
Blackburn MA, Kirby SJ, Waldock MJ (1999) Concentrations of alkyphenol polyethoxylates entering UK estuaries. Marine Pollut Bull 38:109–118
Borga K, Gabrielsen GW, Skaare JU (2001) Biomagnification of organochlorines along a Barents Sea food chain. Environ Pollut 113:187–198
Brigitte G, Johannes W (2001) Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology 166:79–89
Burkhard LP (2003) Factors influencing the design of bioaccumulation factor and biota-sediment accumulation factor field studies. Environ Toxicol Chem 22:351–360
Burkhard LP, Cook PM, Lukasewycz MT (2004) Biota-sediment accumulation factors for polychlorinated biphenyls, dibenzo-p-dioxins, and dibenzofurans in southern Lake Michigan lake trout (Salvelinus namaycush). Environ Sci Technol 38:5297–5305
Burkhard LP, Cook PM, Lukasewycz MT (2005) Comparison of biota-sediment accumulation factors across ecosystems. Environ Sci Technol 39:5716–5721
Campbell CG, Borglin SE, Green FB, Grayson A, Wozei E, Stringfellow WT (2006) Biologically directed environmental monitoring, fate, and transport of estrogenic endocrine disrupting compounds in water: a review. Chemosphere 65:1265–1280
Cargouet M, Perdiz D, Mouatassim-Souali A, Tamisier-Karolak S, Levs Y (2004) Assessment of river contamination by estrogenic compounds in Paris area (France). Sci Total Environ 324:55–66
Cheng CY, Liu LL, Wang WH (2006a) Determination and distribution characteristics of degradation products of nonylphenol polyethoxylates in the rivers of Taiwan. Chemosphere 65:2275–2281
Cheng CY, Liu LL, Wang WH (2006b) Occurrence and seasonal variation of alkylphenols in marine organisms from the coast of Taiwan. Chemosphere 65:2152–2159
Chrzan BG, Bradford PG (2007) Phytoestrogens activate estrogen receptor beta1 and estrogenic responses in human breast and bone cancer cell lines. Mol Nutr Food Res 51:171–177
Colborn T, vom Saal FS, Soto AM (1993) Developmental effects of endocrine disrupting compounds in wildlife and humans. Environ Health Perspect 101:378–384
European Commission (2008) Updated European Union risk assessment report: 4, 4′-isopropylidenediphenol (bisphenol-A). European Commission, Brussels, pp 119–120
Ferguson PL, Iden CR, Brownwell BJ (2001) Distribution and fate of neutral alkylphenol ethoxylate metabolites in a sewage-impacted urban estuary. Environ Sci Technol 35:2428–2435
Folmar LC, Hemmer MJ, Denslow ND, Kroll K, Chen J, Cheek A, Richman H, Meredith H, Grau EG (2002) A comparison of the estrogenic potencies of estradiol, ethynylestradiol, diethylstilbestrol, nonylphenol and methoxychlor in vivo and in vitro. Aquat Toxicol 60:101–110
Hanselman TA, Graetz DA, Wilkie AC (2003) Manure-borne estrogens as potential environmental contaminants: a review. Environ Sci Technol 37:5471–5478
Hu J, Jin F, Wan Y, Yang M, An L, An W, Tao S (2005) Trophodynamic behavior of 4-nonylphenol and nonylphenol polyethoxylate in a marine aquatic food web from Bohai Bay, North China: comparison to DDTs. Environ Sci Technol 39:4801–4807
Imai S, Koyama J, Fujii K (2007) Effects of estrone on full life cycle of Java medaka (Oryzias javanicus), a new marine test fish. Environ Toxicol Chem 26:726–731
Isobe T, Nishiyama H, Nakashima A, Takada H (2001) Distribution and behavior of nonylphenol, octylphenol, and nonylphenol monoethoxylate in Tokyo metropolitan area: their association with aquatic particles and sedimentary distributions. Environ Sci Technol 35:1041–1049
Jin SW, Yang FX, Liao T, Hui Y, Xu Y (2008) Seasonal variations of estrogenic compounds and their estrogenicities in influent and effluent from a municipal sewage treatment plant in China. Environ Toxicol Chem 27:146–153
Jonkers N, Laane RW, De Voogt P (2003) Fate of nonylphenol ethoxylates and their metabolites in two Dutch estuaries: evidence of biodegradation in the field. Environ Sci Technol 37:321–327
Kawaguchi M, Inoue K, Yoshimura M, Sakui N, Okanouchi N, Ito R, Yoshimura Y, Nakazawa H (2004) Stir bar sorptive estraction with in situ derivatization and thermal desorption-gas chromatography–mass spectrometry in the multi-shot mode for determination of estrogens in river water samples. J Chromatogr A 1049:1–8
Labadie P, Budzinski H (2005) Determination of steroidal hormone profiles along the Jalle d’Eysines River (near Bordeaux, France). Environ Sci Technol 39:5113–5120
Legler J et al (2002) Comparison of in vivo and in vitro reporter gene assays for short-term screening of estrogenic activity. Environ Sci Technol 36:4410–4415
Lei B, Huang S, Zhou Y, Wang D, Wang Z (2009) Levels of six estrogens in water and sediment from three rivers in Tianjin area, China. Chemosphere 76:36–42
Li D, Dong M, Shim WJ, Hong SH, Oh JR, Yim UH, Jeung JH, Kanan N, Kim ES, Sung RC (2005) Seasonal and spatial distribution of nonylphenol and IBP in Saemangeum Bay, Korea. Marine Pollut Bull 51:966–974
Li D, Dong M, Shim WJ, Yim UH, Sang Hong H, Kannan N (2008) Distribution characteristics of nonylphenolic compounds in Masan Bay environments, Korea. Chemosphere 71:1162–1172
Maund SJ, Hamer MJ, Lane MCG (2002) Partitioning, bioavailability, and toxicity of the pyrethroid insecticide cypermethrin in sediments. Environ Toxicol Chem 21:9–15
Noppe H, Verslycke T, Wulf E, Verheyden K, Monteyne E, Van Caeter P, Janssen CR, De brabander HF (2007) Occurrence of estrogens in the Scheldt estuary: a 2-year survey. Ecotox Environ Safety 66:1–8
Petrovic M et al (2002) Occurrence and distribution of nonionic surfactants, their degradation products, and linear alkylbenzene sulfonates in coastal waters and sediments in Spain. Environ Toxicol Chem 21:37–46
Rkumarkhanal S, Xie B, Thompson ML, Sung S, Leeuwen JV (2006) Fate, transport, and biodegradation of natural estrogens in the environment and engineered systems. Environ Sci Technol 40:6537–6546
Sole M, de Alda MJL, Castillo M, Porte C, Ladegaard-Pedersen K, Barceló D (2000) Estrogenicity determination in sewage treatment plants and surface waters from the Catalonian area (NE Spain). Environ Sci Technol 34:5076–5083
Stopper H, Schmitt E, Kobras K (2005) Genotoxicity of phytoestrogens. Mutat Res 574:139–155
Ternes TA, Kreckel P, Mueller J (1999) Behaviour and occurrence of estrogens in municipal sewage treatment plants-II. Aerobic batch experiments with activated sludge. Sci Total Environ 225:91–99
Ying GG, Williams B, Kookana R (2002) Environmental fate of alkyphenols and alkyphenol ethoxylates: a review. Environ Int 28:215–226
Zhang X, Li QZ, Li GX, Yan CZ (2009) Levels of estrogenic compounds in Xiamen Bay sediment, China. Marine Pollut Bull 58:1210–1216
Zhou HY, Wong MH (2000) Accumulation of sediment-sorbed PCBs in tilapia. Water Res 34:2905–2914
Acknowledgment
This study was supported by the Knowledge Innovation Project of Chinese Academy of Sciences (No. KZCX2-YW-422).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Gao, Y., Li, Q. et al. Estrogenic Compounds and Estrogenicity in Surface Water, Sediments, and Organisms from Yundang Lagoon in Xiamen, China. Arch Environ Contam Toxicol 61, 93–100 (2011). https://doi.org/10.1007/s00244-010-9588-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-010-9588-0