Abstract
In this study, Poecilia sphenops (gold mollies) were chronically exposed to low pH that mimic those found in natural environments, e.g., rivers and lakes. The Poecilia sphenops were placed in two separate aquaria with pH levels of 5 and 6 and presented with a different chemically mediated behavioral challenge of locating the food source. The results indicated that under pH 5 the Poecilia sphenops had difficulties in locating the odor source of food and at the same time their swimming speed were greatly reduced. The failure by the Poecilia sphenops to locate the food source and their reduced swimming speed can have a negative impact on the survival of the fish by introducing a high probability of starvation. If the fish are starved, it means that even their reproduction rate will be reduced, while the juveniles growth will be arrested under natural conditions of acidification. In addition to this, since the fish’s swimming speed is also impaired, it means that, they will be unable to run away from their predators once found. The combination of starvation and failure to run away from predators could negatively impact the gold mollies severely. Their fitness would be compromised.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Acid rain is an anthropogenic phenomenon in which sulfuric and nitric acids, derived from oxides of sulfur and nitrogen produced by industrial activities, precipitate on the earth’s surface (Ikuta et al. 2003). The rapidly expanding industrial activities throughout the world due to globalization will continue to cause a continued increase in the quantity of these emissions (Japan Environment Agency 1997).
Human changes to global biogeochemical cycles of nitrogen and sulfur have led to acidic deposition from the atmosphere (Rodhe et al. 2002; Bouwman et al. 2003; Holland et al. 2005; Lamarque et al. 2005; Dentener et al. 2006), which has impacted aquatic ecosystems. The absorption of anthropogenic carbon dioxide and atmospheric sulfur and nitrogen compounds into water bodies has contributed to the acidification of rivers, lakes, and oceans (Doney et al. 2007). Acid contamination of water bodies is contributing to the decline in some aquatic species (Blaustein et al. 2003). The mortality rates shown by some species in experimental tests are not the only way that water pollution could contribute to the decline of aquatic organisms. Sublethal levels of several pollutants could affect vital behavior, indirectly decreasing survival probabilities of individuals and thus producing effects at a population level (Manuel et al. 2007). Acute exposure to a low pH directly kills the fish by means of discharge of sodium and chloride ions from body fluids; aluminum ion eluded from soil due to low pH exacerbates this effect on gill membranes (Leivestad and Muniz 1976).
Researchers have focused on the laboratory-determined lethal concentration effects of contaminants, but in reality, the levels in their actual environments are sublethal. Nominal (sublethal) concentrations can have a detrimental effect on several aspects of the behavior of fish (Saglio and Trijasse 1998; Scholz et al. 2000). Few researchers have performed many experiments on the sublethal effects of acid rain exposure on chemosensory-mediated behavior of fish. Many fish perceive and respond to chemicals that are found in their environments. This can be seen in a number of behaviors, such as locating food (the focus of this study), avoiding predation, and locating mates (Lemly and Smith 1985).When given an opportunity, fish avoid water of low pH and high aluminum concentration (Christopher 1999; Johnson and Webster 1977; Jones et al. 1985). Other studies have shown that behavior of aquatic organisms is influenced by chemical signals such as pheromones (Dulka and Stacey 1991; Sargent et al. 1998), food odors (Zipple et al. 1993), and predator odors (Hazlet 1994; Brown and Smith 1998). If the behavior of fish is influenced by chemical signals and predator odor, then impairing the chemoreception of fish will greatly impact their behavior.
In this study, Poecilia sphenops (gold mollies) were chronically exposed to pH levels that mimic those found in natural environments, e.g., rivers and lakes, with a view to investigating the impact of exposure to low pH (pH 5 and 6) on chemosensory behavior of gold mollies.
Materials and Methods
Poecilia sphenops (gold mollies) were purchased from a commercial supplier and housed in five aquaria (76 × 31 × 47 cm) marked A, B, C, D, and E. Aquaria were maintained at a temperature of 24–25°C. Aquaria A and B contained six fish each, and aquaria C (used as a control tank), D, and E had two fish each. Aquaria A and B were a constant source of fish for aquaria C (control), D, and E (experimental). Fish in aquaria A, B, and C were kept at pH 7.9, which is the normal pH level of water at Bowling Green State University. Experimental aquarium D had a pH of 5, while aquarium E had a pH of 6 during the testing period. The fish were fed once a day on fish flakes (Tetrafin goldfish food), 0.20 g per aquarium. The fish were allowed to acclimatize for 3 weeks before undergoing experimentation. The experimental water was filtered and left to stand for 3 days before it was poured into aquaria D and E.
Experimental Setup
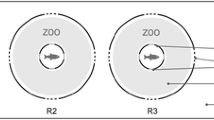
The aquaria were each three-quarters filled with (83 L) municipal dechlorinated water. Analytical-grade sulfuric acid (1 N) with 96.3% purity (J. T. Baker) was used to lower the pH to the desired value of either pH 5 or pH 6. A buffer, MES (morpholinoethanesulfonic acid), was added to maintain a constant pH. Two fish were put in aquarium C (control, pH 7.9), two in aquarium D (experimental, pH 5), and two in E (experimental, pH 6). In each trial a different pair of fish was used. All data obtained in every trial and for every parameter was for one pair of fish. The food stimulus was prepared fresh for each trial by homogenizing 2 g of fish flakes in 1 L of filtered tank water. Thereafter, the food stimulus was strained through a USGS 60-μm-mesh sieve to remove large pieces of food flakes. Filtered food stimuli were poured into 1-L bottles with a spout at the base and were clamped 80 cm above the control and experimental aquaria. Flow of the food stimuli from the 1-L bottle into the aquaria were regulated by using manostat Rite flowmeters, set at a constant flow rate of 1.0 mL/s. The fish in each aquarium (C [control] and D and E [experimental]) were allowed to acclimate for 24 h before trials began and during this period they were not fed. The food odor was administered in the center of the aquarium about 30 cm below the water surface (at X in Fig. 1). After the acclimatization period, 5-min trials were begun by opening the manostat flowmeters to allow the food stimulus into the aquaria (Fig. 1).
Behavioral Data Acquisition
All trials, control and experimental, were recorded using a Sony HI 8-mm videocamera, which was mounted a meter above the aquaria. Spatial parameters were established to determine changes in the behavior of the fish. These parameters were swimming forward (i.e., swimming of the fish toward the food odor source), swimming backward (swimming movements of the fish away from the food odor source), stopping (i.e., when the fish remained motionless), stopping right on food (i.e., when the fish were observed to stop directly on the food odor source in an apparent attempt to eat), swimming directly on food (without zigzag swimming), the time taken in each trial for the fish to locate the food source for the first time, and the fish’s swimming speed. Movements were quantified by dividing the distance the fish covered in swimming by the time it spent to swim from one point to another in front of or away from the food odor source for each frame of video analyzed. Thus the distance covered by the fish while swimming was calculated for each segment of the path. The distance per second values were then averaged for each trial, giving a mean for each parameter. Swimming toward the odor source can be a positive or negative vector (Moore et al. 1991; Moore and Grills 1999). In this food-oriented experiment, fish that came within 5 cm of the flow input were deemed to have been successful in locating the food odor source.
The time the fish took to cover the distance for each parameter in every trial was calculated as seconds. The calculated data were statistically analyzed using two-way MANOVA and differences between parameters were obtained using an LSD post hoc comparison test (Zar 1999) (STATISTICA 5.1 97; Statsoft, Tulsa, OK). Data were analyzed independently for each pH value, and differences were considered significant at probabilities <5% (p < 0.05).
The swimming speed was calculated using a peak Motus Bioengineering motion analysis system (to obtain X and Y spatial coordinates of the fish’s movement), 8 s before the food source was introduced, to ensure that the fish’s swimming speed was not affected by the presence of food. The percentage success in locating the odor source was analyzed using a chi-square test.
Results
Backward Swimming
Fish in the pH 5 treatment spent significantly more time swimming away from the food odor source compared to fish in the control treatment and fish in the pH 6 treatment (Fig. 2; p < 0.05, two-way MANOVA). Fish in the pH 5 treatment spent 19.1 ± 9.06 (SE) s swimming away from the odor source, whereas fish in the control treatment spent 10.58 ± 1.21 s) swimming away from the food odor source (Fig. 2).
Forward Swimming
Fish in both treatments (pH 5 and pH 6) spent the same time swimming toward the food odor source (Fig. 3). Fish in the pH 5 treatment spent 14.35 ± 2.91 (SE) s swimming toward the food odor source, whereas fish in the control and pH 6 treatment spent 15.27 ± 1.73 and 8.46 ± 1.15 s, respectively, swimming forward in front of the food odor source (Fig. 3).
Stopping/Remaining Motionless
Fish in the pH 5 treatment spent significantly more time stopping compared to fish in the control treatment and fish in the pH 6 treatment (Fig. 4; p < 0.05, two-way MANOVA). Fish in the pH 5 treatment spent 29.76 ± 17.54 (SE) s remaining in one position, whereas fish in the control and pH 6 treatments spent 7.60 ± 4.21 and 6.8 ± 3.40 s, respectively, motionless (Fig. 4).
Stopping on Food
Fish in the pH 5 treatment spent almost the same time stopping on the food odor source as fish in the control treatment and the pH 6 treatment (Fig. 5). Fish in the pH 5 treatment spent 7.1 ± 6.26 (SE) s stopping on food, whereas fish in the control and pH 6 treatments spent 6.85 ± 1.63 and 9.05 ± 1.53 s, respectively, stopping on food (Fig. 5).
Swimming on Food
Fish in the pH 5 treatment spent significantly less time swimming on the food odor source than fish in the control and the pH 6 treatments (Fig. 6; p < 0.05, two-way MANOVA). Fish in the pH 5 treatment spent 3.0 ± 1.59 (SE) s swimming on the food odor source, whereas fish in the control and the pH 6 treatments spent 8.33 ± 1.17 and 7.77 ± 1.41 s swimming on the food odor source (Fig. 6).
Time Taken to Locate the Food Odor Source
Fish in the pH 5 treatment took significantly more time to locate the food odor source compared to fish in the control treatment and the pH 6 treatment (Fig. 7; p < 0.0001, two-way MANOVA).Fish in the pH 5 treatment spent 279.8 ± 15.32 (SE) s locating the food odor source, whereas fish in the control and the pH 6 treatments spent 15.27 ± 1.73 and 8.46 ± 1.15 s, respectively, to locate the food odor source (Fig. 7).
Success in Locating the Odor Source
Success in locating the odor source was determined by observing the fish in each trial that stopped right on the food odor source. In the pH 6 treatment (control and experimental), all 10 pairs of fish (100%) located the food odor source, but in the pH 5 treatment, only 2 of 10 pairs of fish (20%) located the food odor source (Fig. 8).
Swimming Speed
Fish in the control group had an average swimming speed of 6.0 ± 1.43 (SE) cm/s. Fish in the pH 6 treatment had an average swimming speed of 3.7 ± 0.63 cm/s, while fish in the pH 5 treatment had an average swimming speed of 2.2 ± 0.52 cm/s. This shows that experimental pH levels of both 5 and 6 affected swimming speed (Fig. 9).
Discussion
Acidification significantly affected the chemosensory behavior of Poecilia sphenops. The results of this study indicate two important findings. First, the gold mollies’ ability to detect and respond to a chemical stimulus of food was impaired. The results show that the fish took more time to find the food odor source. In addition, the fish found the food less often in the pH 5 treatment than in the other treatments. The pH 5 treatment negatively impacted the chemosensory behavior of the fish toward the food odor source. The results demonstrate that under sublethal acidic conditions, the ability of gold mollies to acquire recognition of food odor from chemoreception was impaired. The results agree with the findings of Leduc et al. (2007).
Second, the gold mollies’ swimming speed was reduced in the lower-pH treatments (pH 5), the average swimming speed per fish was much lower than in the control treatment. This explains why, in the pH 5 treatment, the fish took a longer period of time on average to locate the food odor source than in the pH 6 and control treatments. Fish exposed to a conspecific alarm signal or predator odor stop feeding, decrease their swimming speed, and avoid the area where the odor is being emitted (Chivers et al. 1995; Brown and Smith 1998; Chivers and Brown 2005). This means that exposure of Poecilia sphenops to sublethal acidification must have caused an impairment in the ability of the fish to locate the food odor source in time.
A strong response to the feeding stimulus and swimming ability were almost entirely eliminated upon exposure to acidified water, pH 5. Variability in food intake is considered a primary factor governing fecundity for many different fish (Hoar and Randall 1969). The impairment of foraging demonstrated by the reduced chemosensory ability would have a negative impact on the ecology of these fish.
Studies on rainbow trout (Salmo gardineri) and brown trout (Salmo trutta) have shown that a reduction in pH of water bathing the olfactory epithelium from 6.5 to 5.5 can completely inhibit a normally strong neuroelectrical response to amino acids within 10 min (Thommesen 1978, 1983; Moore 1994). The sensory cells of olfaction and gustation are in constant contact with the aquatic media and can also be inhibited by low-pH exposure (Brown et al. 1982; Klaprat et al. 1992; Kasumyan and Doving 2003). It has been shown that acidic pollutants affect fish taste reception by both destroying the taste buds and reducing the sensitivity to the taste stimuli (Klaprat et al. 1992). A neurosensory impairment of the fish could affect the systems used to detect potential threats and to forage and feed (Wolf and Moore 2002). Damaging the fish’s ability to swim fast and their ability to respond to odor would create a higher probability of their being preyed on. The ability to detect, avoid, and escape predators is of prime importance for the survival of fish (Sih 1987; Lima and Dill 1990). The impairment of normal behavioral functions of fish exposed to acidic conditions may compromise their ability to adapt and survive in natural systems (Buckler et al. 1995). Sublethal acidic conditions also affect the physiological processes in fish, including reproduction (Klaprat et al. 1992; Ikuta et al. 2000; Ikuta and Kitamura 1995; Parker and McKeown 1986; Weiner et al. 1986; Tam et al. 1990). If fish’s reproduction is affected, this would lead to a reduction in recruitment and a decline in fish fitness. Fish need to be able to detect conspecific predators that occur in their habitat and be able to escape once given the chance to do so. The fish in the pH 5 treatment spent more time searching for food or took longer to find the food odor source. When fish take longer to locate the food odor source, they spend more time exposed to potential threats or predators (Wolf and Moore 2002; Lemly and Smith 1985; Chivers and Smith 1998; Smith 1999). It is known that predator avoidance in the estuarine environment is affected in physiologically stressed fish (smolts) (Jarvi 1989; Handeland et al. 1996; Mccormick et al. 1998) and that estuarine movements are delayed (Magee et al. 2003). This would definitely have a negative impact on the lifestyle and population of gold mollies.
Low pH significantly altered the feeding and swimming behavior of Poecilia sphenops. In nature, foraging or feeding includes a series of activities including searching, capture, acceptance, or rejection (Lemly and Smith 1985). All these processes constitute the feeding behavior in most species. Chemical stimuli are often used at different levels during an organism’s life (Lemly and Smith 1985). A low threshold response to food odor in the water may initiate a visual search and capture response. Chemicals from food in the mouth may lead to the food’s being accepted and then swallowed or rejected and spat out.
The results of this study reveal that the first two processes of feeding behavior (search and capture) would suffer greatly as a result of impaired chemoreceptors. Interference with chemoreceptors implies interference with other chemically mediated behavior patterns such as mating pheromones, predator odors (or recognition of individual conspecific and predators), homing behavior, and orientation (Lemly and Smith 1985). Previous research on sublethal exposure to pollutants has suggested that sensory impairment could have occurred as a result of inhibition of neurotransmitter receptors necessary for processing of information (Moore and Waring 1996; Hanazato 1999; Scholz et al. 2000). In this case a neurosensory impairment could have occurred which affected the system used to detect food and foraging. Moore (1994) showed that exposure of salmon to water of pH 5.5 significantly reduced the ability of the olfactory epithelium to detect relevant odors. The results show that a short exposure to water with a low pH can induce drastic changes in the ability of fish to respond to the smell of substances (Kasumyan and Sidorov 1995). Such findings might explain the misbalance of biotic communities in acidified water.
Acid rain is occurring at sublethal concentrations, but biological effects that lethal-concentration values do not address still exist (Shebra et al. 2000). These results also reveal that human-induced environmental change due to acidification may affect organisms in ways that are not immediately apparent. In addition, despite the obvious effect at pH 5, in this experiment pH 6 did not show a significant difference from the control. This does not mean that pH 6 has no effect; it probably would have impacted the gold mollies if they had been kept in that environment a bit longer.
References
Blaustein AR, Romansic JM, Kiesecker JM, Hatch AC (2003) Ultraviolet radiation, toxic chemicals and amphibian population declines. Divers Distrib 9:123–140. doi:10.1046/j.1472-4642.2003.00015.x
Brown GE, Chivers DP (2005) Learning as an adaptive response to predation within aquatic ecosystems. In: Barosa P, Castellano I (eds) Ecology of predator prey interactions. Oxford University Press, Oxford, pp 34–54
Brown GE, Smith RJF (1998) Acquired predator and recognition in juvenile rainbow trout (Oncorhynchus mykiss): conditioning hatchery-reared fish to recognize chemical cues of a predator. Can J Fish Aquat Sci 55:611–617. doi:10.1139/cjfas-55-3-611
Brown SB, Evans RE, Thompson BE, Hara TJ (1982) Chemoreception and aquatic pollutants. In: Hara TJ (ed) Chemoreception in fishes. Elsevier, Amsterdam, pp 363–393
Bouwman AF, Van Vuuren DP, Derwent RG, Posch M (2003) A global analysis of acidification and eutrophication of terrestrial ecosystems. Water Air Soil Pollut 141(1–4):349–382
Buckler DR, Cleveland L, Little EE, Brumbaugh WG (1995) Survival sub-lethal responses and tissue residues of Atlantic salmon exposed to acidic pH and aluminum. Aquat Toxicol 31:203–216
Chivers DP, Smith RJ (1998) Chemical alarm signaling in aquatic predator-prey systems: a review and prospectus. EcoScience 5:338–352
Chivers DP, Wisenden BD, Smith CJF (1995) The role of experience in the response of fathead minnows (Pimephales promelus) to extra skin of Iowa darters. Behaviour 132(9–10):665–674
Christopher E (1999) Avoidance of aluminum by rainbow trout. Environ Toxicol Chem 19(4):933–939
Dentener F et al (2006) Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Global Biogeochem. Cycles 20(4):GB4003. doi:10.1029/2005GB002672
Doney SC et al (2007) The impact of anthropogenic atmospheric nitrogen and sulfur deposition on ocean acidification and the inorganic carbon systems. Proc Natl Acad Sci USA 104(37):14580–14585
Dulka JA, Stacey NE (1991) Effect of olfactory tract lesions on gonadotropin and milt response to the female sex-pheromone, 17-alpha, 20-beta-dihydroxy-4-pregnen-3-one, in male goldfish. J Exp Zool 257(2):223–229
Hanazato T (1999) Anthropogenic chemicals (insecticides) disturb natural organic chemical communication in the plankton community. Environ Pollut 105:137–142
Handeland SO, Jarvi T, Ferno A, Stefansson SD (1996) Osmotic stress, anti-predator behavior and mortality of Atlantic salmon (Salmo salar) smolts. Can J Fish Aquat Sci 53:2673–2680
Hazlet BA (1994) Alarm responses in the crayfish Orconectes virilis and Orconectes propinquus. J Chem Ecol 20:1525–1535
Hoar WS, Randall DJ (eds) (1969) Reproduction. In: Fish physiology, vol 3. Academic Press, New York, pp 1–72
Holland EA, Brasswell BH, Sulzman J, Lamarque JF (2005) Nitrogen deposition onto the United States and Western Europe: synthesis of observations and models. Ecol Appl 15(1):38–57
Ikuta K et al (2000) Recent studies on the effects of acidification of fish in Japan. Global Environ Res 4(1):79–87
Ikuta K et al (2003) Effects of low pH on the reproductive behavior of salmonid fishes. Fish Physiol Biochem 28:407–410
Ikuta K, Kitamura S (1995) Effects of low pH exposure of adult salmonids on gametogenesis and embryo development. Water Air Soil Pollut 85:327–332
Japan Environment Agency (1997) Progress report of the 3rd rain monitoring in whereabouts of global environment/acid rain. Chuohoki, Tokyo, pp 203–252
Jarvi T (1989) Synergistic effects on mortality in Atlantic salmon (Salmo salar), smolt caused by osmotic stress and the presence of predators. Environ Boil Fishes 26:149–152
Johnson DW, Webster DA (1977) Avoidance of low pH in selection of spawning sites by brook trout (Salvelinus fontinalis). J Fish Res Board Can 34:2215–2218
Jones KA, Hara TJ, Scherer E (1985) Behavior modifications in arctic char (Salvelinus alpinus) chronically exposed to sublethal pH. Physiol Zool 58:400–412
Kasumyan AO, Doving KB (2003) Taste preferences in fish. Fish and fisheries. N-0136. University of Oslo, Blackwell, London
Kasumyan AO, Sidorov SS (1995) Environmental science and vulnerable ecosystems. 5th SETA-Europe congress, Copenhagen, pp 284
Klaprat D, Evans R, Hara TJ (1992) Environmental contaminants and chemoreception in fishes. In: Hara TJ (ed) Fish chemoreception. Chapman and Hall, New York, pp 321–341
Lamarque JF et al (2005) Assessing future nitrogen deposition and carbon cycle feedback using a multimodel approach: analysis of nitrogen deposition. J Geophys Res 110:D19303
Leduc AOH, Roh E, Breau C, Brown GE (2007) Effects of ambient acidity on chemosensory learning: an example of an environmental constraint on acquired predator recognition in wild juvenile Atlantic salmon (Salmo salar). Ecol Freshwater Fish 16:385–394
Leivestad H, Muniz IP (1976) Fish kill at low PH in Norwegian river. Nature 259:391–392
Lemly AD, Smith RJF (1985) Effects of acute exposure to acidified water on the behavioral response of fathead minnows, Pimephales promelas, to chemical feeding stimuli. Aquat Toxicol 6:25–36
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Canadian J Zool 68:619–640
Manuel E, Ortiz-Santaliestra M, Adolf JF, Miguel ML (2007) Effects of ammonium nitrate exposure and water acidification on the dwarf newt: the protective effect of oviposition behavior on embryonic survival. Aquat Toxicol 85:251–257
Magee JA, Obeclinski M, McCormick SD, Kocik JF (2003) Effects of episodic acidification on Atlantic salmon (Salmo salar) smolts. Can J Fish Aquat Sci 60:214–221
McCormick SD, Hansen LP, Quinn TP, Saunders RL (1998) Movement migration and smolting of Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 55:77–92
Moore A (1994) An electrophysiological study on the effects of pH on olfaction in mature male Atlantic salmon (Salmo salar) parr. J Fish Biol 45:493–502
Moore PA, Grills JL (1999) Chemical orientation to food by the crayfish Orconectes rusticus: influence of hydrodynamics. Anim Behav 58:953–963
Moore A, Waring CP (1996) Sub-lethal effects of the pesticide diazinon on olfactory function in mature male Atlantic salmon parr. J Fish Biol 48:758–775
Moore PA, Scholz N, Atema J (1991) Chemical orientation of lobsters, Homarus americanus, in turbulent odor plumes. J Chem Ecol 17:1293–1307
Parker DB, McKeown BA (1986) The effects of low pH on egg and alevin survival of kokanee and sockeye salmon on Corhyrichus nerka. Comp Biochem Physiol 87:259–268
Rodhe H, Dentener F, Schulz M (2002) The global distribution of acidifying wet deposition. Environ Sci Technol 36:4382–4388
Saglio P, Trijasse S (1998) Behavioral responses to atrazine and diuron in goldfish. Arch Environ Contam Toxicol 31:232–238
Sargent RC, Rush VN, Wisenden BD, Yan HY (1998) Courtship and mate choice in fishes: integrating behavioral and sensory ecology. Am Zool 38:82–86
Scholz N et al (2000) Diazinon disrupts antipredator and homing behaviors in Chinook salmon (Oncorhynchus tshawytscha). Can J Fish Aquat Sci 57:1911–1918
Shebra M, Dunham DW, Harvey HH (2000) Sub-lethal copper toxicity and food response in the freshwater crayfish Cambarus bartonii (Cambaridae, Decapoda, Crustacea). Ecotoxicol Environ Safety 46:329–333
Sih A (1987) Predator and prey life styles: an evolutionary and ecological overview. In: Kerfoot WC, Sih A (eds) Predation: direct and indirect impacts on aquatic communities. University press of New England, Hanover, pp 203–224
Smith RJ (1999) What good is smelly stuff in the skin? Cross function and cross taxa effects in fish “alarm substances”. In: Johnston RE, Muller-Schwarze D, Sorensen PW (eds) Advances in chemical signals in vertebrates. Kluwer, New York, pp 475–487
Tam WH, Fryer JN, Valentine B (1990) Reduction in oocyte production and gonadotrope activity and plasma levels of estrogen and vitellogenin, in brook trout exposed to low environmental pH. Can J Zool 68:2468–2476
Thommesen G (1978) The spatial distribution of odor induced potentials in the olfactory bulb of char and trout (Salmonide). Acta Physiol Scand 102:205–217
Thommesen G (1983) Detection of a blocking effect of low pH in the trout olfactory organ. In: Doving KB (ed) Chemoreception in studies of marine pollution. University of Oslo, Oslo, Norway
Weiner GS, Schreck CB, Li HW (1986) Effects of low pH on reproduction of rainbow trout. Trans Am Fish Soc 115:75–82
Wolf MC, Moore O (2002) Effects of the herbicide metolachlor on the perception of chemical stimuli by Orconectes rusticus. J N Am Benthol Soc 21(3):457–467
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice-Hall, Upper Saddle River, NJ
Zipple HP, Voigt R, Knaust M, Luan Y (1993) Spontaneous behavior, training and discrimination training in gold fish using chemosensory stimuli. J Comp Physiol 172A:81–90
Acknowledgments
I would like to thank all the people who lent their support, guidance, and efforts toward the successful completion of this project. I extend my sincere appreciation to Dr. Paul A. Moore for his insight and assistance throughout the development and completion of this article. I would like to acknowledge Dr. Daniel Pavuk for his input and suggestions during the writing of this article. Finally, I thank my family members Eddie, Emmanuel, and Nathan for being patient with me during the writing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tembo, R.N. The Sublethal Effects of Low-pH Exposure on the Chemoreception of Poecilia sphenops . Arch Environ Contam Toxicol 57, 157–163 (2009). https://doi.org/10.1007/s00244-008-9255-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-008-9255-x