Abstract
Tissue-specific responses against oxidative stress and lipid peroxidation were analyzed in wild adult mullet (Liza saliens) caught in the Portuguese coastal lagoon Esmoriz-Paramos. Parameters measured were catalase (CAT), superoxide dismutase (SOD), and glutathione-S-transferase (GST) activities in liver and gill tissues and lipid peroxidation. The enzyme activities were related to gill histopathological alterations, as well as to heavy metals (Cu and Zn) concentrations in these tissues. Gill epithelium of L. saliens showed histological alterations, such as epithelial hyperplasia resulting in lamellar fusion, epithelial lifting, vasodilatation, and lamellar aneurisms, with a prevalence ranging from 62% to 92%. The highest Cu content was found in liver (379 mg·kg−1), while the highest Zn content was observed in gill (119 mg·kg−1). SOD and CAT activities showed differences between gill and liver. The highest activities found were SOD in gill (10.1 U/mg protein) and CAT in liver (39.2 mmol/min/mg protein). In gill, CAT activity was negatively related to both Cu levels and gill lifting, while a positive relationship was found between SOD activity and fish age. The positive relationship between Cu and CAT activity in liver suggests that an increase in metabolic level is related to Cu-induced oxidative stress. The decrease in gill CAT activity can be due to osmotic stress caused by damaged gill epithelium. CAT activity in liver is an appropriate biomarker of oxidative stress in the Esmoriz-Paramos lagoon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The effects of pollutants in fish can be properly evaluated if bioaccumulation is complemented with other biomarkers. Contaminants usually appear in the environment as very complex mixtures that can cause interactive effects, thus biomarkers offer an integrated measurement of these effects (Orbea et al. 2002; Ferreira et al. 2005). Among pollutants that can accumulate in fish, heavy metals are of great interest because they could trigger oxidative stress in fish (Bláha et al. 2004; Deviller et al. 2005), by reactive oxygen species (ROS) generation (Durmaz et al. 2006; Lesser 2006).
Several studies revealed that exposure to contaminants in aquatic ecosystems can enhance intracellular formation of ROS, which could cause oxidative damage to biological systems (Livingstone 2003; Ferreira et al. 2005). ROS can be detoxified by an enzymatic defense system, which includes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (Halliwell and Gutteridge 1989). SOD is the enzyme that catalyses dismutation of the superoxide anion to O2 and H2O2, and CAT reacts with H2O2 to form water and molecular oxygen (Livingstone 2001; Lushchak et al. 2001; Ozmen et al. 2004). Glutathione-S-transferases form a family of multifunctional phase II biotransformation enzymes, present in cytosol of most cells, that catalyze the conjugation of glutathione to a variety of compounds (Livingstone 2003).
Recent data indicate that changes in the levels of antioxidant enzyme activities can be used as contamination biomarkers in different aquatic organisms (Livingstone 2003; Regoli et al. 2004). Lipid peroxidation is one of the main manifestations of oxidative damage induced by various compounds, including metals (Ercal et al. 2001; Livingstone 2003), and it has also been used as a biomarker of pollution (Sayeed et al. 2003; Bláha et al. 2004; Almroth et al. 2005).
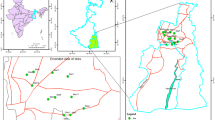
The Esmoriz-Paramos is a coastal lagoon on the Northwest coast of Portugal, which receives untreated industrial and domestic sewage that promotes a decline in water and sediment quality and a decrease in biodiversity. Heavy metals can be up-taken by fish from water, sediments, and suspended particulate material (Hardersen and Wratten 1998) and have been an important source of contamination in this ecosystem.
The most used vertebrate model in ecotoxicological studies is fish, which allows the evaluation of antioxidant responses in tissues and the hepatic oxidative damage caused by metal contamination (Orbea et al. 2002). The liver plays a primary role in the metabolism of xenobiotic compounds and it is a detoxification organ essential for excretion of toxic substances in fish (Hinton and Laurén 1990; Figueiredo-Fernandes et al. 2006a). Gill is the osmoregulatory surface tissue and it is the primary site of uptake of waterborne pollutants (McDonald and Wood 1993; Monteiro et al. 2005). The leaping grey mullet (Liza saliens) is the dominant species in the lagoon that comes into contact with water and sediment pollutants, through its detritus feeding behavior. Previous studies have shown Cu and Zn bioaccumulation in liver and gill of L. saliens from the lagoon and an increase in Zn in gill and Cu in both tissues that were age dependent (Fernandes et al. 2007a).
The aim of the present study was to determine the antioxidant enzyme activities in liver and gill of L. saliens from the Esmoriz-Paramos coastal lagoon and lipid peroxidation as a measurement of liver oxidative damage. Relationships among enzyme activities, gill histopathological alterations, and metal levels were also investigated.
Materials and Methods
Study Area
The Esmoriz-Paramos lagoon, 1500 m long (N–S), 700 m wide (W–E), and 2.5 m in maximum depth, with a catchment area of 78 km2, is located on the northwest coast of Portugal (Almeida 1998). It receives two main water inflows, one from the north and another from the south, and occasional effluents from local housing and small industry. The lagoon communication with the sea is established through a nonpermanent channel with small dimensions, particularly on the shoreline. Major inputs of contaminants into the lagoon are industrial or municipal, mostly untreated sewage from its tributaries and runoff from contaminated soils and surfaces (SIMRIA 2002).
Fish Sampling
Liza saliens were collected during April 2004 in the Esmoriz-Paramos lagoon, using a gill net. Mullets from the sea, 14 km northward from the lagoon, were also caught during the same period, and CAT liver and gill activities were measured and compared with equivalent activities in lagoon mullets. Fish were anesthetized, and gill and liver samples were removed, frozen in liquid nitrogen, and stored at –80°C, until biochemical assay. Gill samples were also randomly taken for histopathological examination and metal analyses. Fish age was determined by reading the annual ring structure of scales removed from the opercular region (Muir and Den Haas 2003).
Biochemical Analysis
Livers were homogenized in ice-cold 50 mM sodium phosphate buffer, 0.1 mM Na2EDTA, pH 7.8. Gills were homogenized in ice-cold 50 mM imidazol buffer, 150 mM sucrose, 10 mM Na2EDTA, pH 7.3 (homogenizer T 1500; Ystral GmH). Mitochondrial fractions were obtained after centrifugation at 15,000 g for 20 min at 4°C. SOD (EC 1.15.1.1) activity was determined by an indirect method involving the inhibition of cytochrome c reduction and spectrophotometric reading at 550 nm (McCord and Fridovich 1969). The concentrations of the reactives were 50 mM buffer, pH 7.8, 50 μM hypoxanthine, 1.98 mU/mL xanthine oxidase, and 10 μM cytochrome c (Ferreira et al. 2005). Enzyme activity is expressed as units per milligram of protein (U/mg pr), where 1 U corresponds to 50% inhibition of the xantine oxidase reaction.
GST (EC. 2.5.1.18) activity was determined according to Habig et al. (1974) adapted to microplate by Frasco and Guilhermino (2002). Concentrations of the reactive were as follows: glutathione (GSH), 10 mM in 0.1 M buffer, pH 6.5, and 1-chloro-2,4-dinitrobenzene (CDNB), 60 mM in ethanol, prepared just before the assay. The reaction mixture was in proportions of 4.95 mL (buffer):0.9 mL (GSH solution):0.15 mL (CDNB solution) (Ferreira et al. 2005). GST activity was measured every 20 s in a spectrophotometer at 340 nm during the first 5 min and quantified using the period of linear change in absorbance. Enzyme activity is expressed as nanomoles per minute per milligram of protein.
CAT (EC 1.11.1.6) activity was determined by measuring the consumption of H2O2 monitored spectrophotometrically at 240 nm, according to Aebi (1974). The reaction volume was 1 mL and contained 67.5 mM potassium phosphate buffer, pH 7.5, and 12.5 mM H2O2 (Ferreira et al. 2005). CAT activity is expressed as millimoles of decomposed hydrogen peroxide per minute per milligram of protein.
Oxidative Damage
The peroxidative damage of lipids creates free radicals that result in malondialdehyde (MDA) production, which was assessed by the thiobarbituric acid method (TBARS) adapted to microplate (Ferreira et al. 2005). Absorbance was measured at 532 nm and the concentration of MDA is expressed as nanomoles of MDA per gram of liver. Total protein was measured by the Lowry method adapted to microplate (Ferreira et al. 2005).
Tissue Metal Content
Gill soft tissue was lyophilized and digested overnight with nitric acid (superpure grade) at 60°C. The digested samples were analyzed in a graphite furnace atomic absorption spectrometer (UNICAMP 939 AA-GF90). Blank determinations were done using the same procedure with Milli-Q50 water. Results are expressed as milligrams per kilogram dry weight. Analytical accuracy and precision were checked using certified reference materials, i.e., DOLT-3 and DORM-2 (National Research Council of Canada).
Light Microscopy
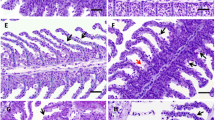
Gill tissue previously fixed in buffered formalin (10%) fluid for 48 h was dehydrated in graded ethanol concentrations and embedded in paraffin wax. Sagittal sections (5 μm thick) were stained with hematoxylin/eosin (H&E). Changes observed in gill tissue were analyzed under a Nikon light microscope.
A score system was used to rank the severity and extension of the gill lesions, according to Fernandes et al. (2007b). The severity of the lesions was scored as follows: 0 = no pathological alterations, 1 = focal mild pathological alterations, 2 = moderate pathological alterations, 3 = severe pathological alterations. The extent of affected lamellae was scored as follows: 0 = 0%, 1 = ≤ 10%, 2 = 11%–49%, 3 = 50%–69%, 4 = ≥70%. For each fish and lesion, the product severity × extension was calculated to establish an assessment value per gill filament varying between 1 and 12.
Statistical Analysis
Data are presented as mean ± standard deviation. Statistical calculations were performed with SPSS software. Differences among metals in tissues, enzymatic activities, and assessment values of gill lesions were tested using the Mann-Whitney U-test and the relationships between them were tested with Spearman correlations. A 5% significance level was employed throughout.
Results
The ranges of age and length of fish collected from the lagoon were 7 to 13 years and 25 to 49 cm, respectively. Gill lesion prevalence and gill histopathological assessment are presented in Table 1. Gill epithelium of L. saliens showed several histopathologic alterations; 77% of the fish presented three or four lesions. The main lesions observed were vasodilatation and epithelial hyperplasia, occasionally resulting in lamellar fusion, both present in 92% of fish. Epithelial lifting and lamellar capillary aneurisms were observed in 69% and 62% of fish, respectively. These histological alterations were observed at varying degrees of extension and severity. Hyperplasia and vasodilatation were scored as the maximum severity (grade 3), whereas the maximum extent (grade 4) was scored for hyperplasia and lifting. The highest mean assessment value of lesion was found for hyperplasia, followed by lifting, while the lowest was found for aneurisms and vasodilatation.
Gill and liver metal contents in L. saliens from the lagoon are summarized in Table 2. The highest Cu content was found in liver (p < 0.01), ranging from 125 to 547 mg·kg−1, and the highest Zn content was observed in gill (p < 0.05), ranging from 88 to 134 mg·kg−1. Branchial and hepatic enzyme activities and lipid peroxidation in fish from the lagoon are presented in Table 3. This table also reports gill and liver CAT activities in fish from the sea. Mullets collected in the lagoon showed similar GST activity levels in liver and in gill, a higher CAT activity in liver than in gill (p < 0.01), and a higher SOD activity in gill than in liver (p < 0.01). Lipid peroxidation and total protein content in liver ranged from 9 to 38 nmol MDA/g and from 90 to 266 mg/g, respectively.
Fish from the sea (22–35 cm long) showed lower liver CAT activity compared with lagoon fish (p = 0.03). In contrast, gill CAT activity was higher in fish from the sea.
A negative correlation between gill CAT activity and both copper levels and lifting assessment value was found in fish from the lagoon (Fig. 1). Similar results were found between gill CAT activity and severity of lifting (r = –0.618, p = 0.032). Also, in gill, a positive correlation was found between SOD and GST activities, as well as between SOD activity and fish age (Fig. 1). Figure 2 shows the two significant positive correlations between liver Cu and CAT activity and between liver Cu and lipid peroxidation. Negative correlations between GST activity and lipid peroxidation and between total liver protein content and fish length (Fig. 2) and fish age (r = −0.572, p = 0.04) were observed. No significant relationship was observed with Zn.
Discussion
Oxidative stress is defined as an adverse reaction resulting from the exposure of molecules, cells, or tissues to excess levels of free radical oxidants, especially ROS (Li et al. 2005; Lesser 2006). ROS produced in biological systems are detoxified by antioxidant defenses. One of the features of these antioxidant enzymes is their induction under conditions of oxidative stress, and such induction can be an important adaptation to pollutant-induced stress (Livingstone 2001). It is generally recognized that ROS production is associated with exposure to several metals (Ercal et al. 2001; Livingstone 2003), which can lead to induction of certain antioxidant enzymes (Ozmen et al. 2004).
This study reveals tissue-specific changes in SOD and CAT activities. However, no significant differences were observed between gill and liver GST activities. Induction of antioxidant enzymes is a common mechanism of adaptive response in fish that vary among tissues (Oruc et al. 2004). CAT activity was high in liver, where the Cu content was high. Paris-Palacios et al. (2000) reported an increase in hepatic CAT activity in fish, Brachydanio rerio, exposed to sublethal concentrations of Cu. The hepatic CAT activity in fish from the Esmoriz-Paramos lagoon was higher than in fish from the sea and higher than that reported in Mugil cephalus caught in polluted environments (Orbea et al. 2002; Ferreira, et al. 2005). The high CAT activity may be a response to increased H2O2 production (Ritola et al. 2002), to protect biological systems against ROS (Romeo et al. 2000). The high hepatic CAT activity found in this work suggests that a metabolic increase was triggered to cope with Cu-induced oxidative stress. In contrast, CAT activity in gill was lower in fish from the lagoon compared with fish from the sea and was negatively correlated with Cu concentrations and gill lifting. The lifting of filamentar and lamellar epithelium constitutes a typical defense mechanism that increases the diffusion distance between blood and waterborne pollutants. Previous studies revealed osmoregulatory disturbances as a consequence of gill permeability and cell integrity changes (Fernandes et al. 2007c) that could have affected CAT activity.
SODs are a group of metalloenzymes that plays a crucial antioxidant role and constitutes a defense system against the natural or chemically induced production of ROS (Roche and Bogé 1996; Livingstone 2001). Several studies using different species exposed to several pollutants showed an increase in SOD activity (Palace et al. 1996; Figueiredo-Fernandes et al. 2006b), namely, when fish were exposed to Cu (Sanchez et al. 2005). The induction of hepatic SOD activity was also described in some studies carried out in field polluted sites (Deviller et al. 2005; Ferreira et al. 2005). Liver SOD activity in our study was low compared to that in M. cephalus caught in polluted environments (Orbea et al. 2002; Ferreira et al. 2005). These results suggest that the decrease in hepatic SOD activity could be due to the high copper content found in livers of fish from the lagoon. Previous experimental studies in Oreochromis niloticus also reported decreased SOD activity in liver (Peixoto et al. 2006). The increase in hepatic CAT activity and the decrease in hepatic SOD activity agreed with the results observed in Geophagus brasiliensis caught in a polluted area (Filho et al. 2001). Porte et al. (2002) also reported an increase in CAT activity and that there is no relationship between pollutants and hepatic SOD activity in wild mullet, Mullus barbatus.
A positive relationship between heavy metals (Cu and Zn) in gill and fish age in L. saliens caught in the Esmoriz-Paramos lagoon was demonstrated in a previous study (Fernandes et al. 2007a). An increase in SOD gill activity with fish age was found in the present work, and the higher SOD activity in gill than in liver could be an indicator of compensatory tissue response to face metal exposure. Oruc et al. (2004) also showed higher SOD activity in gill compared with other tissues as a result of pollutant exposure.
The conjugation of phase I metabolites with GSH is catalyzed by GST, one of the most widely studied conjugation enzymes in vertebrates. GST is involved in detoxification and excretion of foreign compounds and it may also show peroxidase activity (Paris-Palacios et al. 2000; Chung et al. 2004). This might explain the negative relationship between hepatic GST activity and lipid peroxidation found in the present study. The effects of pollutants on GST activity have been somewhat inconclusive, showing induction, no change, or inhibition of this enzyme (Stephensen et al. 2000). Some studies showed that exposure to pollutants can lead to an increase of hepatic GST activity (Sen and Kirikbakan 2004; Camargo and Martinez 2006), whereas others reported no variation (Porte et al. 2002) with respect to copper (Sanchez et al. 2005) and even decreased activity (Filho et al. 2001). Martinez-Lara et al. (1996) found a decrease in GST activity in gilthead seabream (Sparus aurata) exposed to pollutants and suggested that these results could be due to inactivation by ROS generated by pollutants. The values of hepatic GST activity of L. saliens caught in a reference site in Turkey (Sen and Kirikbakan 2004) are similar to those found in the present work. In addition, hepatic GST activities in M. cephalus collected in a polluted site (Ferreira et al. 2006) were high compared to our data. A positive relationship between gill SOD and gill GST activities was found, which may reflect a reinforced response against oxidative stress in this organ.
No relationship between Zn content and oxidative stress enzymes activities was found in gill or liver of L. saliens. This could be related to the fact that Zn is apparently regulated in liver and its increase in gill over time is kept within a range (Fernandes et al. 2007a).
Malondialdehyde (MDA) production is a well-known oxidation product of polyunsaturated fatty acids, influencing cell membrane fluidity as well as the integrity of biomolecules (Ercal et al. 2001; Almroth et al. 2005), and is an important indicator of lipid peroxidation (Freeman and Crapo 1981). The present study revealed a positive relationship between lipid peroxidation measured as MDA and Cu liver content. The induction of hepatic lipid peroxidation caused by chronic dietary exposure to Cu was confirmed in grey mullet Chelon labrosus (Baker et al. 1998). Our results suggest that antioxidant enzymes were not able to prevent the hepatic lipid peroxidation induced by chronic metal exposure.
The decrease in hepatic total protein was related to fish age and fish length, suggesting that it is age dependent. These data can be related to a metabolic activity reduction in older fish, as well as protein degradation due to ROS production.
In conclusion, the present study revealed that fish developed tissue-specific enzyme responses, such as increase in CAT activity in liver and SOD activity in gill, to cope with pollution exposure. CAT activity in liver is an appropriate biomarker of oxidative stress against copper exposure in the Esmoriz/Paramos lagoon. The fact that L. saliens lives in the lagoon for its entire life span enabled long-term evaluation of the stress responses, which are more realistic than acute laboratory toxicity tests. Furthermore, data obtained in this study may be useful to compare biomarker fish responses from other polluted sites.
References
Aebi H (1974) Catalase. In: Bergmayer HU (ed) Methods of enzymatic analysis. Academic Press, London, pp. 671–684
Almeida CSD (1998) Estudo de pesticidas na Barrinha de Esmoriz-Lagoa de Paramos. Desenvolvimento do método de extracção de triazinas em fase sólida e identificação por cromatografia líquida de alta eficiência com detecção por Diodo Array. MSc thesis, ICBAS, Oporto University, Portugal
Almroth BC, Sturve J, Berglund A, Förlin L (2005) Oxidative damage in eelpout (Zoarces viviparous), measured as protein carbonyls and TBARS, as biomarkers. Aquat Toxicol 73:171–180
Baker RTM, Handy RD, Davies SJ, Snook JC (1998) Chronic dietary exposure to copper affects growth, tissue lipid peroxidation, and metal composition of the grey mullet, Chelon labrosus. Mar Environ Res 45(4/5):357–365
Bláha L, Kopp R, Simková K, Mares J (2004) Oxidative stress biomarkers are modulated in silver carp (Hypophthalmichthys molitrix Val.) exposed to microcystin-producing cyanobacterial water bloom. Acta Veterin BRNO 73:477–482
Camargo MMP, Martinez CBR (2006) Biochemical and physiological biomarkers in Prochilodus lineatus submitted to in situ tests in an urban stream in southern Brazil. Environ Toxicol Pharmacol 21(1):61–69
Chung MJ, Walker PA, Hogstrand C (2004) Metal physiology and biochemistry in fish cells: from toxicity to protection by zinc. Comp Biochem Physiol 100C:137–147
Deviller G, Palluel O, Aliaume C, Asanthi H, Sanchez W, Franco Nava MA, Blancheton JP, Casellas C (2005) Impact assessment of various rearing systems on fish health using multibiomarker response and metal accumulation. Ecotoxicol Environ Saf 61:89–97
Durmaz H, Sevgiler Y, Üner N (2006) Tissue-specific antioxidative and neurotoxic responses to diazinon in Oreochromis niloticus. Pest Biochem Physiol 84:215–226
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress Part I: Mechanisms involved in metal induced oxidative damage. Curr Topics Med Chem 1:529–539
Fernandes C, Fontaínhas-Fernandes A, Peixoto F, Salgado MA (2007a) Bioaccumulation of heavy metals in Liza saliens from the Esmoriz-Paramos coastal lagoon, Portugal. Ecotoxicol Environ Saf 66:426–431
Fernandes C, Fontaínhas-Fernandes A, Monteiro S, Salgado MA (2007b) Histopathological gill changes in wild leaping grey mullet (Liza saliens) from the Esmoriz-Paramos coastal lagoon, Portugal. Environ Toxicol 22:443–448
Fernandes C, Fontaínhas-Fernandes A, Monteiro S, Salgado MA (2007c) Changes in plasma electrolytes and gill histopathology in wild Liza saliens from the Esmoriz-Paramos coastal lagoon, Portugal. Bull Environ Contam Toxicol 79:301–305
Ferreira M, Moradas-Ferreira P, Reis-Henriques MA (2005) Oxidative stress biomarkers in two resident species, mullet (Mugil cephalus) and flounder (Platichthys flesus), from a polluted site in River Douro Estuary, Portugal. Aquat Toxicol 71:39–48
Ferreira M, Moradas-Ferreira P, Reis-Henriques MA (2006) The effect of long-term depuration on phase I and phase II biotransformation in mullets (Mugil cephalus) chronically exposed to pollutants in River Douro Estuary, Portugal. Mar Environ Res 61:326–338
Figueiredo-Fernandes A, Fontaínhas-Fernandes A, Rocha E, Reis-Henriques MA (2006a) Effects of gender and temperature on hepatic EROD activity, liver and gonadal histology in Nile tilapia Oreochromis niloticus exposed to paraquat. Arch Environ Contam Toxicol 51:626–632
Figueiredo-Fernandes A, Fontaínhas-Fernandes A, Peixoto F, Rocha E, Reis-Henriques MA (2006b) Effect of paraquat on oxidative stress enzymes in tilapia Oreochromis niloticus at two levels of temperature. Pest Biochem Physiol 85:97–103
Filho DW (1996) Fish antioxidant defences—a comparative approach. Braz J Med Biol Res 29:1735–1742
Filho DW, Torres MA, Tribess TB, Pedrosa RC, Soares CHL (2001) Influence of season and pollution on the antioxidant defenses of the cichlid fish acará (Geophagus brasiliensis). Braz J Med Biol Res 34:719–726
Frasco MF, Guilhermino L (2002) Effects of dimethoate and beta-naphthoflavone on selected biomarkers of Poecilia reticulate. Fish Physiol Biochem 26:149–156
Freeman BA, Carpo JD (1981) Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem 256:10986–10992
Habig WH, Pabst MJ, Jaoby W (1974) Glutatione-S-transferases—first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Halliwell B, Gutteridge HMC (1999) Free radicals in biology and medicine. Oxford University Press, Oxford, UK
Hardersen S, Wratten SD (1998) The effects of carbaryl exposure of the penultimate larval instars of Xathocnemis zealandica on emergence and fluctuating asymmetry. Ecotoxicology 7:297–304
Hinton DE, Laurén DJ (1990) Liver structural alterations accompanying chronic toxicity in fishes: potential biomarkers of exposure. In: McCarthy JF, Shugart LR (eds) Biomarkers of environmental contamination. Lewis, Boca Raton, FL, pp 17–57
Lesser MP (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol 68:253–278
Li XY, Chung IK, Kim JI, Lee JA (2005) Oral exposure to Microcystis increases activity-augmented antioxidant enzymes in the liver of loach (Misgurnus mizolepis) and has no effect on lipid peroxidation. Comp Biochem Physiol 141:292–296
Livingstone DR (2001) Contaminant reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42:656–666
Livingstone DR (2003) Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Rev Méd Vét 154:427–430
Lushchak V, Lushchak LP, Mota AA, Hermes-Lima M (2001) Oxidative stress and antioxidant defences in goldfish Carassius auratus during anoxia and reoxygenation. Am J Physiol Regul Integr Comp Physiol 280:100–107
Martinez-Lara E, Toribio F, López-Barea J, Bárcena JA (1996) Glutathione-S-transferase isoenzyme patterns in the gilthead seabream (Sparus aurata) exposed to environmental contaminants. Comp Biochem Physiol 113:215–220
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymatic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
McDonald DG, Wood CM (1993) Branchial mechanisms of acclimation to metals in freshwater fish. In: Rankin J (ed) Fish ecophysiology. Chapman & Hall, London
Monteiro SM, Mancera JM, Fontaínhas-Fernandes A, Sousa M (2005) Copper induced alterations of biochemical parameters in the gill and plasma of Oreochromis niloticus. Comp Biochem Physiol 141:375–383
Muir A, Den Haas TC (2003) Manual of standard methods for the Chippewas of Nawash calcified structure analysis facility. Fish Assessment Program, Chippewas of Nawash First Nations
Orbea A, Ortiz-Zarragoitia M, Solé M, Porte C, Cajaraville MP (2002) Antioxidant enzymes and peroxisome proliferation in relation to contaminant body burdens of PAHs and PCBs in bivalve molluscs, crabs and fish from the Urdaibai and Plentzia estuaries (Bay of Biscay). Aquat Toxicol 58:75–98
Oruc EO, Sevgiler Y, Uner N (2004) Tissue-specific oxidative stress responses in fish exposed to 2,4-D and azinphosmethyl. Comp Biochem Physiol 137:43–51
Ozmen I, Bayir A, Cengiz M, Sirkecioglu AN Atamanalp M (2004) Effects of water reuse system on antioxidant enzymes of rainbow trout (Oncorhynchus mykiss W., 1792). Veterin Med Czech 49:373–378
Palace VP, Dick TA, Brown SB, Baron CL, Klaverkamp JF (1996) Oxidative stress in Lake sturgeon (Acipenser fulvescens) orally exposed to 2,3,7,8-tetrachlori-dibenzofuran. Aquat Toxicol 35:79–92
Paris-Palacios S, Biagianti-Risbourg S, Vernet G (2000) Biochemical and (ultra)structural hepatic perturbations of Brachydanio rerio (Teleostei, Cyprinidae) exposed to two sublethal concentrations of copper sulphate. Aquat Toxicol 50:109–124
Peixoto F, Alves-Fernandes D, Santos D, Fontaínhas-Fernandes A (2006) Toxicological effects of oxyfluorfen on oxidative stress enzymes in tilapia Oreochromis niloticus. Pest Biochem Physiol 85:91–96
Porte C, Escarpín E, Garcia de la Parra LM, Biosca X, Albaigés J (2002) Assessment of coastal pollution by combined determination of chemical and biochemical markers in Mullus barbatus. Mar Ecol Prog Ser 235:205–216
Regoli F, Frenzilli G, Bochetti R, Annarumma F, Scarcelli V, Fattorini D, Nigro N (2004) Time-course variations of oxyradical metabolism, DNA integrity and lysosomal stability in mussels, Mytilus galloprovincialis, during a filed translocation experiment. Aquat Toxicol 68:167–178
Ritola O, Livingstone DR, Peters LD, Lindstrom-Seppa P (2002) Antioxidant processes are affected in juvenile rainbow trout (Oncorhynchus mykiss) exposed to ozone and oxygen-supersaturated water. Aquaculture 210:1–19
Roche H, Bogé G (1996) Fish blood parameters as a potential tool for identification of stress caused by environmental factors and chemical intoxication. Mar Environ Res 41(1):27–43
Romeo M, Bennani N, Gnassia-Barelli M, La Faurie M, Girard JP (2000) Cadmium and copper display different responses towards oxidative stress in the kidney of the sea bass Dicentrarchus labrax. Aquat Toxicol 48:185–194
Sanchez W, Palluel O, Meunier L, Coquery M, Porcher J-M, Aït-Aïssa S (2005) Copper-induced oxidative stress in three-spined stickleback: relationship with hepatic metal levels. Environ Toxicol Pharmacol 19:177–183
Sayeed I, Parvez S, Pandey S, Bin-Hafeez B, Haque R, Raisuddin S (2003) Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicol Environ Saf 56:295–301
Sen A, Kirikbakan A (2004) Biochemical characterization and distribution of glutathione S-transferase in leaping mullet (Liza saliens). Biochemistry (Moscow) 69(9):993–1000
SIMRIA (2002) Avaliação da Contaminação da Barrinha de Esmoriz. International Report. Saneamento integrado dos municípios da Ria, Aveiro, Portugal
Stephensen E, Svavarsson J, Sturve J, Ericon G, Adolfson-Erici M, Förlin L (2000) Biochemical indicators of pollution exposure in shorthorn sculpin (Myoxocephalus scorpius), caught in four harbours on the southwest coast of Iceland. Aquat Toxicol 48:431–442
Acknowledgments
This study was partially supported by the Foundation for Science and Technology (FCT) through research project POCTI/QUI/15089/1999 and by the Center of Studies for Technological, Environmental and Life Sciences (CETAV), University of Trás-os-Montes and Alto Douro, Portugal. The authors would like to thank Esmoriz fire brigade for their support in fish sampling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernandes, C., Fontaínhas-Fernandes, A., Ferreira, M. et al. Oxidative Stress Response in Gill and Liver of Liza saliens, from the Esmoriz-Paramos Coastal Lagoon, Portugal. Arch Environ Contam Toxicol 55, 262–269 (2008). https://doi.org/10.1007/s00244-007-9108-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-007-9108-z