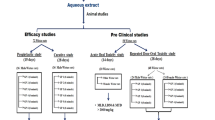
Abstract
The crude extract of Trachyspermum ammi seeds (Ta.Cr) was studied for its antiurolithic activity using the in vivo and in vitro experiments. In the in vivo experiments, Ta.Cr treatment showed a diuretic activity at the dose of 30 and 100 mg/kg and exhibited curative effect in male hyperoxaluric Wistar rats, which received 0.75% ethylene glycol (EG) in drinking water given for 3 weeks, with 1% ammonium chloride (AC) for initial three days. In the in vitro experiments, Ta.Cr delayed the slopes of nucleation and inhibited the calcium oxalate (CaOx) crystal aggregation in a concentration-dependent manner like that of potassium citrate. Ta.Cr also inhibited DPPH free radicals like standard antioxidant drug butylated hydroxytoluene (BHT), and significantly reduced cell toxicity and LDH release in Madin–Darby canine kidney (MDCK) cells, exposed to oxalate (0.5 mM) and COM (66 µg/cm2) crystals. In isolated rabbit urinary bladder strips, Ta.Cr relaxed high K+ (80 mM) and CCh (1 µM)-induced contractions, showing antispasmodic activity. The findings of this study suggest that the antiurolithic activity of crude extract of Trachyspermum ammi seeds may be mediated by a number of mechanisms, including a diuretic, an inhibitor of CaOx crystal aggregation, an antioxidant, renal epithelial cell protection, and an antispasmodic, thus, showing the therapeutic potential in urolithiasis, for which there is no viable non-invasive option in modern medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary stone formation is one of the earliest disorders. Its incidence and prevalence is constantly increasing, with no regard for geographical, cultural, or racial boundaries. Calcium oxalate (CaOx) alone or in combination with the nucleus of calcium phosphate accounts for approximately 80% of kidney stones, while uric acid and struvite stones account for 9 and 10%, respectively, leaving only 1% for all others such as cystine, ammonium acid urate and others [1, 2].
Clinical and experimental data provide strong evidence for the development of reactive oxygen species (ROS) production and oxidative stress (OS) in the kidneys of stone-forming patients and rats, respectively. Initially, ROS prevents stone formation by the production of crystallization inhibitors. However, decreased antioxidant capacity or persistently supersaturated urine, may result in OS and urolithiasis [3]. It is also evident that OS and inflammation are associated with both nephrolithiasis and cardiovascular diseases. In addition, kidney stone formation can lead to chronic kidney disease, hypertension, diabetes and myocardial infarction (MI) [4]. Antilipidemic drug; atorvastatin, reduce in renal epithelial injury and CaOx crystal deposition in hyperoxaluric rats [5]. Renal cells exposed to CaOx crystals secrete superoxide in real-time as measured by an electrochemical superoxide biosensor [6] while the presence of antioxidants considerably reduced the cell injury and improved the antioxidant status of the cells when exposed to Ox, CaOx or CaP crystals [7]. There is limited success in the treatment with pharmaceutical medicine while surgical procedure is expensive and beyond the reach of a common man, particularly in developing countries. According to WHO, traditional medicine comprising mainly herbs provides an economical and affordable source of drugs for three-quarters of the world population and its therapeutic use is increasing day by day [8, 9]. In this study, we selected the seeds of Trachyspermum ammi (L.), which is traditionally used as diuretic, litholytic, antiseptic, in stomachache, colic among other multiple uses [10,11,12]. The phytochemical constituents are carbohydrates, calcium, iron, carotene, nicotinic acid, riboflavin, beta-pinene, alpha-pinene, potassium, protein, thiamine and thymol [12].
Trachyspermum ammi has been scientifically evaluated for its anthelmintic, antiaggregatory of human platelets [13], antihistaminic [14], dislipidemic [15], hepatoprotective, bronchodialatory, antihypertensive, antispasmodic [16], antioxidant and antimutagenic [17] activities. In 1990, a study was conducted to see the antiurolithic effect of different plants including Trachyspermum ammi. At the end of the experiment, it was concluded that all other plants were quite effective, Trachyspermum ammi, which give only 29% protection as opposed to others [18], the authors, therefore, concluded that their data did not support the traditional use of Trachyspermum ammi in the treatment of urolithiasis. In this study, the Trachyspermum ammi was used in the powder form, with oral gavages and 3% glycolic acid and measured calcium and oxalate content in kidney tissue, which is not a very sensitive approach to evaluate antilithiatic properties. Kaur, T. has isolated anticalcifying protein from Trachyspermum ammi and study its antiurolithic action [19]. Therefore, this study has been undertaken to investigate anti-urolithic activities of the crude extract of Trachyspermum ammi seeds (Ta.Cr) using the in vivo calcium oxalate and diuretic activity in animal models and several in vitro experiments including antioxidant, crystallization assays, renal epithelial (MDCK) cells protective and spasmolytic using rabbit urinary bladder strips, to investigate the mechanisms likely to contribute in its potential antiurolithic effect and to rationalize medicinal use of Ta.Cr in the management of kidney stones.
Methodology
Chemicals
Chemicals were obtained from the following specified sources: 1,1,3,3,-tetraethoxy propane, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 5-5′-dithiobis, ammonium chloride, bovine serum albumin, calcium oxalate monohydrate crystals (COM), butylated hydroxyl toluene (BHT), sodium oxalate, ethylene glycol (EG), carbachol (carbamylcholine chloride, CCh), ferrous sulfate heptahydrate, Folin–Ciocalteu’s phenol reagent, Hydrogen peroxide (H2O2), petroleum spirit and n-butanol (Merck, Darmstadt, Germany). Nuclear fast red, potassium citrate tribasic hydrate, silver nitrate (AgNO3), thiobarbituric acid, potassium chloride, verapamil hydrochloride, 5–5′-dithiobis, thymol, trichloroacetic acid, 2-nitrobenzoic acid (DTNB), (Sigma Chemical Company, St. Louis, MO, USA). For the estimation of blood urea nitrogen, calcium and magnesium Randox Kits (Randox Laboratories Ltd. Ardmore, Diamond Road, Crumlin, Co. Antrim, UK) were used. Trinity Biotech (Plc, IDA business park, Bray, Co. Wicklow, Ireland) kit was used to estimate oxalate while citrate kit was obtained from R-Biopharm AG, D-64293 Darmstadt. Reagents of histopathology: eosin, hematoxylin, xylene, paraffin wax, and chemicals for physiological salt solutions were calcium chloride (CaCl2), glucose, ethylenediamine tetraacetic acid (EDTA), magnesium chloride (MgCl2), potassium dihydrogen phosphate (KH2PO4), magnesium sulfate (MgSO4), potassium chloride (KCl), sodium bicarbonate (NaHCO3), sodium dihydrogen phosphate (NaH2PO4), were obtained from Merck, Darmstadt, Germany. Sodium chloride (NaCl) was obtained from BDH Laboratory Supplies, Poole, England, and potassium chloride (KCl) was from Sigma Chemical Co, St Louis, MO, USA.
Animals
Wistar rats (190–220 g) of either sex were housed at the animal house of the Aga Khan University and kept in cages (47 × 34 × 18 cm3) containing saw dust (renewed after every 2 days). Controlled temperature of 23–25 ℃ and 12 h light–dark cycles were maintained in the animal house. A standard diet containing (Kg/10 kg): bran (3.85 kg), flour (3.85 kg), molasses (115 g), salt (57.7 g), nutrivet L (19.25 g), potassium meta bisulphate (11.5 g), oil (385 g), fishmeal (1.73 kg), and powdered milk (1.54 kg), and tap water was given ad libitum to animals. However, no food was given 24 h before and during 6 h of diuretic study, and during collecting 24 h urine samples.
The study was approved by the Board of Advance Studies & Research, University of Karachi through reference No. BASR/No./2274-A/Sc, Resol. No. 09 (08) as part of the research proposal for PhD research. All experiments were performed in agreement with the rulings of the Institute of Laboratory Animal Resources, Commission on Life Sciences, [20]. Research Council (1996) and all efforts were made to minimize suffering for animals used in this research.
Extraction method
Dried seeds of Trachyspermum ammi were purchased from a local herbal store and identified by Prof. Dr. Jhandar Shah, Taxonomist/Ex Vice-chancellor Shaheed Benazir Bhutto University, Sheringal, Pakistan. The sample of the seeds was submitted to the herbarium of the Department of Biological and Biomedical Sciences (DBBS), the Aga Khan University, Karachi, with voucher specimen No. TA-SE-06–08-73. The clean seeds were extracted with 70% methanol (70% methanol and 30% water, v/v) and stored for three days, with intermittent shaking. It was filtered twice: once with a muslin cloth and once with Whatman qualitative grade 1 filter paper. This procedure was repeated three times, and then the combined filtrate was evaporated on a rotary evaporator at low pressure (60 mmHg) to a thick, semi-solid brown mass, i.e. the crude extract of Trachyspermum ammi seeds (Ta.Cr). The final yield of the crude extract was approximately 17% w/w [24].
In vivo diuretic activity
Wistar rats (180–220 g) of either sex were used for the diuretic activity of Ta.Cr according to the method described previously [21]. Briefly, animals were divided into six groups, each having 6–8 rats. Normal saline and positive control groups were given saline (20 ml/kg of body weight) by gavage and hydrochlorothiazide (HCT; 10 mg/kg), respectively. While groups 3–6 were given 10, 30, 100 and 300 mg/kg body weight of Ta.Cr in saline. Afterward, each rat was individually placed in the metabolic and diuretic cage. At 2-h interval, the urine was collected in graduated cylinders for 6 h. The volume of the total excreted urine for 6 h was determined. Then its pH was determined using pH meter (Orion* 320 PerpHecT* Thermo Scientific), while Na+ and K+ concentrations were measured on Corning’s flame photometer (Flame Photometer 410).
Studies on animal models of urolithiasis
Antiurolithic activity of the extract was determined using the rat model of CaOx urolithiasis as mentioned in our previous studies [22, 23]. Male Wistar rats weighing 180–220 g were divided into 8 groups each containing 6 rats to receive various treatments for preventive and curative effects of Ta.Cr.
In preventive study model, normal water was given to rats of group 1, which served as normal control. Whereas Group II; served as the diseased control group, were given water containing EG (0.75% w/v) for 21 days along with NH4Cl (AC) (1% w/v) for the first 5 days. Group I and II received i.p injection of normal saline (2.5/kg) while group number III and IV, received i.p injections of Ta.Cr 30 and 100 mg/kg, respectively, once in a day along with stone-inducing treatment (EG + AC), like that of group II (Disease control).
In the curative study, CaOx stones were induced in the kidneys of both untreated control (Group VI) and treated (Groups VII and VIII) rats with EG (0.75%) and NH4Cl (1%) administration for 21 days, while the group V received normal tap water for the first three weeks. Thereafter, stone-inducing treatment was withdrawn and, normal and diseased control groups were given normal saline 2.5 ml/kg, while group number VII and VIII received Ta.Cr 30 and 100 mg/kg, respectively, for another 2 weeks.
Animal weights and their activity were observed regularly in both the preventive and curative study models, whereas 24 h urine samples were collected at the start day 0, 21 and 35. 3-h morning urine sample was collected after both 21 and 35 days, for crystalluria measurement.
24 h urine samples were stored at − 20 ℃ after volume and pH were determined. Blood was taken from rats under ether anesthesia via cardiac puncture to separate serum creatinine and blood urea nitrogen (BUN). Then experimental rats were sacrificed and their kidneys were fixed in 10% neutral-buffered formalin, processed, embedded in paraffin wax, sectioned at 5 m, and stained for microscopic examination with haematoxylin and eosin (H&E) and Pizzolato’s method for calcium oxalate crystals [24].
Deposition of calcium oxalate crystal in kidneys
Crystal distribution within the kidneys was determined by using the semi-quantitative scoring methods used previously [22, 23]. Briefly, the crystal deposits in stained sections with visible in a field of 10 × magnification were counted, and severity grades were assigned as 0 = no crystals, 1 = 1–10 crystals, 2 = 11–30, 3 = 31–50, 4 = 51–75 and 5 = more than 75 crystals. Most of the crystals were located in the cortical and outer modularly region of the kidney.
Urine and serum analyses
Urine samples for Ca++, citrate, Ox, Mg++, and uric acid (UA) and serum samples for and blood urea nitrogen (BUN) and creatinine contents were measured through commercially available kits, while urinary protein and inorganic phosphate were measured through Lowry’s [25] and molybdenum blue (MB) reaction [26], respectively.
In vitro crystallization assays
Kinetic study
The effects of Ta.Cr on calcium oxalate (CaOx) crystallization was verified by the measurement of the change in turbidity with time, due to the crystal nucleation and aggregation of Ca++ and Ox after mixing metastable solutions of Ca++ and Ox. Stock solutions of CaCl2 (8.5 mM) and Na2C2O4 (1.5 mM), containing sodium acetate (10 mM) and NaCl (200 mM) were adjusted to pH 5.7 [27]. To inspect the event of CaOx crystallization, platelet aggregometer (Chrono-Log Corporation, USA), was used [28]. CaCl2 solution (0.5 ml) was stirred continuously both in the absence and presence of different concentrations of standard drug (potassium citrate) or extracts at 37 ℃. Crystallization was induced by the addition of Na2C2O4 solution (0.5 ml) to get the final concentration of 0.75 mM of Ox and 4.25 mM of Ca++. The change in turbidity of the solution with time was concurrently started on a chart, which was moving for around 15 min, at the speed of 30 mm/h with constant stirring of the solutions. All the experiments were repeated three times. Linear regression analysis was used to calculate the slopes of aggregation phases (SA) and nucleation (SN). The percentage inhibition was calculated as [(1-Se/Sc) × 100], where Se is the slope in the presence of plant extract and Sc is the slope of the control experiment.
Incubation assay
To examine the effect of incubation of extract or control drug; potassium citrate, on CaOx crystal formation, stock solutions of Na2C2O4 and CaCl2; similar to that used in kinetic study, were used. CaCl2 solutions, containing various concentrations of potassium citrate or Ta.Cr, were aliquoted (0.5 ml) in a 24 well (flat-bottomed) plate. (Iwaki Microplate with lid, Scitech Div., Asahi Techno Glass, Japan). Na2C2O4 solution (0.5 ml) was added to each well [29]. Three concentration of each test material was prepared. The plates were then incubated for 45 min. at a temperature of 37 ℃ in a shaking water bath. Abundance and the morphology of the crystals in each tube were then examined under an inverted microscope (Nikon Corporation, Tokyo, Japan).
Antioxidant activity
Antioxidant activity of the Ta.Cr was evaluated in vitro by lipid peroxidation and free radical scavenging activities.
Lipid peroxidation inhibitory activity
Lipid peroxidation inhibitory potential was evaluated in homogenized kidney tissues of Wistar rats (Zero-Max®). The homogenate was prepared in ice-cold PBS (50 mmol/l, pH 7.4) as reported earlier [30]. Briefly, the homogenate was centrifuged for 10 min at 10,000 × g and the supernatant was incubated for 01 h in 10 µM FeSO4 and 0.1 mM ascorbic acid at 37 ℃, in the absence (control) or presence of different concentrations (50 & 150 µg/ml) of the test material. The reaction was blocked/stopped by adding 0.5 ml TCA (28%) and 0.75 ml TBA (1%) in succession and then heated for 20 min at 90 ℃. This incubation caused the formation of pink-colored malondialdehyde–thiobarbituric acid (MDA–TBA), which was extracted with n-butanol (3 ml). Then the color intensity of the MDA-TBA complex was measured by spectrophotometer (DU 730, Beckman Coulter) at 532 nm. The same formula, given above for the free radical scavenging activity, was used to determine the inhibition ratio.
Free radical scavenging activity
To determine the free radical scavenging potential, 1 ml of 0.1 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical solution prepared in methanol, was added to 3 ml of the different concentrations of test material [31]. These solutions were then incubated for 30 min at room temperature. Then absorbance was evaluated at 517 nm. A rise in DPPH radical-scavenging activity was indicated by a decrease in the absorbance of DPPH solution. This activity is shown as % DPPH radical-scavenging and calculated by using the following equation keeping DPPH solution as control:
Effect on MDCK (Madin–Darby canine kidney) cell lines
MDCK cells were purchased from ATCC (Manassa, VA, U.S.A.) and maintained as sub-confluent monolayers in 5% CO2 at 37 ℃. 75 cm2 Falcon tissue culture flasks were used for cell cultures growth, in in a 1:1 mixture of modified Eagle’s medium (DMEM) nutrient mixture and F-12 medium (DMEM/F-12) having 10% fetal bovine serum (Gibco BRL), 2% streptomycin/penicillin, pH 7.4. The medium was changed regularly at every 3 to 4 days.
Cell viability assay
Media was aliquoted to selected wells of a 96 well plate, containing MDCK cells and viability of the cells were assessed by XTT cell viability Assay Kit obtained from Biotium, Inc., Hayward, CA, USA). Cells were incubated for 24 h with different concentration of Ta.Cr. Then 50 µL of the working activation solution, prepared by mixing of one bottle of XTT solution and one of the activation Reagent PMS, was added to all the wells and incubated for 2–4 h. Optical density was read at 450–490 nm on a Bio-Rad 3550 microplate reader.
Lactate dehydrogenase (LDH) assay
Media was aliquoted to selected wells of a 96-well plate. CytoTox 96 Non-Radioactive Cytotoxicity assay kit was used for determination of percent LDH release. The substrate, supplied with the kit, was added to the blanks (acclimatization media), positive control (MDCK cells containing lysis solution-only), and all samples. The plate was incubated for 30 min at normal temperature in the dark. Stop solution, supplied with kit, was added to blanks, positive control and all samples. Bio-Rad 3550 microplate reader (Bio-Rad, Hercules, CA) was used to read the optical density at 490 nm.
Antispasmodic activity in the urinary bladder
Antispasmodic activity of Ta.Cr was assessed against high K+ (80 mM) and carbachol (CCh; 1 µM)-induced contractions in rabbit’s urinary bladder strips [32]. Each strip of the bladder was mounted in a 10–15 ml tissue bath containing Krebs–Henseleit solution. Tissue baths were continuously bubbled with carbogen and maintained at 37 ℃. Throughout the experiment, a tension of 1 gm was applied to each tissue. Power lab (AD Instruments Australia) was used to record the isometric responses of the tissues. Before the addition of any drug, the tissues were kept for a period of about 01 h to equilibrate. During this period, the isolated tissues were washed with fresh bathing fluid at an interval of every 20–25 min.
After equilibrium, the reparations were stabilized, by treating with 1 µM CCh repeatedly, until constant responses were obtained. Then the spasmolytic activity of the Ta.Cr was determined by adding Ta.Cr on CCh and high K+-induced sustained contraction in rabbit bladder, in a cumulative way to get the concentration-dependent inhibitory response [33]. IC50 values (concentration causing 50% inhibition) were calculated from these curves and calculated as a measure of the spasmolytic potency of the extract.
Ca++ CRCs were made in the absence (control curve) and the presence of increasing concentrations of Ta.Cr, for confirmation of the Ca++ channel blocking (CCB) activity. For this purpose, the tissues were stabilized in a normal Krebs–Henseleit solution, which was then replaced with Ca++-free Krebs–Henseleit solution containing EGTA (0.1 mM) to remove Ca++ from the tissues. Then this solution was replaced by K+-rich and Ca++-free Krebs–Henseleit solution. This solution was refilled 3–5 times with 10 min intervals and then, the Ca++ CRCs were constructed in the absence (control curve) and the presence of different concentrations of the verapamil or Ta.Cr [34]. Isometric responses were recorded on the PowerLab 4/24 data acquisition system attached to the computer, running Lab Chart 6 (AD Instruments, Sydney, Australia).
Similarly, for the determination of anticholinergic activity, the cumulative CCh-CRCs were constructed in the presence of the increasing concentration of the test material (Gilani et al., 2008).
Data analysis
All these data are expressed as mean ± standard error of mean (SEM, n = number of experiments) and the median effective concentration (EC50 value) with 95% confidence intervals (CI). The statistical comparisons between the two groups are made through Student’s t test, while one-way analysis of variance (ANOVA) followed by post hoc Dunnett’s test, was used for comparisons among more than two groups. p value less than 0.05 (p < 0.05) is considered as significant. Concentration–response curves (CRCs) were analyzed by non-linear regression using GraphPad Prism version 4.03 (GraphPad Software, San Diego, CA, USA).
Results
In vivo diuretic activity
Ta.Cr (30 and 100 mg/kg) increased the urine output in Wistar rats, whereas, HCT (10 mg/kg) was used as a standard drug (Fig. 1A). Also, Ta.Cr also increased (p < 0.05) urine excretion of Na+ at the dose of 100 mg/kg; however, slight but no significant increase was observed in the K+ excretion (Fig. 5C and D). No significant change was observed in the urine pH of any group (Fig. 1B).
Effect on rat model of urolithiasis
Preventive study
In the preventive study, all the parameters were recorded before (day 0) and 3 weeks after the treatment. The parameters recorded before the treatments were not different (p > 0.05) among the groups. After the treatment, the stone-forming group (diseased control) was compared with normal control and also with the treated groups.
At the end of the 3 weeks’ treatment, a significant loss in body weights was caused by the lithogenic treatment (EG and AC) in the stone-forming group (group II) as compared to saline (normal control). The co-administration of Ta.Cr (30 and 100 mg/kg) prevented the net loss in body weight, 24 h urine volume and water intake were elevated in the stone-forming (lithogenic) group compared to that of saline (Normal control) animals. Urine pH was also reduced (p < 0.05) in the stone-forming group. A co-treatment with Ta.Cr prevented these urinary changes compared to that of saline (Normal control) group (Table 1). In 3 h morning urine, significantly more and bigger CaOx crystals mostly of COD were observed in the stone-forming group as compared to the saline control, whereas Ta.Cr significantly reduced urinary crystal count (Fig. 2). Similarly, oxalate excretion was also raised significantly in stone-forming rats while calcium excretion was decreased. Ta.Cr significantly decreased the urinary oxalate and restored the urinary calcium. Urinary contents of phosphate, citrate, UA and magnesium remained unchanged; however, Ta.Cr at a dose of 100 mg/kg significantly reduced urinary Na+ and K+ concentration (Table 1).
Ethylene glycol treatment damaged renal function as evident from increased serum creatinine, BUN and total urinary protein loss. However, Ta.Cr significantly reduced the increased serum creatinine, BUN and total protein loss with respect to disease control group (Table 1).
In histological preparations of H and E staining, many crystalline deposits were observed under polarized light in almost all regions of the kidneys, especially in the cortex and papilla of rat’s kidneys tissues in the untreated control group. However, in Ta.Cr-treated groups (30–100 mg/kg), the densities of crystals were much lower than that of the untreated control group (Figs. 3 and 4).
Curative study model
In the curative study, all the parameters were recorded three times during the experimental protocol; once at day 0, then at day 21, and lastly at day 35. Before the treatment, parameters were compared with each other. After 21 days of stone induction, the parameters were compared with the Saline (Normal control). After 35 days, when the stone-inducing treatment was withdrawn, the diseased control (negative control) was compared with Saline (Normal control) and also with the treated groups.
On day 0, all the parameters were similar in all the groups. The EG (0.75%) and AC (1%) consumption for the first 21 days initiated the lithogenic parameters in the animals in untreated groups as compared to the saline group like in the preventive study. This was suggested by a net loss in the body weights and a significant increase in urine volume, water intake, oxalate, and uric acid while a decrease in urine pH and calcium content of the urine. Serum creatinine, BUN and total serum protein were also increased significantly compared to the normal saline group. Post-induction treatment with Ta.Cr (10 and 30 mg/kg) reversed the loss in body weights, unpaired urinary and serum functions (Table 2), crystal deposition in the kidneys more quickly than in the untreated group (Figs. 5 and 6).
In vitro experiment
Effect on in vitro crystallization
Kinetic study
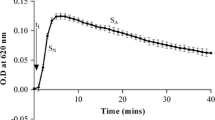
Effect of Ta.Cr on different phases of CaOx crystallization as revealed by time-course measurement of turbidity under standard conditions (4.25 mM Ca++ and 0.75 mM Ox) is given in Fig. 7A while Fig. 7B shows representative tracing of the experiment. After induction of the crystallization with disodium oxalate, an initial increase in the turbidity was observed in the control curve, reached its maximum at about 150 ± 15 s, followed by a slow decrease, which is typical of the pattern reported in other studies [9, 25]. Ta.Cr decreased both slopes of nucleation (1–4 mg/ml) and inhibits the crystal aggregation (0.5–4 mg/ml) in a concentration-dependent manner, while potassium citrate (2–4 mM) increased nuclei induction time and also slow down the crystals nucleation and aggregation (Fig. 7C).
Effect Trachyspermum ammi (Ta.Cr) and potassium citrate (K-Cit) on calcium oxalate crystallization. A and B are the typical tracing of the control and in the presence of Ta.Cr and K.Cit. Panel (C) is the concentration–response curves of Ta.Cr and potassium citrate on SA of the turbidity curves, while (D) shows the % inhibition on the SN. Symbols shown are mean ± S.E.M. (n = 3). SN and SA represent the slope of nucleation and slop of aggregation, respectively
Incubation study
In the incubation study, mixing the metastable solutions of Ca++ and Ox resulted in the formation of CaOx crystals predominately of calcium oxalate monohydrate (COM) (Fig. 8A). Ta.Cr caused a concentration-dependent (1–4 mg/ml) decrease in the CaOx crystal formation and also decreased the number and size of COM (Fig. 8B), similar to potassium citrate (Fig. 8C and D).
Antioxidant effect
Effect on free radicals
Ta.Cr caused inhibition of DPPH free radicals with IC50 value of 3.4 µg/ml (95% CI, 3.166 to 3.692) and while the control drug BHT inhibited DPPH with IC50 value of 3.2 µg/ml (95% CI, 2.7 to 3.8) (Fig. 9).
Concentration–response curves of the free radical scavenging activity of the butylated hydroxytoluene (BHT) and the crude extract of Trachyspermum ammi (Ta.Cr) while bar-chart representing lipid peroxidation inhibitory activity of two different concentrations of Ta.Cr and butylated hydroxytoluene (BHT). Inhibition is measured as % of the respective control experiments. The values shown are mean ± SEM (n = 3)
Lipid peroxidation inhibitory activity
Ta.Cr inhibited lipid peroxidation, induced in rat’s kidney tissue homogenate by 20.33 ± 4.7% and 73.07 ± 5.03% while BHT caused 30.85 ± 2.60 and 71.60 ± 3.84% inhibition of lipid peroxidation at 50 and 150 µg/ml, respectively (Fig. 9).
Effect on kidney epithelial cell lines (MDCK)
Ta.Cr had no toxic effect on MDCK cells up to a concentration of 0.1 mg/ml. However, it induced significant cell viability inhibition at and after increasing the concentration beyond 0.3 mg/ml (Fig. 10A).
Effects of various concentrations of Trachyspermum ammi MDCK cell survival in acclimatization media. Ta.Cr has no toxic effect on MDCK cells at up to 0.3 mg/ml for 24 h (A). MDCK cell survival fraction decreased significantly vs. that in untreated controls after exposure to 0.5 mM oxalate (B) and 66 mg/cm2 COM (C). Ta.Cr increases the cell survival fraction of MDCK cells at 0.03 and 0.1 mg/ml concentration
Effect of Ox and COMs
The cell viability was significantly decreased (p < 0.001) after exposure to 0.5 mM oxalate and 66 µg/cm2 of COM vs. untreated control. However, the co-exposure of Ta.Cr increased (p < 0.05) cell viability at a dose of 0.1 mg/ml (Fig. 10B and C).
Effect on LDH release
LDH release has significantly increased after exposure to 0.5 mM oxalate or 66 µg/cm2 COM vs. untreated control. However, after co-exposure of Ta.Cr at the concentration of 0.1 mg/ml, the LDH decreased significantly (Fig. 11).
Percent increase in lactate dehydrogenase (LDH) release against control by MDCK cells exposed to A Ox. 0.5 mM and B COM 66 μg/cm2 alone and with 0.03 and 0.1 mg/ml of Ta.Cr for 24 h. Data shown are mean plus or minus SEM of two separate experiments with 3 independent replicates. ***p < 0.05, **p < 0.05 and ***p < 0.001 control versus exposed groups
Effect on urinary bladder
Ta.Cr relaxed both CCh (1 µM) and high K+ (80 mM)-induced contractions in rabbit’s isolated urinary bladder strips at a concentration of 1 mg/ml with an EC50 values of 0.17 (0.14–0.20, 95% CI) and 0.13 mg/ml (0.10–0.16, 95% CI) (Fig. 12A), similarly, verapamil, relaxed both high K+ and CCh induced contractions (Fig. 12C) with IC50 values of 0.08 µM (0.06–0.12) and 0.34 µM (0.25–0.47), respectively, and found more potent against the high K+ than the CCh-induced contraction. Ta.Cr (0.03–0.1 mg/m1) caused a rightward shift of the Ca++-CRCs accompanied by suppression of the maximum contractile effect, like that caused by verapamil (0.01–0.03 µM) as shown in Fig. 12B and D, respectively.
Concentration–response curves of crude extract of Ta.Cr (A) and verapamil (B) on K+(80 mM) and CCh (1 µM)-induced contractions and Ca.++ CRCs constructed in the absence (control curve) and presence of increasing concentrations of Ta.Cr (C) and verapamil (D) in isolated rabbit urinary bladder. The symbols represent mean ± SEM (n = 4–6)
Discussion
The crude extract of of Trachyspermum ammi (Ta.Cr) was evaluated for its possible diuretic and antiurolithic activities using the in vivo and in vitro experimental models to rationalize it’s traditional use as aniturolithic agent.
In the in vivo diuretic experiment Ta.Cr significantly increased urine output at a dose of 30 and 100 mg/kg similar to positive control; hydrochlorothiazide (10 mg/kg), while, administration of the next higher dose (300 mg/kg) did not cause any significant change in the diuretic effect, compared to the saline control, which could be due to the possible co-existence of anti-diuretic component(s) in Ta.Cr. The presence of synergistic and/or side effect neutralizing properties in plants is very well studied [9] which is probably meant by nature not to allow the therapeutic effect to go beyond a certain limit beyond which it could have caused adverse effects, as is the case with chemical drugs [9, 35]. Diuretics increase urine volume, which results in the reduction of the supersaturation of stone-forming salts, and also help in the expulsion of crystals [36, 37].
The antiurolithic effect of Ta.Cr was evaluated in the in vivo rat model by using ethylene Glycol (EG) in drinking water [46,47,48]. Male Wistar rats were selected because they are more sensitive to ethylene glycol toxicity; therefore, changes in their urine electrolytes and CaOx supersaturation occur to a greater extent than in other strains [38].
In this study, hyperoxaluria was induced by administration of EG (0.75%) for 21 days, and 1% ammonium chloride (AC) for the first 5 days, as the addition of 1% AC for more than 5 days lead to extreme weight loss and ultimately death of rats, which is consistent with the other studies [39].
In the preventive study, the administration of EG and AC resulted in crystalluria with larger crystals due to hyperoxaluria; an increase in water intake and urine output might be due to renal impairment [40], as there was a significant increase in serum creatinine, blood urea nitrogen and total urinary protein loss in the control group as compared to normal, which has been restored by the Ta.Cr treatment. These data suggest that lithogenic treatment (EG) increases oxalate and decreases Ca++ excretion in urine [39, 41], which was prevented by Ta.Cr.
There were hypertrophy and widespread deposition of CaOx crystals in the kidneys of untreated rats. The renal tubules were markedly dilated, which might be due to the obstruction in distal renal tubular flow by large crystals [42]. Several in vivo and in vitro studies have revealed that hyperoxaluria; a key risk factor for CaOx stone formation, results in greater production of hydroxyl free radicals and superoxide, leading to oxidative stress, cell membrane rapture and cell death [42, 43] which leads to CaOx crystal adherence and retention in renal tubules [25, 42]. Thus, it can be hypothesized that the antioxidant activity of Ta.Cr may be contributing factor to its inhibitory effect against CaOx crystal deposition in renal tubules.
In the curative study, Ta.Cr treatment was started after 21 days of lithogenic period. Ta.Cr increased the recovery in the treated group as compared to the untreated group, which was clearly shown by the significant decrease in urinary oxalate, gain in body weight, renal crystal deposition and improvement in renal functions compared to the untreated rats.
In further possible mechanisms, which might be contributing to the antiurolithic activity of the Ta.Cr, we performed a number of in vitro experiments, which include crystallization studies, anti-oxidant studies and spasmolytic effect on urinary bladder.
The effect of Ta.Cr on CaOx crystallization kinetics was studied by the time course measurement of turbidity using an aggregometer, like that of the previous studies [25, 44]. Ta.Cr inhibited CaOx crystal nucleation and aggregation in a concentration-dependent manner, similar to K + citrate, an inhibitor of CaOx crystallization and clinically used for the treatment of urolithiasis [45]. Similarly in the incubation study, Ta.Cr decreased crystal count and changed CxOx monohydrate crystals, which are more likely to attach with the kidney epithelial cells than CaOx dihydrate, resulting in the formation of kidney stones [46, 47] like that of citrate and Mg2+ [48]. Calcification is a multifactorial phenomenon [48, 49], arising as a result of a series of proceedings initiated by supersaturation, including crystal nucleation, growth, aggregation and retention [50]. Although supersaturation is not the only critical step evolved in the formation of kidney stones and several studies has identified many inhibitors of calcium oxalate and calcium phosphate crystallization, characterized as ionic or macromolecular inhibitors [51], yet it is the primary prerequisite for the formation of the crystals in the urinary tract [52]. Various crystal inhibitors like potassium-sodium citrate and magnesium oxide are known to decrease the CaOx supersaturation and inhibit “crystal nucleation, growth and aggregation” causing a decrease in urinary crystallization of stone forming patients [53]. Therefore, agents causing inhibition of crystal growth and aggregation can serve as possible therapeutic options for the management of recurrent kidney stones formation. Ta.Cr inhibits CaOx crystal nucleation and aggregation along with a decrease in the count and morphological change in crystals. These results indicate crystallization inhibitory of activity of Ta.Cr extract could be one of the contributing factors in its antiurolithic activity.
The in vitro and in vivo studies revealed that oxalate, calcium oxalate and hydroxy apatite crystals cause injury in kidney cells [54, 55]. This injury is caused by the production of ROS and is considered to be the risk factor for the crystallization and crystals deposition in the kidney as it promotes sites for crystal nucleation, aggregation, retention and stone development [53, 56]. Therefore, antioxidants such as catechin, vitamin E and selenium can protect against oxalate and crystal deposition-induced oxidative injury [42, 57]. Antioxidant activity of Ta.Cr was measured by free radical scavenging and lipid peroxidation inhibitory activity, where Ta.Cr showed of DPPH free radicals scavenging activity and inhibited ferrous-ascorbate-induced lipid peroxidation of rat kidney tissue homogenate like BHT, a standard antioxidant [41].
Several studies revealed that oxalate and COM crystals are not only the major constituent of oxalate stones but also have cytotoxic effect on renal epithelial cells [42, 58]. Here pretreatment with the Ta.Cr significantly increases the survival rate and decreased LDH release in MDCK cells, when exposed to Ox and COM crystals. This protective effect could be the result of its antioxidant activity.
In Medical Expulsive Therapy (MET) for kidney stones expulsion, spasmolytics drugs are more commonly used [59]. Alpha-adrenergic antagonists (alpha-blockers) or Ca++ channel blockers (CCB) increases stone expulsion rates and decreases colic event [60]. Therefore, we evaluate the antispasmodic effect of Ta.Cr, on K+ (80 mM) and CCh (1 µM) induced contraction, using rabbit urinary bladder. K+ concentration greater than 30 mM, is known to cause smooth muscle relaxation through the opening of voltage-dependent L-type calcium channels, thus allowing an influx of extracellular Ca++, resulting in the contraction of the smooth muscles [61]. A substance that inhibits the high K+ induced contraction is considered to be CCB [62], whereas, CCh is a cholinergic drug that can induce contraction in the urinary bladder through activation of muscarinic (predominately M3) receptors [63]. Ta.Cr inhibited the high K+ and CCh induced contraction at similar doses like that of verapamil, as standard CCB [63], indicating CCB activity. To confirm the CCB action, CRCs of Ca++ were constructed, using the rabbit urinary bladder, in the absence (control curve) and presence of the increasing concentration of Ta.Cr. It shifted the CRC to the right with suppression of the maximum response, like standard calcium channel blocker (verapamil), confirming the presence of CCB constituent in Ta.Cr.
Conclusion
Results of our study suggest the presence of antiurolithic effects in Ta.Cr against CaOx kidney stone formation may be mediated through multiple pathways, which rationalize its traditional medicinal use in the management of urolithiasis. However, more studies may be required to investigate the possible molecular pathways and establish its in-depth safety and efficacy for clinical use.
Future studies should focus on the activity-guided fractionation for the isolation of respective compounds for different activities, which can help resolve the dilemma that why crude extracts of the plants are more effective in these kinds of complex disorders than the pure single molecule.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Lemann J Jr (1993) Composition of the diet and calcium kidney stones. N Engl J Med 328(12):880–882. https://doi.org/10.1056/NEJM199303253281212
Evan AP (2010) Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol 25(5):831–841. https://doi.org/10.1007/s00467-009-1116-y
Khan SR (2013) Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol 189(3):803–811
Khan SR (2012) Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol Res 40(2):95–112. https://doi.org/10.1007/s00240-011-0448-9
Tsujihata M, Momohara C, Yoshioka I, Tsujimura A, Nonomura N, Okuyama A (2008) Atorvastatin inhibits renal crystal retention in a rat stone forming model. J Urol 180(5):2212–2217. https://doi.org/10.1016/j.juro.2008.07.024
Gaspar S, Niculite C, Cucu D, Marcu I (2010) Effect of calcium oxalate on renal cells as revealed by real-time measurement of extracellular oxidative burst. Biosens Bioelectron 25(7):1729–1734. https://doi.org/10.1016/j.bios.2009.12.013
Khan SR (2005) Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res 33(5):349–357. https://doi.org/10.1007/s00240-005-0492-4
Hollenberg D, Zakus D, Cook T, Xu XW (2008) Re-positioning the role of traditional, complementary and alternative medicine as essential health knowledge in global health: do they still have a role to play? World Health Popul 10(4):62–75
Gilani AH, Rahman AU (2005) Trends in ethnopharmocology. J Ethnopharmacol 100(1–2):43–49. https://doi.org/10.1016/j.jep.2005.06.001
Usmanghani K, Saeed A, Alam MT (1997) Indusyunic medicine. University of Karachi Press, Karachi
Duke JA (2002) Handbook of medicinal herbs. CRC Press
Duke JA (1992) Handbook of phytochemical constituents of GRAS herbs and other economic plants. CRC Press Inc., Boca Raton, pp 292–293
Srivastava KC (1988) Extract of a spice—omum (Trachyspermum ammi)-shows antiaggregatory effects and alters arachidonic acid metabolism in human platelets. Prostaglandins Leukot Essent Fatty Acids 33(1):1–6. https://doi.org/10.1016/0952-3278(88)90115-9
Boskabady MH, Shaikhi J (2000) Inhibitory effect of Carum copticum on histamine (H1) receptors of isolated guinea-pig tracheal chains. J Ethnopharmacol 69(3):217–227. https://doi.org/10.1016/s0378-8741(99)00116-6
Agrewala JN, Pant MC (1986) Effect of feeding Carum copticum seeds on serum lipids, high density lipoproteins & serum cholesterol binding reserve in the albino rabbits. Indian J Med Res 83:93–95
Gilani AH, Jabeen Q, Ghayur MN, Janbaz KH, Akhtar MS (2005) Studies on the antihypertensive, antispasmodic, bronchodilator and hepatoprotective activities of the Carum copticum seed extract. J Ethnopharmacol 98(1–2):127–135. https://doi.org/10.1016/j.jep.2005.01.017
Zahin M, Ahmad I, Aqil F (2010) Antioxidant and antimutagenic activity of Carum copticum fruit extracts. Toxicol In Vitro 24(4):1243–1249. https://doi.org/10.1016/j.tiv.2010.02.004
Ahsan SK, Shah AH, Tanira MOM, Ahmad MS, Tariq M, Ageel AM (1990) Studies on some herbal drugs used against kidney stones in Saudi folk medicine. Fitoterapia 61(5):435–438
Kaur T, Bijarnia RK, Singla SK, Tandon C (2009) In vivo efficacy of Trachyspermum ammi anticalcifying protein in urolithiatic rat model. J Ethnopharmacol 126(3):459–462. https://doi.org/10.1016/j.jep.2009.09.015
Williamson EM, Okpako DT, Evans FJ (1996) Selection, preparation, and pharmacological evaluation of plant material. John Wiley & Sons
Khan A, Bashir S, Gilani AH (2012) An in vivo study on the diuretic activity of Holarrhena antidysenterica. Afr J Pharm Pharmacol 6(7):454–458
Khan A, Gilani AH (2010) Pharmacological basis for the medicinal use of Origanum vulgare Linn. In: Urolithiasis [abstract]. Paper presented at the 16th world congress of basic and clinical pharmacology, Copenhagen, Denmark
Khan A, Khan SR, Gilani AH (2012) Studies on the in vitro and in vivo antiurolithic activity of Holarrhena antidysenterica. Urol Res 40(6):671–681. https://doi.org/10.1007/s00240-012-0483-1
Pizzolato P (1971) Mercurous nitrate as a histochemical reagent for calcium phosphate in bone and pathological calcification and for calcium oxalate. Histochem J 3(6):463–469. https://doi.org/10.1007/BF01014785
Hess B, Meinhardt U, Zipperle L, Giovanoli R, Jaeger P (1995) Simultaneous measurements of calcium oxalate crystal nucleation and aggregation: impact of various modifiers. Urol Res 23(4):231–238. https://doi.org/10.1007/BF00393304
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Ebisuno S, Komura T, Yamagiwa K, Ohkawa T (1997) Urease-induced crystallizations of calcium phosphate and magnesium ammonium phosphate in synthetic urine and human urine. Urol Res 25(4):263–267. https://doi.org/10.1007/BF00942096
Guerra A, Meschi T, Allegri F, Schianchi T, Adorni G, Novarini A, Borghi L (2004) Calcium oxalate crystallization in untreated urine, centrifuged and filtered urine and ultrafiltered urine. Clin Chem Lab Med 42(1):45–50. https://doi.org/10.1515/CCLM.2004.009
Huang DJ, Chen HJ, Hou WC, Lin CD, Lin YH (2004) Active recombinant thioredoxin h protein with antioxidant activities from sweet potato (Ipomoea batatas [L.] Lam Tainong 57) storage roots. J Agric Food Chem 52(15):4720–4724. https://doi.org/10.1021/jf0498618
Borchert VE, Czyborra P, Fetscher C, Goepel M, Michel MC (2004) Extracts from Rhois aromatica and Solidaginis virgaurea inhibit rat and human bladder contraction. Naunyn Schmiedebergs Arch Pharmacol 369(3):281–286. https://doi.org/10.1007/s00210-004-0869-x
Ajith TA, Usha S, Nivitha V (2007) Ascorbic acid and alpha-tocopherol protect anticancer drug cisplatin induced nephrotoxicity in mice: a comparative study. Clin Chim Acta 375(1–2):82–86. https://doi.org/10.1016/j.cca.2006.06.011
Farre AJ, Colombo M, Fort M, Gutierrez B (1991) Differential effects of various Ca2+ antagonists. Gen Pharmacol 22(1):177–181. https://doi.org/10.1016/0306-3623(91)90331-y
Van Rossum JM (1963) Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther 143:299–330
Kalaiselvi P, Udayapriya KL, Selvam R (1999) Uric acid-binding proteins in calcium oxalate stone formers and their effect on calcium oxalate crystallization. BJU Int 83(9):919–923. https://doi.org/10.1046/j.1464-410x.1999.00084.x
Dasaeva LA, Shilov EM, Shatokhina SN (2003) Diuress for the treatment of early stages of urolithiasis. Klin Med (Mosk) 81(10):50–52
Goldfarb DS, Coe FL (2005) The medical management of stone disease. In: Davison AM, Cameron JS, Ritz E, Grünfeld J, Winearls CG, Ponticelli C, van Ypersele C (eds) Oxford textbook of clinical nephrology. Oxford University Press, New York, pp 1199–1279
Tsai CH, Chen YC, Chen LD, Pan TC, Ho CY, Lai MT, Tsai FJ, Chen WC (2008) A traditional Chinese herbal antilithic formula, Wulingsan, effectively prevents the renal deposition of calcium oxalate crystal in ethylene glycol-fed rats. Urol Res 36(1):17–24. https://doi.org/10.1007/s00240-007-0122-4
Vanachayangkul P, Chow N, Khan SR, Butterweck V (2011) Prevention of renal crystal deposition by an extract of Ammi visnaga L. and its constituents khellin and visnagin in hyperoxaluric rats. Urol Res 39(3):189–195. https://doi.org/10.1007/s00240-010-0333-y
Bashir S, Gilani AH (2011) Antiurolithic effect of berberine is mediated through multiple pathways. Eur J Pharmacol 651(1–3):168–175. https://doi.org/10.1016/j.ejphar.2010.10.076
Fan J, Glass MA, Chandhoke PS (1999) Impact of ammonium chloride administration on a rat ethylene glycol urolithiasis model. Scanning Microsc 13(2–3):299–306
Park HK, Jeong BC, Sung MK, Park MY, Choi EY, Kim BS, Kim HH, Kim JI (2008) Reduction of oxidative stress in cultured renal tubular cells and preventive effects on renal stone formation by the bioflavonoid quercetin. J Urol 179(4):1620–1626. https://doi.org/10.1016/j.juro.2007.11.039
Thamilselvan S, Khan SR, Menon M (2003) Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res 31(1):3–9. https://doi.org/10.1007/s00240-002-0286-x
Wiessner JH, Hasegawa AT, Hung LY, Mandel GS, Mandel NS (2001) Mechanisms of calcium oxalate crystal attachment to injured renal collecting duct cells. Kidney Int 59(2):637–644. https://doi.org/10.1046/j.1523-1755.2001.059002637.x
Tiselius HG (2003) Epidemiology and medical management of stone disease. BJU Int 91(8):758–767. https://doi.org/10.1046/j.1464-410x.2003.04208.x
Wesson JA, Worcester EM, Wiessner JH, Mandel NS, Kleinman JG (1998) Control of calcium oxalate crystal structure and cell adherence by urinary macromolecules. Kidney Int 53(4):952–957. https://doi.org/10.1111/j.1523-1755.1998.00839.x
Wesson JA, Ward MD (2006) Role of crystal surface adhesion in kidney stone disease. Curr Opin Nephrol Hypertens 15(4):386–393. https://doi.org/10.1097/01.mnh.0000232879.50716.6f
Guerra A, Meschi T, Allegri F, Prati B, Nouvenne A, Fiaccadori E, Borghi L (2006) Concentrated urine and diluted urine: the effects of citrate and magnesium on the crystallization of calcium oxalate induced in vitro by an oxalate load. Urol Res 34(6):359–364. https://doi.org/10.1007/s00240-006-0067-z
Wang AY (2009) Vascular and other tissue calcification in peritoneal dialysis patients. Perit Dial Int 29(Suppl 2):S9–S14
Khan SR (1997) Animal models of kidney stone formation: an analysis. World J Urol 15(4):236–243. https://doi.org/10.1007/BF01367661
Marangella M, Bagnis C, Bruno M, Vitale C, Petrarulo M, Ramello A (2004) Crystallization inhibitors in the pathophysiology and treatment of nephrolithiasis. Urol Int 72(Suppl 1):6–10. https://doi.org/10.1159/000076583
Carvalho M, Vieira MA (2004) Changes in calcium oxalate crystal morphology as a function of supersaturation. Int Braz J Urol 30(3):205–208. https://doi.org/10.1590/s1677-55382004000300005
Kato Y, Yamaguchi S, Yachiku S, Nakazono S, Hori J, Wada N, Hou K (2004) Changes in urinary parameters after oral administration of potassium-sodium citrate and magnesium oxide to prevent urolithiasis. Urology 63(1):7–11. https://doi.org/10.1016/j.urology.2003.09.057
Aihara K, Byer KJ, Khan SR (2003) Calcium phosphate-induced renal epithelial injury and stone formation: involvement of reactive oxygen species. Kidney Int 64(4):1283–1291. https://doi.org/10.1046/j.1523-1755.2003.00226.x
Escobar C, Byer KJ, Khaskheli H, Khan SR (2008) Apatite induced renal epithelial injury: insight into the pathogenesis of kidney stones. J Urol 180(1):379–387. https://doi.org/10.1016/j.juro.2008.02.041
Byer K, Khan SR (2005) Citrate provides protection against oxalate and calcium oxalate crystal induced oxidative damage to renal epithelium. J Urol 173(2):640–646. https://doi.org/10.1097/01.ju.0000143190.49888.c7
Santhosh Kumar M, Selvam R (2003) Supplementation of vitamin E and selenium prevents hyperoxaluria in experimental urolithic rats. J Nutr Biochem 14(6):306–313. https://doi.org/10.1016/s0955-2863(03)00033-0
Babich H (1982) Butylated hydroxytoluene (BHT): a review. Environ Res 29(1):1–29. https://doi.org/10.1016/0013-9351(82)90002-0
Ohgaki K, Horiuchi K, Hikima N, Kondo Y (2010) Facilitation of expulsion of ureteral stones by addition of alpha1-blockers to conservative therapy. Scand J Urol Nephrol 44(6):420–424. https://doi.org/10.3109/00365599.2010.497769
Seitz C, Liatsikos E, Porpiglia F, Tiselius HG, Zwergel U (2009) Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol 56(3):455–471. https://doi.org/10.1016/j.eururo.2009.06.012
Bolton TB (1979) Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev 59(3):606–718. https://doi.org/10.1152/physrev.1979.59.3.606
Godfraind T, Miller R, Wibo M (1986) Calcium antagonism and calcium entry blockade. Pharmacol Rev 38(4):321–416
Brown JH, Taylor P (2006) Muscarinic receptor agonists and antagonists. Goodman and Gilman’s manual of pharmacology and therapeutics, 11th edn. McGraw-Hill Professional, New York, pp 183–200
Fleckenstein A (1977) Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol 17(1):149–166. https://doi.org/10.1146/annurev.pa.17.040177.001053
Acknowledgements
This study was supported by the Higher Education Commission (HEC) of Pakistan as (i) Indigenous M.Phill/PhD and (ii) International Research Support Initiative Program (IRSIP) scholarships awarded to Aslam Khan for carrying out research at Department of Biological and Biomedical Sciences, Aga Khan University Medical College, Karachi, Pakistan and Center for the Study of Lithiasis, Colleges of Medicine, University of Florida, Fl, USA, respectively.
Author information
Authors and Affiliations
Contributions
Dr. Anwarul-Hassan Gilani, supervised the research work and critically revise and correct the manuscript. Dr. Aslam Khan, did the experimental work and wrote the first draft of the mannuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, A., Gilani, A.H. An insight investigation to the antiurolithic activity of Trachyspermum ammi using the in vitro and in vivo experiments. Urolithiasis 51, 43 (2023). https://doi.org/10.1007/s00240-023-01415-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00240-023-01415-9