Abstract
This article describes an updated computer model which attempts to simulate known renal reabsorption and secretion activity through the nephron (NEPHROSIM) and its possible relevance to the initiation of calcium-containing renal stones. The model shows that, under certain conditions of plasma composition, de novo nucleation of both calcium oxalate (CaOx) and calcium phosphate (CaP) can take place at the end of the descending limb of the Loop of Henle (DLH), particularly in untreated, recurrent idiopathic CaOx stone-formers (RSF). The model incorporates a number of hydrodynamic factors that may influence the subsequent growth of crystals nucleated at the end of the DLH as they progress down the renal tubules. These include the fact that (a) crystals of either CaOx or CaP nucleated at the end of the DLH and travelling close to the walls of the tubule travel at slower velocities than the fluid flowing at the central axis of the tubule, (b) the transit of CaOx crystals travelling close to the tubule walls may be delayed for up to at least 25 min, during which time the crystals may continue to grow if the relative supersaturation with respect to CaOx (RSS CaOx) is high enough and (c) such CaOx crystals may stop moving or even fall back in upward-draining collecting ducts (CD) owing to the Stokes gravitational effect. The model predicts, firstly, that for small, transient increases in plasma oxalate concentration, crystallisation only takes place in the CD and leads to the formation of small crystals which are comfortably passed in the urine and, secondly, that for slightly greater increases in the filtered load of oxalate, spontaneous and/or heterogeneous nucleation of CaOx may occur both at the end of the DLH and in the CD. This latter situation leads to the passage in the final urine of a mixture of large crystals of CaOx (arising from nucleation at the end of the DLH) and small crystals of CaOx (as a result of nucleation originating in the CD). As a result of the higher calcium and oxalate concentrations in the urine of RSF, these patients have an increased probability of initiating CaOx crystallisation in the DLH and so of going on to form the large crystals and aggregates found in their fresh urines, but not in the fresh urines from normal subjects (N). These predictions are supported by evidence from clinical studies on six RSF and six normal controls (NC) who were maintained for 4 days on a fixed basal diet. Their patterns of CaOx crystalluria were measured on the second day of the basal diet and after a small dose of sodium oxalate was given before breakfast on the fourth day of the study. The model also shows that the tubular fluid of RSF is more likely than that of N to reach the conditions necessary for de novo nucleation of CaP at the end of the DLH. This may occur following either a small increase in ultrafiltrable phosphate, as a result of ingestion of a high phosphate-containing meal, or a small decrease in the proximal tubular reabsorption of phosphate resulting, for example, from increased parathyroid activity. CaP crystals initiated at this point may heterogeneously nucleate the crystallisation of CaOx under the high metastable conditions of RSS CaOx which frequently exist in the urines of RSF. Under certain conditions, it is predicted that CaP crystals, initiated at the end of the DLH and travelling close to the tubular walls where their transit time is increased, might also be able to grow and agglomerate sufficiently to become trapped at some point in the CD and lead to the formation of Randall’s Plugs in the Ducts of Bellini. Currently, work is under way to incorporate data on the growth and aggregation of crystals of CaP into NEPHROSIM to confirm the likelihood of this phenomenon occurring. The model shows that an increase in plasma calcium is unlikely to lead to spontaneous nucleation of either CaOx or CaP at the end of the DLH unless the concentration of plasma calcium reaches values usually associated with the cases of primary hyperparathyroidism. The most likely cause of spontaneous CaOx crystal formation at the end of the DLH is a small increase in plasma oxalate; the most likely cause of spontaneous CaP crystal formation at the end of the DLH is either an increase in plasma phosphate or a decrease in the fractional reabsorption of phosphate in the proximal tubule. The model predicts that the maximum volume of CaOx crystalluria that is likely to occur in a given urine is a function of both the RSS CaOx and the oxalate/calcium ratio in the final urine. These data explain why the volume of CaOx crystalluria is in the order UK normals < UK recurrent stone-formers < Saudi Arabian recurrent stone-formers which, in turn, probably accounts for the very high incidence of CaOx-containing stones found in Saudi Arabia compared with that in the UK.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last century, many theories have been proposed to account for the initiation of calcium oxalate (CaOx) stones in the urinary tract, including those based on (a) the role of urinary macromolecules in the nucleation of stones (the so-called “matrix theory”) [1, 2], (b) those based on a lack or deficiency of inhibitors of crystallisation in urine (the so-called “inhibitor theory”) [3, 4] and (c) those based on the role of supersaturation and crystalluria (the so-called “supersaturation-crystallisation theory”) [5, 6]. Although the matrix and inhibitor theories are now generally considered to be of less importance than the supersaturation-crystallisation theory in terms of stone initiation, they have not been completely discounted [7, 8].
In an attempt to elaborate on the supersaturation-crystallisation theory, Finlayson and Reid [9] outlined two possible scenarios for the initiation of CaOx stones—the so-called “free-particle” and “fixed-particle” models. In the “free-particle” model, they described stone-formation as being initiated when tubular fluid becomes so supersaturated with respect to CaOx that de novo nucleation of that salt takes place in the tubule. As the nuclei traverse the remainder of the nephron, they are postulated to grow (and possibly aggregate [10, 11]) under increasing conditions of supersaturation and, if these processes take place rapidly enough, a critical particle may be formed that is large enough to become trapped at some narrow segment of the tubule before the particle can be expelled from the Duct of Bellini of the collecting tubule concerned. Once trapped, this particle may continue to grow until it becomes a stone under the prevailing conditions of supersaturation in collecting duct urine [10]. However, on the basis of their very simple mathematical model of the kidney, Finlayson and Reid calculated that the above process was unlikely to occur within the normal transit time of tubular fluid from the glomerulus to the Ducts of Bellini which in their model was estimated to be around 10 min.
As an alternative, Finlayson and Reid proposed that crystal entrapment could only occur if the initial small nuclei of CaOx, which were formed as a result of excessive supersaturation of tubular fluid with respect to that salt, somehow became attached to the epithelial cells lining the tubule at some point where damage had occurred at the cell surface–either brought about by abrasion caused by the CaOx crystals themselves, or as a result of cell necrosis caused by infection or chemical damage. They termed this scenario the “fixed-particle” model of stone-formation. This model then dominated the stone field for almost 20 years during which period many papers were published on the nucleation and growth of CaOx crystals on monolayers of kidney cells grown in culture in the laboratory [12–17].
In 1994, Kok and Khan [18] re-examined the Finlayson and Reid model and adjusted some of the original authors’ initial assumptions by including more up-to-date data on tubule dimensions and flow rates of fluid through the nephron which had become available at that time. In their revised model, the transit time of tubular fluid was reduced to between 2.5 and 4.5 min depending on the length of the Loop of Henle in the nephron concerned. In summary, they still accepted the majority of the findings of Finlayson and Reid but they concluded that the “free-particle” model might be still feasible if crystal agglomeration (previously ignored by Finlayson and Reid) was to occur during the time in which CaOx crystals, initiated high up in the tubule, travelled down the remainder of the nephron. (Indeed, it must be emphasised at this point that aggregation of crystals is a much more rapid way of increasing particle size within a given time period than is crystal growth alone, which tends to be a relatively slow process). Kok and Khan postulated that once a large particle had been retained in this way, it then became attached to the tubular epithelial cells either by some form of crystal–cell interaction or by damaging the tubular cell lining and breaking through to reach the basement membrane of the cells [18]. In a sense, the Kok and Khan version of stone initiation constituted a compromise between the “free-” and “fixed-particle” models.
In 2004, Robertson [19] re-visited the two earlier models and modified that of Kok and Khan to include three hydrodynamic factors which had been previously ignored in both models. The three additional factors were (a) the effect of frictional drag on tubular fluid passing close to the tubule walls compared with that at the central axis of the tubule, (b) a similar frictional drag effect of the tubular walls on any particles travelling close to the tubule walls and (c) the effect of gravity on particles travelling in upward-draining segments of the collecting ducts. He considered that these factors could potentially delay the passage of crystals and so increase the risk of large crystals and aggregates of CaOx forming over the prolonged time that it would take for crystals formed high up in the nephron to be extruded from the Ducts of Bellini. Introduction of these factors into the mathematical model of urine flow and the estimation of the concentrations of calcium and oxalate ions at various points in the renal tubule (after allowing for reabsorption and secretion along the length of the nephron of all the ions required for the calculation of the relative supersaturation (RSS) of CaOx) suggested that any crystals that might be nucleated towards the end of the descending limb of the Loop of Henle and which travelled close to the tubular walls may be delayed long enough to grow sufficiently large to become trapped further down the nephron, particularly in upward-draining sections of the collecting ducts.
As an alternative to these models of stone initiation, within the past 10 years or so attention has been refocused on the observation made in 1936 by Alexander Randall of lesions in the renal papillae of 19.6 % of 1,154 pairs of kidneys which he examined at autopsy [20, 21]. These lesions occurred as sub-epithelial plaques and consisted of deposits of calcium phosphate (CaP) or carbapatite (CaP-CO3). Of the 2,308 kidneys examined, 2.8 % had small calculi consisting of CaOx attached to them. Randall termed these type 1 papillary lesions (now referred to as Randall’s Plaques type 1). Another type of lesion (now referred to as Randall’s Plaques type 2 or Randall’s Plugs) was also described by Randall and later confirmed by Cifuentes-Delatte [22]. These plugs were intratubular in origin and were found in the Ducts of Bellini at the ends of collecting ducts. They consisted mainly of CaP-CO3 but often had small CaOx stones attached to them. In the 20 years or so following the initial observations of Randall, numerous authors confirmed his findings but since Randall’s Plaques appeared to account for only a relatively small proportion of calcium-containing stones (10–20 %), this theory fell into obscurity for almost 50 years until it was revived by the Chicago-Indianapolis groups about 10 years ago [23, 24]. Since then, much has been written about the role of Randall’s Plaques in the initiation of stones (including the contents of this Special Issue of Urolithiasis) so that one might be tempted into believing that Randall’s plaques are the SOLE cause of stones. This paper attempts to redress this imbalance by showing that other possible explanations may exist for the initiation of CaOx stones which do not necessarily require the involvement of Randall’s Plaques or Randall’s Plugs, although the initiation of CaOx stones might involve the participation of crystals of CaP acting as heterogeneous nuclei for the precipitation and subsequent growth of CaOx crystals in tubular fluid.
Materials and methods
In this paper, the original Excel program of Robertson [19] for modelling renal function (now termed NEPHROSIM) has been further developed to calculate more accurately the levels of RSS of various stone-forming salts in tubular fluid at 0.1–0.2 mm increments throughout the nephron using more up-to-date micropuncture evidence on the tubular reabsorption of the various ions that are important in the calculation of RSS of the common stone-forming salts. The details of the renal characteristics used in the model (which are similar to those employed by Kok and Khan [18]) are shown in Table 1. In addition, NEPHROSIM attempts to mimic as far as possible the effects of those hormones on the tubular reabsorption of ions that might be important in the stone-forming process. It also allows for (a) secretion, particularly of oxalate in the latter part of the proximal tubule [25–27], (b) changes in dietary intake that may influence the filtered loads of calcium, phosphate, oxalate and urate, (c) acid–base changes in various sections of the nephron and (d) variations in water reabsorption at different sites along the nephron. In this paper, the data are calculated based on the dimensions of nephrons containing a medium-length Loop of Henle (Table 1). Similar calculations using NEPHROSIM are currently being carried out on the RSS CaOx and RSS CaP in nephrons with long and short Loops of Henle. These will be reported in a later publication.
The baseline blood and urine data employed in the study are shown in Table 2. These correspond to the non-fasting blood levels and 24-h urine composition of (a) an average normal male in the UK with no history of urolithiasis (UK N), (b) an average untreated recurrent idiopathic CaOx/CaP stone-former in the UK with hypercalciuria, moderate phosphaturia, a slightly raised urinary pH, mild hypocitraturia and pronounced mild hyperoxaluria (UK RSF) [28] and (c) an average untreated recurrent idiopathic CaOx-UA stone-former in the Kingdom of Saudi Arabia with marked hyperoxaluria, marked hyperuricosuria, marked hypocitraturia and an acid urinary pH (KSA RSF) [29]. Marked hyperoxaluria is a common feature in the populations of the oil-rich Gulf States in the Middle East and is largely dietary in origin; hypercalciuria, on the other hand, is very uncommon [29].
The RSS values of the various stone-forming salts and acids were calculated using the most up-to-date form of the SUPERSAT program originally devised in 1969 [30, 31]. This program also calculates the RSS of CaOx, OCP, brushite, magnesium ammonium phosphate, uric acid, sodium urate, ammonium urate and calcium urate. These results will be published elsewhere.
The model was tested in the following ways:
-
1.
Increasing the concentration of plasma oxalate on RSS CaOx in an average untreated UK RSF following ingestion of a high oxalate/calcium ratio meal which leads to increased absorption of oxalate both in the stomach [32, 33] and in the large intestine [34, 35].
-
2.
Increasing the concentration of ultrafiltrable phosphate on RSS OCP and urinary excretion of phosphate in an average untreated UK RSF following the ingestion of a high phosphate meal.
-
3.
Increasing the urinary excretion of phosphate on RSS OCP in an average untreated UK RSF by decreasing the proximal tubular reabsorption of phosphate in an attempt to simulate the effect of increasing the circulating level of parathyroid hormone (PTH).
-
4.
Increasing the concentration of ultrafiltrable calcium on RSS OCP in an average untreated UK RSF as might occur following ingestion of a high calcium meal or after an increase in the circulating level of PTH.
-
5.
Initiation of CaP and/or CaOx crystals of various sizes at the end of the descending limb of the Loop of Henle.
-
6.
Following the progress of these crystals as they traverse the remainder of the nephron under various conditions of RSS and orientation of the collecting ducts.
Results and discussion
Supersaturation studies
The effect of progressively increasing plasma oxalate from 1.25 to 2.75 μmol/l on the RSS of CaOx (RSS CaOx) along the nephrons (containing medium-length Loops of Henle) of an average untreated recurrent idiopathic CaOx/CaP stone-former in the UK (UK RSF) is shown in Fig. 1. The data are plotted in relation to the thermodynamic solubility product (SP) and the empirical spontaneous formation product (FP) of CaOx [31]. This shows that the ultrafiltrate of plasma is initially well undersaturated with respect to CaOx, but that RSS CaOx increases in the proximal tubule (Prox) owing to the secretion of oxalate in the latter section of that portion of the nephron [25–27]. RSS CaOx further increases in the descending limb of the Loop of Henle (DLH) owing to the reabsorption of water, reaching a peak at the end of the DLH and beginning of the ascending limb (ALH). Under average “normal” conditions in a UK RSF, the tubular fluid at this point is not quite sufficiently supersaturated to cause spontaneous homogeneous precipitation of CaOx. RSS CaOx then decreases slightly in the ALH and distal tubule (D), as a result of the reabsorption of calcium, but increases progressively along the collecting ducts (CD) during the final water adjustment in the kidneys before being extruded as highly supersaturated urine at the Ducts of Bellini.
RSS CaOx (plotted on log scale) at various positions along the nephron from the glomerulus to the Duct of Bellini in an average UK RSF at various levels of plasma oxalate concentration (filled circle 1.25 μmol/l; filled square 1.50 μmol/l; filled triangle 1.75 μmol/l; filled diamond 2.00 μmol/l; asterisk 2.25 µmol/l; times 2.50 µmol/l; plus 2.75 µmol/l)
Figure 1 also shows that increasing plasma oxalate causes the RSS CaOx profile through the nephron to increase but that only at the highest plasma level of oxalate studied (2.75 μmol/l) does RSS CaOx reach the Formation Product of CaOx at the end of the DLH, whereas it exceeds the FP at the some point in the CD in samples with plasma oxalate concentrations greater than about 1.4 μmol/l. Figure 1 also shows that there are two sections of the nephron where spontaneous precipitation of CaOx may take place—firstly, at the end of the DLH (but only if plasma oxalate is transiently increased), and secondly, at some point in the CD depending the actual RSS CaOx in that section of the nephron.
Figure 2 shows the patterns of RSS CaOx along the nephron constructed for the average UK N, the average UK RSF and the average KSA RSF using the baseline data in Table 2. The patterns of RSS CaOx show slightly lower curves for the average UK N but slightly higher curves for the average UK RSF and average KSA RSF as might be expected from the corresponding lower (UK N) and higher (UK RSF and KSA RSF) average urinary oxalate excretions in these subjects.
RSS CaOx (plotted on log scale) at various positions along the nephron from the glomerulus to the Duct of Bellini in an average UK N (open circle), UK RSF (filled square) and KSA RSF (filled triangle) at their respective average non-fasting plasma oxalate concentrations. (1.50 μmol/l, 1.65 μmol/l and 1.79 μmol/l)
Figures 3 and 4 show the corresponding data for the effect on the RSS of calcium phosphate along the nephron of an average untreated UK RSF of incrementally increasing the concentration of ultrafiltrable phosphate from 0.8 to 2.0 mmol/l (Fig. 3) or progressively decreasing the fractional tubular reabsorption of phosphate from 1.3 to 1.1 (Fig. 4); the latter is an attempt to simulate an increase in the concentration of circulating PTH with consequent hyperphosphaturia. The RSS of calcium phosphate is based on that of octocalcium phosphate (OCP) which has been shown to be the first form of calcium phosphate to be precipitated under urine-like conditions [31, 36]. OCP later transforms to the more stable hydroxyapatite under urinary conditions. The data are shown in relation to the thermodynamic Solubility Product (SP) and the empirical spontaneous Formation Product (FP) of OCP [31].
RSS OCP (plotted on log scale) at various positions along the nephron from the glomerulus to the Duct of Bellini in an average UK RSF at various levels of ultrafiltrable phosphate concentration (filled circle 0.8 mmol/l; filled square 1.2 mmol/l; filled triangle 1.6 mmol/l; filled diamond 2.0 mmol/l)
Figures 3 and 4 show similar but not identical patterns of RSS OCP along the nephron. They show that the ultrafiltrate of plasma is initially slightly supersaturated with respect to OCP but that RSS OCP decreases in the proximal tubule owing to the reabsorption of phosphate and the slight acidification of tubular fluid. The extent of the decrease is modulated either by increasing the concentration of ultrafiltrable phosphate or by decreasing the tubular reabsorption of phosphate in the proximal tubule. RSS OCP increases sharply again in the descending limb of the Loop of Henle (DLH) owing to the reabsorption of water and, more importantly (see below), the re-alkalinisation of the tubular fluid, reaching a peak at the end of the DLH and beginning of the ascending limbs (ALH) as postulated by Asplin et al. [37]. In some situations, such as increasing ultrafiltrable phosphate or decreasing the proximal tubular reabsorption of phosphate, calcium phosphate crystallisation may be initiated spontaneously at this point. RSS OCP decreases sharply in the ALH and distal tubules (D) as a result of the reabsorption of calcium, but then increases progressively again along the collecting ducts (CD) during the final water and pH adjustments before being extruded as supersaturated urine at the Ducts of Bellini. If the final pH of the tubular fluid extruded at the Ducts of Bellini is greater than about 6.15, the urine becomes excessively supersaturated with respect to CaP (not shown here) and CaP crystals are formed spontaneously and/or by heterogeneous nucleation caused by CaOx crystals precipitated higher up in the nephron. This could account for the formation of Randall’s Plugs at the ends of the CD consisting mainly of CaP but often mixed with CaOx.
Figure 5 shows the patterns of RSS OCP along the nephron constructed for the average UK N, the average UK RSF and the average KSA RSF using the baseline data in Table 2. The patterns of RSS OCP are similar to those in Figs. 3 and 4 but they show a slightly lower overall curve for the average UK N, as might be expected from their slightly lower urine pH values and phosphate excretions. They also show a higher curve for the KSA RSF in the early part of the nephron, as might be expected from their slightly higher ultrafiltrable phosphate concentrations, but a significantly lower curve in the CD owing to the marked acidification of their urine that occurs in that section of the nephron. The latter observation accounts for the low CaP content of stones from Saudi Arabia since any crystals of CaP which might have formed at the end of the DLH will be likely to be dissolved under the very acidic conditions in the later part of the nephron and the consequently very low RSS OCP found in the final urine of patients from the oil-rich Gulf States [29].
RSS OCP (plotted on log scale) at various positions along the nephron from the glomerulus to the Duct of Bellini in an average UK N (open circle), UK RSF (filled square) and KSA RSF (filled triangle) at their respective average non-fasting ultrafiltrable phosphate concentrations (1.00 mmol/l, 1.60 mmol/l and 1.60 mmol/l)
However, this acidification in the urines of the KSA RSF has another significant effect. Figure 6 shows the patterns of RSS of uric acid (RSS UA) along the nephron constructed for the average UK N, the average UK RSF and the average KSA RSF using the baseline data in Table 2. The curves show that for all subjects, RSS UA remains markedly undersaturated in all sections of the nephron until the marked acidification which takes place in the tubular fluid in the CDs of the KSA RSF. UA may then be heterogeneously nucleated by the CaOx crystals that are inevitably found in the tubular fluid of the CDs in most KSA RSF patients. This, together with their high urinary excretions of uric acid, would account for the high UA content of stones from RSF in Saudi Arabia, Kuwait and the United Arab Emirates [29].
In the average UK RSF, the RSS of sodium urate and the RSS of brushite of tubular fluid towards the end of the CD (not shown) are in the metastable regions of supersaturation of both salts, which possibly account for the occasional occurrence of these salts in Randall’s Plugs [22].
Figures 7, 8, 9 and 10 show the effects of increasing the concentrations of plasma oxalate, ultrafiltrable calcium and ultrafiltrable phosphate and decreasing the fractional tubular reabsorption of phosphate in the proximal tubule on the maximum RSS of CaOx and OCP at the end of the DLH in an average untreated UK RSF.
Figure 7 shows that increasing plasma oxalate from 1.65 (the average value in idiopathic RSFs in the UK) to 2.70 μmol/l will lead to spontaneous precipitation of CaOx at the end of the DLH. This only requires the intestinal absorption and/or metabolic production of about 16 μmol of additional oxalate. Assuming 5–10 % absorption of oxalate from a diet of around 2 mmol/day (i.e., about 100–200 μmol of oxalate), this requires only 8–16 % of absorbed oxalate over a short time to increase plasma oxalate to the critical level that will cause CaOx to be precipitated spontaneously at the end of the DLH in an average UK RSF. Dietary intakes of oxalate greater than 2 mmol/day will greatly increase the chance of plasma oxalate reaching the critical level for spontaneous precipitation of CaOx at the end of the DLH. The Chicago-Indianapolis group claim that they never found crystals of CaOx in the DLH–ALH section of the kidney [23, 24], but they studied their patients after an overnight fast so that the plasma oxalate of these patients would be too low to cause spontaneous crystallisation of CaOx both at that site and later in the CD, as clearly predicted by the data in Figs. 1 and 2.
Figure 8 shows the effects of increasing the concentration of ultrafiltrable calcium incrementally from 1.2 to 1.9 mmol/l on the maximum RSS OCP at the end of the DLH in an average UK RSF. This corresponds to a range of 2.00–3.17 mmol/l in total plasma calcium. This shows that spontaneous precipitation of CaP can only occur if the concentration of ultrafiltrable calcium reaches at least 2.8 mmol/l (i.e., the levels that are found mainly in primary hyperparathyroidism). These data strongly suggest that the slight variations that can normally occur in plasma calcium (because of its tightly controlled regulation by calcium homeostatic mechanisms in the ALH and D sections of the nephron) are unlikely per se to be a primary factor in the initiation of CaP precipitation in the Loop of Henle in idiopathic stone-formers, although the concentration of calcium does reach a peak at that point as a result of water reabsorption in the DLH. Much more importantly as a risk factor for the precipitation of CaP at the end of the DLH is that the pH of tubular fluid also reaches a peak at that point (around 7.39), having fallen in the proximal tubule from the original 7.38 in plasma ultrafiltrate to around 6.71, and then steadily risen again to 7.39 at the end of the DLH). This alkalinisation has a logarithmic effect on the concentration of PO4 − ions (the form of phosphate ions that precipitate in OCP) and a further cubic effect on RSS OCP based on its formula [31]. This confirms the assertion made by the Leeds group in 1969 that the changes in urinary pH are much more important than the changes in calcium excretion in causing CaP to precipitate both in inorganic solutions which simulate urine and in urine itself [31, 38].
Figures 9 and 10 show that either increasing the concentration of non-fasting ultrafiltrable phosphate from 1.6 (the average value in non-fasting idiopathic RSF in the UK) to 1.9 mmol/l or by decreasing the fractional tubular reabsorption of phosphate in the proximal tubule from 1.215 (the average value estimated for idiopathic RSF in the UK) to 1.190 will lead to spontaneous precipitation of CaP at the end of the DLH. The former only requires the intestinal absorption of about 4.5 mmol of phosphate. Assuming an average intestinal absorption of phosphate of about 67 % and an average daily intake of about 50 mmol/day, this would require about 13 % of the daily absorbed phosphate (33.5 mmol/day) to be absorbed over a short period of time (about 3 h; i.e. about 1.5 mmol/h) to cause CaP to be precipitated spontaneously at the end of the DLH in an average UK RSF. Alternatively, a decrease in the fractional reabsorption of phosphate from 1.215 to 1.190 would cause about 10 mmol of additional phosphate to be excreted per day from the proximal tubule (about 0.4 mmol/h). This would lead to an increase in the concentration of phosphate at the end of the DLH sufficient to cause spontaneous precipitation of CaP at that point. Figures 9 and 10 suggest that the changes in either the intake of phosphate and/or its renal handling are much more important than the changes in plasma calcium in causing possible precipitation of CaP in the Loop of Henle in idiopathic stone-formers. This is essentially because diurnal fluctuations in plasma phosphate are much more exaggerated through the day than those in plasma calcium.
In summary, at this point it would seem that spontaneous precipitation of both CaOx and CaP can occur at the end of the DLH in RSF but is less likely to do so in normal subjects. In RSF, a relatively small increase in plasma oxalate (such as could be caused by ingestion of a high oxalate meal) or an increase in plasma phosphate (such as caused by ingestion of a high phosphate meal) or a decrease in the tubular reabsorption of phosphate (such as might be caused by a small increase in the circulating concentration of PTH, as is often observed in patients living in the northern hemisphere during the winter months) could be sufficient to initiate the precipitation of CaOx and CaP, respectively. Furthermore, as a result of the high RSS of both salts at the end of the DLH, spontaneous precipitation of one could lead to heterogeneous nucleation of the other.
The question is—does this matter? For, even if spontaneous precipitation of either or both calcium salts does occur at the end of the DLH, would the particles not just be excreted before they can grow large enough to be trapped at some point before reaching the Ducts of Bellini as predicted by Finlayson and Reid? However, when hydrodynamic factors, such as (a) the effect of frictional drag on tubular fluid passing close to the tubule walls, (b) a similar frictional drag effect of the tubular walls on any particles travelling close to the tubule walls and (c) the effect of gravity on particles travelling in upward-draining segments of the renal tubule, are taken into account, the situation changes [19]. The version of NEPHROSIM used in this study contains updated data on the relative flow rates of tubular fluid and of the growth of any crystals nucleated at the end of the DLH as they pass through the remainder of the nephron. In general, they confirm the conclusions made using the original model [19].
Particle growth and transit studies
Figure 11 shows the profile of fluid transit times between the start of the ALH and the Ducts of Bellini at various positions relative to the central axis of the tubule. This shows that at a fraction of 0.9 of the tubule radius from the central axis, it takes approximately 5 times longer for fluid to traverse a given segment of tubule than the fluid at the central axis; at a position of 0.95 of the tubule radius, it takes approximately 10 times longer; and at 0.98 of the tubule radius, it takes approximately 25 times longer. Thus, for particles moving in a fluid stream close to the tubule walls, there can be a highly significant delay in the transit time through the segments of the tubule beyond the DLH owing to the drag effect of the walls on the flow of fluid. This delay is further exacerbated by the potential friction effect of the tubule walls on any particles contained in that fluid. Finally, if the final CD is upward-draining against the force of gravity (such as often is the case in the papillae in the lower pole of the kidney), this will tend to oppose the passage of crystals through these CDs and provide additional time for the individual crystals to grow and perhaps agglomerate. This gravitational effect is known as the Stokes factor. It may even lead to crystals, which have grown large enough, to stop or possibly fall back in the tubule and so be delayed further in their passage. All these factors are taken into account in NEPHROSIM. The only factors that have not been included are those caused by (a) the sharp bends in the tubules described by Schulz et al. [39], (b) the kinks in tubules identified by Graves [40] and (c) crystal agglomeration as proposed by a number of researchers because it has not yet been possible to build the mathematics of this into the model [10, 11, 18]. All of these phenomena will potentially delay further the passage of crystals through the nephrons concerned.
Taking the above three hydrodynamic factors into account, Table 3 shows the effect of increasing the initial plasma oxalate concentration in an average untreated UK RSF on the transit times from the start of the ALH to the Ducts of Bellini of crystals of CaOx of various sizes which have been initiated either spontaneously by homogeneous nucleation or heterogeneously by crystals of CaP at the end of the DLH and which have travelled the remainder of the nephron in contact with the walls of the tubule. The table shows, firstly, that the minimum size of initial particles of CaOx and/or CaP nucleated at the end of the DLH needs to be around 8 μm in diameter at the average level of plasma oxalate in UK RSF (1.65 μmol/l) for CaOx particles to stop moving or fall back in upward-draining collecting ducts before they are expelled from the Ducts of Bellini. But this minimum size requirement for initial particles formed at the end of the DLH progressively decreases as plasma oxalate increases such that, at plasma oxalate levels greater than 2, the initial particles only require to be in the range 1–5 μm in diameter to potentially stop or fall back at some point in the CD depending on the actual level of plasma oxalate at the time. These particle sizes are easily achievable in a short space of time, particularly if CaP is also precipitated at the same point. The table shows at which point in the CDs where the crystals either stop or fall back in upward-draining tubules and the diameter of the CaOx crystals at the point of stoppage or fall-back. This shows that particles initially as small as 1 μm in diameter may stop or fall back in upward-draining CDs within 14–25 min, if they travel very close to the tubule wall and if the RSS CaOx is sufficiently high to promote rapid crystal growth. At the point of stoppage or fall-back, the individual crystal diameters predicted by the model range from about 34 to 48 μm in diameter but may be much larger than this before eventually being extruded from the Ducts of Bellini, particularly if aggregation takes place when crystals falling back in upward-draining tubules make contact with other smaller crystals which are still moving in the direction of flow of the tubular fluid. These are similar to the individual crystal sizes reported in fresh, warm urines from RSF by a number of researchers [10, 41, 42]. They contrast with the much smaller crystals of CaOx reported in fresh, warm urine collected from normal subjects [10]. Studies on two RSF who had catheters inserted into their renal pelves (for other medical reasons) showed that the large crystals and aggregates were present in their pelvi-calyceal urine and had not just formed in the bladder.
There are at least two possible explanations for this difference in the size of crystals excreted by recurrent stone-formers and normal subjects. Firstly, some may argue that the smaller crystals in the urines of normal subjects may be due to the present of inhibitors of crystallisation of calcium salts that are present in normal urine but absent or deficient in the urines of recurrent stone-formers. However, the evidence against this is that, at the high levels of RSS CaOx necessary to cause spontaneous precipitation of CaOx, common inhibitors, such as pyrophosphate, are much less active than at lower levels of RSS CaOx and RSS OCP [43]. This has been observed both in vitro with pyrophosphate [43] and in vivo after the administration of a bisphosphonate [44] and strongly suggests that the role of inhibitors in urines with a high RSS CaOx is actually much less than that proposed by some researchers in the field. A second possible explanation for the difference between the CaOx crystal sizes excreted by normals and RSF emerges from NEPHROSIM, which shows that there are two possible points in the nephron where CaOx crystallisation may be initiated, particularly in UK RSF and KSA RSF (Figs. 1, 2). The first is at the end of the DLH (if there is a small transient increase in plasma oxalate) and the second is in the latter part of the CD. Those crystals initiated at the end of the DLH, by spontaneous nucleation (or by heterogeneous nucleation caused by crystals of CaP), are the ones which may be delayed in transit for the reasons presented above and grow into the large crystals and aggregates frequently observed in fresh urine from RSF. In contrast, the model shows that those crystals initiated in the CD (when RSS CaOx increases again owing to reabsorption of water in that section of the tubule) will not grow fast enough in the remaining transit time of fluid through the CD and will be excreted as relatively small crystals of only a few microns in diameter, such as observed in the urines of most RSF (alongside the large crystals initiated earlier in the nephron, described above) and even in the urines of some normal subjects [10].
The predictions of the model are supported by the findings from a study carried out about 45 years ago by the Leeds group, in which six idiopathic calcium RSF and six normal control subjects (NC) were fed a fixed basal diet for 4 days. On the second day of the study, urine samples were collected into warm vacuum flasks at 0900 hours (just before breakfast) and then at 3-hourly intervals over the next 12 h and finally at 0900 hours the following morning. The size profiles of CaOx crystals contained in the urine samples (maintained at 37 °C) were measured within 10 min of voiding using a Coulter Counter. The size and appearance of the crystals and aggregates of CaOx were also checked by light microscope immediately after voiding. On the fourth day of the study, a small oral dose of sodium oxalate (2.6 mmol–equivalent to the amount contained in about 30 g of dry spinach or 40 g of boiled spinach) was administered to the RSF and NC immediately before breakfast at 0900 hours and fresh, warm urine samples collected according to the protocol on the basal day. Again, the size profiles of CaOx crystals in the urine samples were measured within 10 min of voiding using a Coulter Counter and the size and appearance of the crystals and aggregates of CaOx checked by light microscope.
Figure 12a shows the mean size distributions of CaOx crystals and aggregates in the fresh, warm urine from the NC and the RSF at 1200 hours on the second day of the basal diet and Fig. 12b shows the corresponding data on Day 4 of the study, 3 h after the sodium oxalate bolus was given. On the basal diet, both groups showed a peak of small CaOx crystals (mean diameter ~6 μm) in the urine samples taken at 1200 hours although the peak in the RSF was much higher than that in the NC. However, the RSF had an additional peak of much larger CaOx crystals and aggregates (mean diameter ~25 μm, but some as large as 50 μm, which was the upper limit of measurement of the Coulter Counter system used at that time). After the oxalate load, the NC showed an increase in the peak of small crystals of CaOx but still had no large crystals or aggregates in their urines at that point or during the remainder of the day (the data are not shown here). The RSF, on the other hand, showed a marked increase in the peak of large crystals and aggregates and three out of the six had attacks of renal colic within a few hours following the oxalate load. Urinary oxalate excretion increased within the first 3 h in both groups but slightly more so in the RSF than in the NC. Furthermore, the NC had a slight diuresis, which helped to moderate the increase in oxalate concentration in their urine (0.22 to 0.28 mmol/l) caused by the ingestion of the sodium oxalate. The RSF, on the other hand, had little or no diuresis and their urinary oxalate concentrations increased from 0.30 to 0.52 mmol/l. From these data, it was calculated that in the first 3 h after the oxalate load, the RSF absorbed 5–10 % more of the ingested oxalate than did the NC. The almost immediate increase in urinary oxalate after the oxalate load supports the observation that oxalate may be rapidly absorbed in the stomach [32, 33] and that for some as yet unknown reason, the RSF absorbed more oxalate than the NC in this section of the intestinal tract. Interestingly, there was a second peak in oxalate excretion in both NC and RSF at around 1800 hours (data not shown) which is assumed to derive from a second phase in the absorption of oxalate taking place in the large bowel [34, 35]. The peak in oxalate concentration at 1800 hours was once again higher in the RSF than in the NC. The relative patterns of crystalluria in the NC and RSF were broadly similar to those shown in Fig. 12b.
The average volume of CaOx crystals and aggregates plotted against particle size in the fresh warm urines collected at 1200 hours from a group of 6 normal control subjects (NC) and a group of 6 recurrent calcium stone-formers (RSF) on a day when they were on a basal diet (a) and at 1200 hours on a subsequent day after they had been given an oral dose of sodium oxalate at 0900 hours prior to breakfast
Based on the patterns of RSS CaOx in Fig. 2, one explanation of these data is that, on the basal diet, the tubular oxalate concentrations at the end of the DLH were likely to be too low in the NC (based on their final urinary oxalate concentrations) to cause any CaOx crystals to nucleate spontaneously at that point. Any crystals that might form in NC tubular fluid would only do so towards the end of the CD, if either the RSS CaOx or the RSS OCP in the tubular fluid reached the critical level for spontaneous nucleation of CaOx or CaP or both. However, any crystals so-formed would be small, such as shown in Fig. 12a, and would be easily excreted from the Ducts of Bellini. Even after the oxalate load, the RSS CaOx levels in the NC would still be unlikely to reach the FP of CaOx and would only become high enough to nucleate spontaneously in the CD. Based on the above reasoning, this would only lead to an increased number of small crystals in the urines of the NC, as observed in Fig. 12b. In the RSF, however, because of their higher absorption of oxalate and subsequently higher urinary oxalate concentrations, CaOx nucleation would be likely to be increased both at the end of the DLH and in the CD. Those crystals initiated at the end of the DLH could be potentially delayed by the hydrodynamic processes described above and under the conditions of the increased throughput of oxalate give rise to the formation of many more large individual crystals and aggregates, such as observed in the RSF urines after the oxalate load. The much higher RSS CaOx values in the CD of the RSF compared with those in the CD of the NC would result in a higher crystal growth rate of any small crystals formed there and would lead to fewer small crystals actually being excreted compared with the numbers formed on the basal diet as shown in Fig. 12a, b. This can actually be shown to occur in the model but there is not space in this paper to show the data.
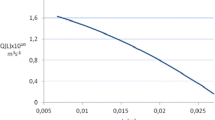
There is one final point to be made about the relative amounts of CaOx crystalluria likely to be observed in the urines of the average UK N, UK RSF and KSA RSF. Firstly, it is important to emphasise that RSS CaOx is only partly responsible for the amount of CaOx crystalluria that is likely in each of these groups. A second and equally significant factor is the oxalate/calcium ratio of the tubular fluid or urine in which the crystals form, for it is possible to have the same RSS CaOx over a wide range of oxalate/calcium ratios. The oxalate/calcium ratios in the final urines of the UK N, UK RSF and KSA RSF in Table 2 are 0.060, 0.061 and 0.161, respectively. At these ratios where oxalate is present at about 1/15 of that of calcium, as in the urines from the UK N and UK RSF, and at about 1/6 of that of calcium, as in the urines in KSA, it is the lower concentration of the two ions (i.e., oxalate) which controls the amount of crystalluria that can be expected. In a one-to-one crystallisation situation, such as is the case with CaOx, no matter how much more additional calcium is excreted in urine, the amount of CaOx crystalluria that can be produced is limited by the much lower amount of oxalate present in the urine. Figure 13 shows that for any given RSS CaOx, the amount of crystalluria increases as the oxalate/calcium ratio increases reaching a maximum at an oxalate/calcium ratio of 1; thereafter, it decreases as the concentration of calcium, which is now less than that of oxalate, takes over as the factor controlling the volume of crystals that can be produced. This latter situation rarely arises in humans except in a few cases of primary hyperoxaluria with chronic renal failure where the concentration of oxalate in urine can occasionally exceed that of calcium. In cat urine, on the other hand, where the oxalate/calcium ratio is normally around 1 [45], small increases in the concentration of either ion can equally affect the amount of CaOx crystalluria and so the excretions of both ions are important in this species.
Figure 13 shows the maximum volume of CaOx crystals that can be precipitated at two levels of RSS CaOx (11 and 17) in relation to a range of oxalate/calcium ratios in the urine. An RSS CaOx of 11 corresponds roughly to that in the urine of a UK N and an RSS CaOx of 17 roughly corresponds to those in the urines of a UK RSF and a KSA RSF, respectively (see the data at the end of the respective CDs in Fig. 2). Figure 13 predicts that, although the UK N and UK RSF have almost the same oxalate/calcium ratios, a UK RSF will have about twice the amount of crystalluria found in the urine of a UK N because of the higher RSS CaOx values in his urine. On the other hand, the KSA RSF, who has the same RSS CaOx as the UK RSF, will have roughly 50 % more crystalluria than the latter because of his much high oxalate/calcium ratio and he will have about three times more crystalluria than the UK N from a combination of his higher RSS CaOx and higher oxalate/calcium ratio in his urine. In summary, CaOx crystalluria would be predicted to be in the order of UK N < UK RSF < KSA RSF—which is actually found to be the case [10, 28, 29]. This accounts for the much higher incidence of stone-formation in Saudi Arabia compared with that in the UK [29].
Conclusions
This latest model of kidney function (NEPHROSIM) shows that under certain conditions de novo nucleation of both CaOx and CaP can take place at the end of the DLH, especially in patients with a history of recurrent calcium stone-formation. The model takes into account the fact that (a) crystals of either CaOx or CaP nucleated at the end of the DLH may move at different speeds in relation to the fluid flow rate at the central axis of the tubule, (b) crystals of CaOx (or CaP) moving close to the tubule walls move more slowly than crystals at the tubule axis, (c) CaOx crystal transit may be delayed for anything up to at least 25 min and the crystals may continue to grow if the RSS CaOx is high enough, and (d) CaOx crystals may stop moving or even fall back in upward-draining sections of tubule, especially the CD. Under average “normal” conditions of urine composition in UK RSF and KSA RSF, spontaneous precipitation of CaOx at the end of the DLH is unlikely to occur. Otherwise, the RSF would be forming stones all the time! The model predicts that (a) for small, transient increases in plasma oxalate concentration, crystallisation only takes place in the CD and leads to the formation of small crystals in the urine but (b) for larger increases in the filtered load of oxalate, spontaneous and/or heterogeneous nucleation of CaOx may occur at both the end of the DLH and in the CD. This scenario will lead to large crystals of CaOx in the final urine arising from nucleation occurring in the DLH, as described above, and to small crystals of CaOx from nucleation taking place in the CD. As a result of the higher calcium and oxalate concentrations in the urine of RSF, they have an increased probability of initiating crystallisation in the DLH and so of going on to form the large crystals and aggregates of CaOx (and CaP [10, 28]) found in their fresh urines but not in the fresh urines from NC [10].
The model also shows that the tubular fluid of RSF is more likely than that of NC to reach the conditions necessary for de novo nucleation of CaP at the end of the DLH. This may occur following either a small increase in ultrafiltrable phosphate following ingestion of a high phosphate meal or a small decrease in the proximal tubular reabsorption of phosphate such as would result from an increase in parathyroid activity. CaP crystals initiated at this point may heterogeneously nucleate the crystallisation of CaOx under the high metastable conditions of RSS CaOx which frequently exist in the urines of RSF, particularly if they also have a simultaneous increased filtered load of oxalate. Under certain conditions, it would be predicted that CaP crystals, initiated at the end of the DLH and travelling close to the tubular walls where their transit time may be increased, might also be able to grow and agglomerate sufficiently to become trapped at some point in the CD and thereby lead to the formation of Randall’s Plugs in the Ducts of Bellini as proposed by Tiselius [46]. Currently, work is under way to incorporate the nucleation and growth of crystals of CaP into the NEPHROSIM model to test this phenomenon.
Abbreviations
- CaOx:
-
Calcium oxalate
- CaP:
-
Calcium phosphate
- CaP-CO3 :
-
Carbapatite
- RSS:
-
Relative supersaturation
- RSS CaOx:
-
Relative supersaturation with respect to calcium oxalate
- RSS OCP:
-
Relative supersaturation with respect to octocalcium phosphate
- RSS UA:
-
Relative supersaturation with respect to uric acid
- NC:
-
Normal control
- RSF:
-
Recurrent stone-former
- UK N:
-
Average normal male in the UK
- UK RSF:
-
Average untreated male recurrent CaOx/CaP stone-former in the UK
- KSA RSF:
-
Average untreated male recurrent CaOx/UA stone-former in the Kingdom of Saudi Arabia
- FP:
-
Empirical thermodynamic formation product
- SP:
-
Thermodynamic solubility product
- Prox:
-
Proximal tubule
- DLH:
-
Descending limb of the Loop of Henle
- ALH:
-
Ascending limb of the Loop of Henle
- D:
-
Distal tubule
- CD:
-
Collecting duct
References
Boyce WH, King JS Jr (1963) Present concepts concerning the origin of matrix and stones. Ann NY Acad Sci 104:563–578
Keutel HJ, King JS Jr, Boyce WH (1964) Further studies of uromucoid in normal and stone urine. Urol Int 17:324–341
Howard JE, Thomas WC Jr, Mukai T, Johnston RA Jr, Pascoe BJ (1962) The calcification of cartilage by urine, and a suggestion for therapy in patients with certain kinds of calculi. Trans Assoc Am Physicians 75:301–306
Fleisch H, Bisaz S (1964) The inhibitory effect of pyrophosphate on calcium oxalate precipitation and its relation to urolithiasis. Experientia 15:276–277
Vermeulen CW, Lyon ES, Gill WB (1964) Artificial urinary concretions. Invest Urol 1:370–386
Robertson WG, Peacock M, Nordin BEC (1968) Activity products in stone-forming and non-stone-forming urine. Clin Sci 34:579–594
Kolbach AM, Afzal O, Sorokina E, Kleinman JG, Wesson JA (2012) Relative deficiency of acidic forms of osteopontin from stone former urine. Urol Res 40:447–454
Ryall RL (2008) The future of stone research: rummaging in the attic, Randall’s plaque, nanobacteria, and lessons from phylogeny. Urol Res 36:77–97
Finlayson B, Reid F (1978) The expectation of free and fixed particles in urinary stone disease. Invest Urol 15:442–448
Robertson WG, Peacock M, Nordin BEC (1969) Calcium crystalluria in recurrent renal stone-formers. Lancet 2:21–24
Kok DJ, Papapoulos SE, Bijvoet OLM (1990) Crystal agglomeration is a major element in calcium oxalate urinary stone formation. Kidney Int 37:51–56
Kumar S, Sigmon D, Miller T, Carpenter B, Khan S, Malhotra R, Scheid C, Menon M (1991) A new model of nephrolithiasis involving tubular dysfunction/injury. J Urol 146:1384–1389
Bigelow MW, Wiessner JH, Kleinman JG, Mandel NS (1998) Calcium oxalate crystal attachment to cultured kidney epithelial cell lines. J Urol 160:1528–1532
Verkoelen CF, van der Boom BG, Houtsmuller AB, Schröder FH, Romijn JC (1998) Increased calcium oxalate monohydrate crystal binding to injured tubular epithelial cells in culture. Am J Physiol 274:F958–F965
Scheid C, Koul H, Hill WA, Luber-Narod J, Jonassen J, Honeyman T, Kennington L, Kohli R, Hodapp J, Ayvazian P, Menon M (1996) Oxalate toxicity in LLC-PK1 cells: role of free radicals. Kidney Int 49:413–419
Lieske JC, Leonard R, Swift HS, Toback FG (1996) Adhesion of calcium oxalate monohydrate crystals to anionic sites of renal epithelial cells. Am J Physiol 270:F192–F199
Koul H, Kennington L, Honeyman T, Jonassen J, Menon M, Scheid C (1996) Activation of c-myc gene mediates the mitogenic effects of oxalate in LLC-PK1 cells, a line of renal epithelial cells. Kidney Int 50:1525–1530
Kok DJ, Khan SR (1994) Calcium oxalate nephrolithiasis, a free or fixed particle disease. Kidney Int 46:847–854
Robertson WG (2004) Kidney models of calcium oxalate stone-formation. Nephron Physiol 98:21–30
Randall A (1936) An hypothesis for the origin of renal calculus. N Engl J Med 214:234–237
Randall A (1937) The origin and growth of renal calculi. Ann Surg 105:1009–1027
Cifuentes Delatte L, Minon-Cifuentes J, Medina JA (1985) Papillary stones: calcified renal tubules in Randall’s plaques. J Urol 133:490–494
Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL, Grynpas M (2003) Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin Loops of Henle. J Clin Invest 111:607–616
Evan AP, Lingeman JE, Coe FL, Shao Y, Parks JH, Bledsoe SB, Phillips CL, Bonsib S, Worcester EM, Sommer AJ, Kim SC, Tinmouth WW, Grynpas M (2005) Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int 67:576–591
Knight TF, Sansom SC, Senekjian HO, Weinman EJ (1981) Oxalate secretion in the rat proximal tubule. Am J Physiol 240:F295–F298
Holmes RP, Ambrosius WT, Assimos DG (2005) Dietary loads and renal oxalate handling. J Urol 174:943–947
Worcester EM, Evan AP, Coe FL, Lingeman JE, Krambeck A, Sommers A, Phillips CL, Milliner D (2013) A test of the hypothesis that oxalate secretion produces proximal tubule crystallization in primary hyperoxaluria type 1. Am J Physiol Renal Physiol 305:F1574–F1584
Robertson WG, Peacock M, Nordin BEC (1971) Calcium oxalate crystalluria and urine saturation in recurrent stone-formers. Clin Sci 40:365–374
Robertson WG (2012) Stone-formation in the Middle Eastern Gulf States—a review. Arab J Urol 10:265–272
Robertson WG (1969) Measurement of ionized calcium in biological fluids. Clin Chim Acta 24:149–157
Robertson WG (1969) Physicochemical aspects of renal stone-formation. Ph.D. Thesis, University of Leeds, UK
Hautmann RE (1993) The stomach: a new and powerful oxalate absorption site in man. J Urol 149:1401–1404
Chen Z, Ye Z, Zeng L, Yang W (2003) Clinical investigation on gastric oxalate absorption. Chin Med J (Engl) 116:1749–1751
Freel RW, Hatch M, Earnest DL, Goldner AM (1980) Oxalate transport across the isolated rat colon. A re-examination. Biochim Biophys Acta 600:838–843
Hatch M, Freel RW, Vaziri ND (1993) Characteristics of the transport of oxalate and other ions across rabbit proximal colon. Pflugers Arch 423:206–212
Tomson MB, Nancollas GH (1978) Mineralization kinetics: a constant composition approach. Science 200:1059–1060
Asplin JR, Mandel NS, Coe FL (1996) Evidence of calcium phosphate supersaturation in the Loop of Henle. Am J Physiol 270:F604–F613
Robertson WG, Nordin BEC (1969) Activity products in urine. In: Hodgkinson A, Nordin BEC (eds) Proceedings of the renal stone research symposium. Churchill, London, pp 221–232
Schulz E, Hengst E, Brundig P, Haerting R, Pirlich W, Günther H (1987) Disturbed urinary transport in the pelvi-calcyceal system in calcium oxalate stone patients. Urol Res 15:109–113
Graves FT (1982) An experimental study of the anatomy of the tubules of the human kidney and its relation to calculus formation. Br J Urol 54:569–574
Sengbusch R, Timmermann A (1957) Das Kristalline Calciumoxalat im menschlichen Harn und seine Beziehung zur Oxalatstein-Bildung. Urol Int 4:76–95
Tiselius HG (1999) Crystalluria in patients with calcium stone disease. J Urol 161:1432
Robertson WG (unpublished data)
Robertson WG, Peacock M, Marshall RW, Knowles F (1974) The effect of ethane-1-hydroxy-1,1-diphosphonate (EHDP) on calcium oxalate crystalluria in recurrent renal stone-formers. Clin Sci 47:13–22
Buckley CM, Hawthorne A, Colyer A, Stevenson AE (2011) Effect of dietary water intake on urinary output, specific gravity and relative supersaturation for calcium oxalate and struvite in the cat. Br J Nutr 106(Suppl 1):S128–S130
Tiselius HG (2013) The role of calcium phosphate in the development of Randall’s plaques. Urolithiasis 41:369–377
Conflict of interest
The author declares that he has no conflict of interests relating to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robertson, W.G. Potential role of fluctuations in the composition of renal tubular fluid through the nephron in the initiation of Randall’s plugs and calcium oxalate crystalluria in a computer model of renal function. Urolithiasis 43 (Suppl 1), 93–107 (2015). https://doi.org/10.1007/s00240-014-0737-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-014-0737-1