Abstract
Vitamin D receptor (VDR) plays a key role in calcium metabolism, and is closely related to urinary stone formation (urolithiasis). Previous studies have investigated the associations between VDR single nucleotide polymorphisms (SNPs) (polymorphisms at BsmI, ApaI, FokI, or TaqI cutting sites) and urolithiasis in different populations. However, the results remain inconsistent and controversial. Therefore, meta-analysis was performed to evaluate these associations. Twenty studies that investigated the associations between VDR SNPs and urolithiasis were retrieved. Odds ratios (ORs) with 95 % confidence intervals (CIs) were calculated under the most appropriate genetic model. The TaqI polymorphism was associated with an increased risk of urolithiasis (tt + Tt vs. TT: OR = 1.253; 95 % CI = 1.033–1.520, p = 0.022, I 2 = 0), whereas the ApaI, BsmI, and FokI polymorphisms were not. Stratifying for ethnicity, a slightly increased risk was found among Asians as compared to Whites (OR 1.263, 1.232, respectively, p < 0.01). Deviation from Hardy–Weinberg equilibrium (HWE) was the major source of heterogeneity. In summary, this updated meta-analysis suggests the TaqI polymorphism is associated with urolithiasis risk, whereas BsmI, ApaI, and FokI polymorphisms are not.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urolithiasis is a global and multifactorial disease. Approximately 13 % of men and 7 % of women have suffered from urinary stones at least once in their lifetime, and the incidence of urolithiasis continues to increase [37]. Urinary stone formation is influenced by diet, environmental factors, and genetic factors [14]. The genes responsible for heritability of urolithiasis are still not determined; however, several genetic loci that appear to have a minor contribution to urolithiasis have been indentified, such as single nucleotide polymorphisms (SNPs) in osteopontin (OPN) [12], calcium-sensing receptor (CASR) [43], and vitamin D receptor (VDR) genes [44]. Among them, the VDR gene is most widely studied.
Located on chromosome 12q12-14, the human VDR gene encodes VDR, which belongs to the nuclear steroid receptor superfamily. VDR regulates the biological activity of vitamin D, a key player in calcium metabolism [35]. Patients with an excessive intake of vitamin D are more likely to suffer from urinary stones [2]. Given that vitamin D exerts its effects through VDR, the VDR gene is a candidate gene for urolithiasis.
There are many validated SNPs in the VDR gene in the dbSNP database, but only four SNPs (located in BsmI, ApaI, FokI, and TaqI cutting sites) have been extensively studied. This is likely because these four SNPs are currently the most relevant in influencing the function or expression of VDR. Located at the translational start site of the VDR gene, the FokI polymorphism alters the VDR protein sequence in f allele carriers, producing a protein three amino acids longer and associated with a reduced response to vitamin D in target cells [3]. For BsmI, ApaI, and TaqI polymorphisms (all located at the 3′-UTR region), the B allele, A allele, and t allele correlate with enhanced mRNA stability or transcriptional activity, and greater vitamin D activity [23, 33].
In recent years, many epidemiological studies have investigated the relationship between VDR SNPs and urolithiasis, but the results are still controversial. To date, only one meta-analysis, based on associations between VDR polymorphisms and risk of urolithiasis, has been published [19]. This meta-analysis included all studies, with an average of 1,124 cases and 1,209 controls (published up to September 2010), although nearly half of the studies did not show Hardy–Weinberg equilibrium (HWE). A marginally significant association of FokI and TaqI polymorphisms with urolithiasis risk was found. Unfortunately, the results were insignificant when α level was adjusted according to the Bonferroni’s method of multiple comparisons (α = 0.05/3 = 0.0167) [8]. When considering that deviation from HWE may increase the type I error rate, and even draw a false-positive conclusion [41], their results would be strengthened through evaluating the impact of non-HWE studies to final results. In addition, three other relatively large sample studies [1, 5, 44], with an average of 552 cases and 554 controls, published in the past 3 years merit inclusion. Therefore, a larger and more precise meta-analysis, investigating the association of these polymorphisms with urolithiasis risk, was performed.
Materials and methods
Search strategy
Eligible studies were extracted via a search of PubMed, EMBASE, Medline, and Chinese National Knowledge Infrastructure databases (up to June 2013) using the following keywords: (vitamin D receptor OR VDR) AND (polymorphisms OR SNPs OR variants) AND (urolithiasis OR nephrolithiasis). The equivalent Chinese terms were used in the Chinese database. The references of retrieved articles were also searched for additional studies.
Inclusion criteria and assessment of study quality
Studies selected met the following criteria: (1) focused on associations between at least one of these four SNPs (BsmI, ApaI, FokI, and TaqI) and urolithiasis risk; (2) case–control study; (3) full-text article published in English or Chinese; (4) reported genotype frequencies or distributions; and (5) comprises patients and healthy individuals; for data unavailable in relevant studies, a direct communication with the corresponding author was made.
The quality of studies was independently assessed by two of the co-authors using the refined criteria (Table S1) originally proposed by Thakkinstian et al. [39] and discrepancies were resolved through discussions. Scores given ranged from 0 (lowest) to 10 (highest). Reports scoring <6 were classified as low quality, and those scoring ≥6 were classified as high quality.
Data extraction
Data from relevant studies are carefully and independently extracted by two authors according to the above-mentioned criteria. Discrepancies were resolved through discussions. Data extracted from these articles included the name of the first author, year of publication, country, ethnicity, genotyping methods, age, sample size, and numbers of various genotypes in case and control groups.
Statistical analysis
First, we calculated OR1, OR2, and OR3 for the genotypes according to the Ammarin Thakkinstian’s study [38]:
-
(a)
OR1 aa versus AA, OR2 Aa versus AA and OR3 aa versus Aa for ApaI;
-
(b)
OR1 ff versus FF, OR2 Ff versus FF and OR3 ff versus Ff for FokI;
-
(c)
OR1 tt versus TT, OR2 Tt versus TT and OR3 tt versus Tt for TaqI;
-
(d)
OR1 bb versus BB, OR2 Bb versus BB and OR3 bb versus Bb for BsmI.
The OR1, OR2, OR3 were used to determine the most appropriate genetic model.
-
(a)
If OR1 = OR3 ≠ 1 and OR2 = 1, a recessive model was suggested (aa vs. Aa + AA).
-
(b)
If OR1 = OR2 ≠ 1 and OR3 = 1, a dominant model was suggested (aa + Aa vs. AA).
-
(c)
If OR2 = 1/OR3 ≠ 1 and OR1 = 1, then a complete overdominant model suggested (aa + AA vs. Aa).
-
(d)
If OR1 < OR2 < 1 and OR1 < OR3 < 1, or if OR1 > OR2 > 1 and OR1 > OR3 > 1, then a codominant model was indicated (aa vs. Aa vs. AA).
-
(e)
If none of the above met, we calculated the multiple pairwise comparisons (aa vs. AA, aa vs. Aa, Aa vs. AA).
HWE was assessed by the Chi-squared goodness-of-fit test for only the control group of each study (p < 0.05 was considered significant). The strength of the associations between each SNPs and the risk of urolithiasis were assessed by odds ratios (OR) and 95 % confidence intervals (CI) under the appropriate genetic model (the significance level was adjusted to α = 0.05/3 = 0.0167 for multiple pairwise comparisons, whereas α = 0.05 under other genetic models). The presence of heterogeneity between studies was tested by the Chi-square-based Q test and I 2. The I 2 statistic was calculated to quantify the proportion of the total variation due to heterogeneity (I 2 > 30 % was considered heterogeneous). The pooled effect was calculated by a fixed-effects model (the Mantel–Haenszel method) when there is no heterogeneity (I 2 < 30 %) [11]. Otherwise, the random-effects model (the DerSimonian and Laird method) was used [21]. To explore the potential effect of heterogeneity, we performed stratification analyses by ethnicity, age, and quality criteria. Furthermore, we performed meta-regression to explore source of heterogeneity. The between-studies variance (τ2) was used to quantify the degree of heterogeneity between studies, and the percentage of τ2 was used to describe the extent of heterogeneity explained [45]. By alternately removing each study, sensitivity analysis was performed to appraise the stability of the final results. Begg’s funnel plot and Egger’s test were carried out to evaluate potential publication bias. All the statistical analyses were performed using the STATA statistical software 12.0.
Results
Study characteristics
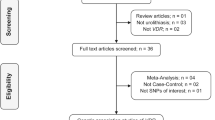
A flowchart of the screening process is shown in Fig. 1. A total of 20 case–control studies were included in the meta-analysis. Among them, 9 studies with 1,543 cases and 1,764 controls [1, 15, 25, 26, 28, 32, 34, 44], 12 studies with 2,051 cases and 2,229 controls [1, 5–7, 10, 20, 22, 27, 28, 32, 34, 44], 11 studies with 1,290 cases and 1,836 controls [5, 15, 18, 22, 24–26, 32–34, 44], 8 studies with 1,064 cases and 1,228 controls [9, 15, 24, 26–28, 30, 44] focused on associations between ApaI, FokI, TaqI, BsmI polymorphisms in the VDR gene and urolithiasis risk, respectively. Table 1 presents the characteristics of the included articles. Thirteen studies were conducted in Asian countries, six in European countries, and one in the United States. Most studies (12) focused on adults, whereas four focused on children, and four did not provide data on age. Genotyping methods included PCR–RFLP (19 studies) and PCR single-strand conformational polymorphism (one study).
Almost half of the studies deviated from HWE (Table 1). There is currently no consensus regarding the inclusion of studies that deviate from HWE. Thus, a sensitivity analysis was performed later to test the robustness of the results and determine whether to exclude these studies.
Quantitative synthesis
For the ApaI polymorphism, the OR1, OR2, and OR3 were 0.944 (p = 0.774), 0.946 (p = 0.697), and 1.149 (p = 0.168), respectively. For the FokI polymorphism, OR1, OR2, and OR3 were 1.244 (p = 0.343), 1.590 (p = 0.028), and 0.834 (p = 0.255), respectively. Thus, no opposite genetic model was attributed to these two SNPs. Accordingly, multiple pairwise comparisons were applied. For the TaqI polymorphism, OR1, OR2, and OR3 were 1.115 (p = 0.497), 1.217 (p = 0.031), and 0.933 (p = 0.653), respectively, suggesting a dominant model. For the BsmI polymorphism, OR1, OR2, and OR3 were 1.309 (p = 0.078), 1.238 (p = 0.154), and 1.075 (p = 0.663), respectively, suggesting a dominant model.
The pooled effects of ApaI and FokI polymorphisms were calculated using the random effects model because studies regarding these SNPs were heterogeneous. In contrast, pooled effects of the TaqI and BsmI polymorphisms were calculated by the fixed-effects model (Table S2–S4). As confounding factors may affect the overall results, subgroup analyses were performed according to ethnic group, age, and quality criteria.
The meta-analysis of VDR polymorphisms and urolithiasis risk are presented in Table S2–S4. For the TaqI polymorphism, the combined results (Fig. 2) suggested tt + Tt carriers have an increased risk of urinary stones (tt + Tt vs. TT: OR = 1.195; 95 % CI = 1.008–1.416, p = 0.041). In the subgroup analyses, significant increased risks were found in studies among Asians when compared with Whites (OR = 1.288; 95 % CI = 1.039–1.597), in studies in which the controls showed HWE compared with those that did not (OR = 1.304; 95 % CI = 1.046–1.626). As for the FokI polymorphism, the overall results were non-significant (Table S3). However, when stratified by ethnic group, age, and quality criteria, child cases had a significantly higher frequency of the ff genotype (ff vs. Ff: OR = 2.824; 95 % CI = 1.843–4.328, p < 0.001) and cases in non-HWE studies had a significantly higher frequency of the ff genotype (ff vs. Ff: OR = 2.756; 95 % CI = 1.418–5.357, p = 0.003). Given that all studies among children deviated from HWE, the results were considered unreliable, and were evaluated in the following analyses. In addition, there was no statistical evidence of an association between ApaI and BsmI polymorphisms with urolithiasis risk (Table S2, S4).
Meta-regression
To explore the source of heterogeneity, we performed meta-regression according to the ethnicity, sample size, quality assessment score, and HWE. The results revealed that HWE (p = 0.009), but not ethnicity (p = 0.512), and quality assessment score (p = 0.744) contribute to the source of heterogeneity for the FokI polymorphism (Ff vs. FF). Furthermore, HWE could explain 54.82 % of the variance between studies (τ2), suggesting studies where controls did not show HWE potentially offer different outcomes. No potential source of heterogeneity was found for other polymorphisms.
Sensitivity meta-analysis
To evaluate the impact of each study to the combined results and determine whether to exclude the studies that deviated from HWE, sensitivity analyses were carried out through removing each particular study. Among five non-HWE studies on associations between the TaqI polymorphism and the risk of stones (Fig. 3), exclusion of the Mossetti [24] and Basiri [5] studies apparent altered the overall results, while exclusion of the Jackman [18], Seyhan [33], and Seo [32] studies did not. Given that deviation from HWE may alter the assumed type I error rate, and even draw a false-positive conclusion, the Mossetti and Basiri studies were excluded. Nevertheless, the association between the TaqI polymorphism and urolithiasis risk remained statistically significant (tt + Tt vs. TT: OR = 1.253; 95 % CI = 1.033–1.520, p = 0.022, I 2 = 0). Notably, there was an inverse overall pooled effect of the FokI polymorphism through similar approaches (Ff vs. FF: overall: OR = 0.934; 95 % CI = 0.754–1.158, p = 0.534, I 2 = 10.6 %), while no significant association between the FokI polymorphism and urolithiasis risk among children was observed (OR = 1.968; 95 % CI = 0.933–3.897, p = 0.052, I 2 = 0). Similarly, no relationships to urolithiasis were observed in the remaining polymorphisms. In addition, heterogeneity of the included studies for each polymorphism had been reduced through this approach, indicating the modified results were much more reliable than previous results.
Publication bias
Begg’s funnel plot and Egger’s test were preformed to evaluate publication bias. The funnel plot of the TaqI polymorphism (Figure S1) showed no apparent asymmetric, and the p values of Begg’s test and Egger’s test were 0.484 and 0.851, respectively, also indicating no publication bias. The results of the remaining SNPs were similar.
Discussion
Although numerous epidemiological studies relating VDR polymorphisms to urolithiasis have been published, there have been conflicting results. This is possibly because of small sample sizes, selection bias, and improper design. To provide more comprehensive and reliable results, meta-analysis was performed. The previous meta-analysis by Liu et al. [19] found a marginally significant association between FokI and TaqI polymorphisms with urolithiasis risk. However, the meta-analysis presented here suggests that TaqI polymorphisms have a significant association with urolithiasis risk, whereas FokI, ApaI, and BsmI polymorphisms appear unrelated with urolithiasis risk. To the best of our knowledge, the meta-analysis presented here is currently the largest meta-analysis to investigate associations between these polymorphisms and urolithiasis risk. In addition, we adjusted α, carefully evaluated the impact of non-HWE studies, and succeeded in reducing heterogeneity. Therefore, the findings presented here potentially reveal more relevant associations.
When considering the potential impact of genetic background on the meta-analysis, subgroup analyses were performed according to ethnicity. Our results showed an increased risk associated with the TaqI polymorphism in Asians, but not Whites. These differences may result from different genetic backgrounds. In addition, the relatively higher frequency of exposure to UVR in Asia countries [29] may also contribute to the effects, as vitamin D is largely derived from processes initiated by UVR exposure [16, 36]. Gender is also a well-known risk factor for urolithiasis. Wang et al. [44] reported that the ff FokI polymorphism showed significant differences in females but not males, suggesting gender may influence the function of the VDR. However, only two studies could be divided into subgroups based on gender, insufficient to establish a credible association. Thus, gender subtypes should be noted in future studies investigating the association between SNPs and a susceptibility to urinary stones.
In the present study, statistically significant heterogeneity was found in FokI and ApaI polymorphisms. Deviation from HWE was determined as the main source of heterogeneity. The HWE law states that in the absence of forces, such as mutation and inbreeding, two alleles (T and t, with frequencies p and q, respectively) should be in equilibrium in a large population. Thus, the proportion of genotypes TT, Tt, and tt should be p2, 2pq, and q2, respectively [38]. Most departures from the HWE are due to genotyping error [17], selection bias in controls, and population stratification [4], all issues that would alter the assumed type I error rate and even result in erroneous results (for example, the FokI polymorphism in this study) [31, 41]. To avoid deviations from HWE and erroneous results, we recommend the following: (1) cases should represent the entire population of patients and be accurately diagnosed, (2) controls should be unrelated healthy individuals matched for age and gender from the same populations, (3) controls should not be hospital-based employees or patients (even if they do not suffer from related diseases), and (4) in studies including different races, subgroup analyses should be performed.
In general, two methods are available for handling departures from HWE: excluding all non-HWE studies and evaluating the impact of each non-HWE study by sensitivity analyses. Given that almost half of the relevant studies were not in HWE, we preferred the later method and excluded studies that influenced the overall results. In contrast to the previous meta-analysis performed, the results here showed that the f allele of the FokI polymorphism is negatively associated with urolithiasis risk.
Although genetic factors create a predisposition to urolithiasis, only a few relevant genetic loci have been identified. Recently, the results of two genome-wide association studies (GWAS) indicated four risk susceptibility loci: 21q22.13 (CLDN14) in Europeans and Japanese [40, 42], 5q35.3 (RGS14-SLC34A1-PFN3-F12), 7p14.3 (INMT-FAM188B-AQP1) and 13q14.1 (DGKH) in Japanese [42]. These studies recommended that more risk susceptibility loci, and the molecular mechanisms of urinary calculi induced by these variants, should be further investigated.
There are some limitations in this meta-analysis. First, due to insufficient original data on gender, age, lifestyle, and other genetic factors, this meta-analysis was based on unadjusted estimates that were relatively inaccurate. Second, although environment and diet may partially contribute to urinary stones and modify gene expression at different biological levels [13], gene–gene and gene–environment interactions could not be investigated. Thus, additional research regarding gene–gene and gene–environment interactions is required. Third, possible publication bias may exist because only published studies in English and Chinese were included, although the funnel plot showed no apparent asymmetric.
In conclusion, our meta-analysis indicates that the TaqI tt + Tt genotype had a modest, but statistically significant relationship to urolithiasis risk. In addition, a deviation from HWE was identified as the major source of heterogeneity. In the future, more research on other relevant SNPs besides VDR (e.g., those in CLDN14, SLC34A1, AQP1, and DGKH) should be assessed for their relevance and molecular mechanisms during urolithiasis formation.
References
Aji K, Song G, Yasen A, Azad B, Tursun H (2012) Association of vitamin D receptor gene polymorphisms with urolithiasis in Uyghur children from southern Xinjiang, China. Zhongguo dang dai er ke za zhi 14:956–959
Anderson RA (2002) A complementary approach to urolithiasis prevention. World J Urol 20:294–301
Arai H, Miyamoto KI, Taketani Y, Yamamoto H, Iemori Y, Morita K, Tonai T, Nishisho T, Mori S, Takeda E (1997) A vitamin D receptor gene polymorphism in the translation initiation codon: effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res 12:915–921
Attia J, Thakkinstian A, D’Este C (2003) Meta-analyses of molecular association studies: methodologic lessons for genetic epidemiology. J Clin Epidemiol 56:297–303
Basiri A, Shakhssalim N, Houshmand M, Kashi AH, Azadvari M, Golestan B, Pargoo EM, Pakmanesh H (2012) Coding region analysis of vitamin D receptor gene and its association with active calcium stone disease. Urol Res 40:35–40
Bid HK, Chaudhary H, Mittal RD (2005) Association of vitamin-D and calcitonin receptor gene polymorphism in paediatric nephrolithiasis. Pediatr Nephrol 20:773–776
Bid HK, Kumar A, Kapoor R, Mittal RD (2005) Association of vitamin D receptor-gene (FokI) polymorphism with calcium oxalate nephrolithiasis. J Endourol 19:111–115
Bland JM, Altman DG (1995) Multiple significance tests: the Bonferroni method BMJ. Br Med J 310:170
Chen W-C, Chen H-Y, Hsu C-D, Wu J-Y, Tsai F-J (2001) No association of vitamin D receptor gene Bsm I polymorphisms with calcium oxalate stone formation. Mol Urol 5:7–10
Chen WC, Chen HY, Lu HF, Hsu CD, Tsai FJ (2001) Association of the vitamin D receptor gene start codon Fok I polymorphism with calcium oxalate stone disease. BJU Int 87:168–171
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Gao B, Yasui T, Okada A, Tozawa K, Hayashi Y, Kohri K (2005) A polymorphism of the osteopontin gene is related to urinary calcium stones. J Urol 174:1472–1476
Goldfarb DS, Fischer ME, Keich Y, Goldberg J (2005) A twin study of genetic and dietary influences on nephrolithiasis: a report from the Vietnam Era Twin (VET) Registry. Kidney Int 67:1053–1061
Goodman HO, Holmes RP, Assimos DG (1995) Genetic factors in calcium oxalate stone disease. J Urol 153:301–307
Gunes S, Bilen CY, Kara N, Asci R, Bagci H, Yilmaz AF (2006) Vitamin D receptor gene polymorphisms in patients with urolithiasis. Urol Res 34:47–52
Holick MF (1994) McCollum Award Lecture, 1994: vitamin D–new horizons for the 21st century. Am J Clin Nutr 60:619–630
Hosking L, Lumsden S, Lewis K, Yeo A, McCarthy L, Bansal A, Riley J, Purvis I, Xu C-F (2004) Detection of genotyping errors by Hardy–Weinberg equilibrium testing. Eur J Hum Genet 12:395–399
Jackman SV, Kibel AS, Ovuworie CA, Moore RG, Kavoussi LR, Jarrett TW (1999) Familial calcium stone disease: Taq I polymorphism and the vitamin D receptor. J Endourol 13:313–316
Lin Y, Mao Q, Zheng X, Chen H, Yang K, Xie L (2011) Vitamin D receptor genetic polymorphisms and the risk of urolithiasis: a meta-analysis. Urol Int 86:249–255
Liu CC, Huang CH, Wu WJ, Huang SP, Chou YH, Li CC, Chai CY, Wu MT (2007) Association of vitamin D receptor (Fok-I) polymorphism with the clinical presentation of calcium urolithiasis. BJU Int 99:1534–1538
Mantel N, Haenszel W (2004) Statistical aspects of the analysis of data from retrospective studies of disease. Chall Epidemiol Issues Sel Read 1:533–553
Mittal RD, Mishra D, Srivastava P, Manchanda P, Bid H, Kapoor R (2010) Polymorphisms in the vitamin D receptor and the androgen receptor gene associated with the risk of urolithiasis. Indian J Clin Biochem 25:119–126
Morrison NA, Qi JC, Tokita A, Kelly PJ, Crofts L, Nguyen TV, Sambrook PN, Eisman JA (1994) Prediction of bone density from vitamin D receptor alleles. Nature 367:284–287
Mossetti G, Vuotto P, Rendina D, Numis F, Viceconti R, Giordano F, Cioffi M, Scopacasa F, Nunziata V (2003) Association between vitamin D receptor gene polymorphisms and tubular citrate handling in calcium nephrolithiasis. J Intern Med 253:194–200
Nishijima S, Sugaya K, Naito A, Morozumi M, Hatano T, Ogawa Y (2002) Association of vitamin D receptor gene polymorphism with urolithiasis. J Urol 167:2188–2191
Özkaya O, Söylemezoğlu O, Mısırlıoğlu M, Gönen S, Buyan N, Hasanoğlu E (2003) Polymorphisms in the vitamin D receptor gene and the risk of calcium nephrolithiasis in children. Eur Urol 44:150–154
Relan V, Khullar M, Singh S, Sharma S (2004) Association of vitamin D receptor genotypes with calcium excretion in nephrolithiatic subjects in northern India. Urol Res 32:236–240
Rendina D, Mossetti G, Viceconti R, Sorrentino M, Castaldo R, Manno G, Guadagno V, Strazzullo P, Nunziata V (2004) Association between vitamin D receptor gene polymorphisms and fasting idiopathic hypercalciuria in recurrent stone-forming patients. Urology 64:833–838
Roy C, Gies H, Toomey S (1995) The solar UV radiation environment: measurement techniques and results. J Photochem Photobiol B Biol 31:21–27
Ruggiero M, Pacini S, Amato M, Aterini S, Chiarugi V (1999) Association between vitamin D receptor gene polymorphism and nephrolithiasis. Miner Electrolyte Metab 25:185–190
Schaid DJ, Jacobsen SJ (1999) Blased Tests of Association: comparisons of allele frequencies when departing from Hardy-Weinberg proportions. Am J Epidemiol 149:706–711
Seo IY, Kang I-H, Chae S-C, Park SC, Lee Y-J, Yang YS, Ryu SB, Rim JS (2010) Vitamin D Receptor Gene AlwI FokI ApaI and TaqI polymorphisms in patients with urinary stone. Urology 75:923–927
Seyhan S, Yavascaoglu I, Kilicarslan H, Dogan HS, Kordan Y (2007) Association of vitamin D receptor gene Taq I polymorphism with recurrent urolithiasis in children. Int J Urol 14:1060–1062
Shaogang W, Jihong L, Shaoqun H, Zhangqun Y (2003) Association of vitamin D receptor gene polymorphisms with calcium oxalate calculus disease. J Huazhong Univ Sci Technol 23:38–41
Simpson D (1983) Citrate excretion: a window on renal metabolism. Am J Physiol 244:F223–F234
Snellman G, Melhus H, Gedeborg R, Olofsson S, Wolk A, Pedersen NL, Michaelsson K (2009) Seasonal genetic influence on serum 25-hydroxyvitamin D levels: a twin study. PLoS ONE 4:e7747
Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC (2003) Time trends in reported prevalence of kidney stones in the United States: 1976–19941. Kidney Int 63:1817–1823
Thakkinstian A, McElduff P, D’Este C, Duffy D, Attia J (2005) A method for meta-analysis of molecular association studies. Stat Med 24:1291–1306
Thakkinstian A, McEvoy M, Minelli C, Gibson P, Hancox B, Duffy D, Thompson J, Hall I, Kaufman J, Leung TF, Helms PJ, Hakonarson H, Halpi E, Navon R, Attia J (2005) Systematic review and meta-analysis of the association between {beta}2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J Epidemiol 162:201–211
Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, Sulem P, Halldorsson BV, de Vegt F, d’Ancona FC, den Heijer M, Franzson L, Christiansen C, Alexandersen P, Rafnar T, Kristjansson K, Sigurdsson G, Kiemeney LA, Bodvarsson M, Indridason OS, Palsson R, Kong A, Thorsteinsdottir U, Stefansson K (2009) Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 41:926–930
Trikalinos TA, Salanti G, Khoury MJ, Ioannidis JP (2006) Impact of violations and deviations in Hardy-Weinberg equilibrium on postulated gene-disease associations. Am J Epidemiol 163:300–309
Urabe Y, Tanikawa C, Takahashi A, Okada Y, Morizono T, Tsunoda T, Kamatani N, Kohri K, Chayama K, Kubo M, Nakamura Y, Matsuda K (2012) A genome-wide association study of nephrolithiasis in the Japanese population identifies novel susceptible Loci at 5q35.3, 7p14.3, and 13q14.1. PLoS Genet 8:e1002541
Vezzoli G, Terranegra A, Arcidiacono T, Gambaro G, Milanesi L, Mosca E, Soldati L (2010) Calcium kidney stones are associated with a haplotype of the calcium-sensing receptor gene regulatory region. Nephrol Dial Transplant 25:2245–2252
Wang S, Wang X, Wu J, Lin Y, Chen H, Zheng X, Zhou C, Xie L (2012) Association of vitamin D receptor gene polymorphism and calcium urolithiasis in the Chinese Han population. Urol Res 40:277–284
Whitehead A, Whitehead J (1991) A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med 10:1665–1677
Conflict of interest
The authors have no financial and non-financial conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
W. Liu and M. Chen contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, W., Chen, M., Li, M. et al. Vitamin D receptor gene (VDR) polymorphisms and the urolithiasis risk: an updated meta-analysis based on 20 case–control studies. Urolithiasis 42, 45–52 (2014). https://doi.org/10.1007/s00240-013-0619-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-013-0619-y